Abstract
The coordinated activity of neural ensembles across multiple interconnected regions has been challenging to study in the mammalian brain with cellular resolution using conventional recording tools. For instance, neural systems regulating learned behaviors often encompass multiple distinct structures that span the brain. To address this challenge we developed a three-dimensional (3D) silicon microprobe capable of simultaneously measuring extracellular spike and local field potential activity from 1,024 electrodes. The microprobe geometry can be precisely configured during assembly to target virtually any combination of four spatially distinct neuroanatomical planes. Here we report on the operation of such a device built for high-throughput monitoring of neural signals in the orbitofrontal cortex and several nuclei in the basal ganglia. We perform analysis on systems-level dynamics and correlations during periods of conditioned behavioral responding and rest, demonstrating the technology's ability to reveal functional organization at multiple scales in parallel in the mouse brain.
Keywords: basal ganglia, high-density electrodes, multielectrodes, orbitofrontal cortex, silicon probes
extracellular microelectrode arrays provide large-scale measurements of neural spiking and local oscillatory fields that are currently not possible with other recording techniques (Buzsáki 2004). Generally, conventional electrode arrays with tens of sites are deployed in single anatomical areas, or divided across multiple areas (Paz et al. 2009). But these configurations provide a sparse view of how local neural information processing is coordinated across anatomically distributed circuits. Thus there is a strong need to increase the number of electrodes at multiple spatial scales, at the level of both local microcircuits as well as macroscopic circuits containing distally located interconnected regions (Stevenson and Kording 2011).
The challenge of realizing recordings at multiple length scales has been addressed to some degree by implanting a large number of electrode microwires (Schwarz et al. 2014), or by relying on microfabrication techniques to increase the density of electrode arrays (Berényi et al. 2014; Blanche et al. 2005; Csicsvari et al. 2003; Wise et al. 2008). However, further increasing the electrode number, density, or the number of regions sampled in parallel may pose difficulties with these approaches in terms of device fabrication, assembly, or tissue displacement. An alternative approach to monitoring activity is to image calcium dynamics; however, calcium imaging is inherently limited in its temporal resolution, and given existing methods it is not possible to observe more than two separate regions in parallel in the mammalian brain (Lecoq et al. 2014). Scalability with current electrode and optical-based tools is especially challenging in the mouse. Mice are an attractive mammalian model for studying brain network dynamics in light of their genetic accessibility, but their relatively small size presents a significant obstacle in terms of accessible space and weight load on the head. Fortunately, a variety of behavioral tasks have been adapted for recordings in head-fixed animals (Dombeck et al. 2007; Dombeck and Reiser 2012), which could mitigate potential size and weight limitations of a large-scale recording system. One approach to developing such a system in a mouse would involve placement of multiple high-density, minimally invasive electrode arrays throughout the brain. These recording tools could dramatically expand the ability to study brain dynamics in the awake behaving mouse, helping to link different scales of brain function, and informing computational and network science approaches to modeling neural systems.
Here we leverage advancements in silicon device microfabrication and assembly techniques to develop a three-dimensional (3D) microelectrode array that can be precisely configured to record from four distinct regions in the mouse brain. The microprobe is built to densely sample activity from local microcircuits with miniaturized silicon prongs containing up to 64 closely packed electrodes, as well as macroscopic circuits using over a dozen such prongs separated by submillimeter length scales. We introduce a 3D microprobe containing 1,024 electrodes monitored in parallel, surpassing the number of simultaneously recorded signals of other in vivo electrode arrays. We evaluate the technology's capabilities by capturing task-evoked and resting state network dynamics in awake head-fixed mice responding to olfactory and appetitive stimuli. This technology has broad applicability in systems-level neuroscience due to its customizable 3D configuration and ability to provide large-scale and high-throughput electrophysiological data.
MATERIALS AND METHODS
3D silicon microprobe recording system development.
To develop a technology suitable for mapping brain activity at multiple scales in the mouse, we addressed several technical challenges related to high-density electrode array fabrication, instrumentation to monitor electrical signals, and assembly. First, we relied on wafer-scale microfabrication techniques for the production of newly designed silicon devices with microelectrodes dispersed along implantable silicon prongs (Fig. 1A). The fabrication was carried out in a silicon microelectromechanical systems (MEMS) foundry (Innovative Micro Technology) on 150-mm silicon-on-oxide wafers. The fabrication process was adapted from a previously described method resulting in submicron electrical interconnects (Du et al. 2011), but was improved in terms of device yield thanks to the larger wafer diameter (now producing over 200 functional probes per wafer) and maximum number of electrodes per device (now increased to 256). Fabrication involved a combination of material deposition (Au for the electrodes, leads and wire bond contacts, stress-free silicon nitride for the upper and lower dielectric layers), photolithography for masking, and dry etching processes with various chemistries to selectively and nonisotropically pattern metal, dielectrics, and silicon. Following fabrication, devices were released from a carrier substrate by immersing in water heated to 80°C, cleaned with deionized water and ethanol, and stored for later use.
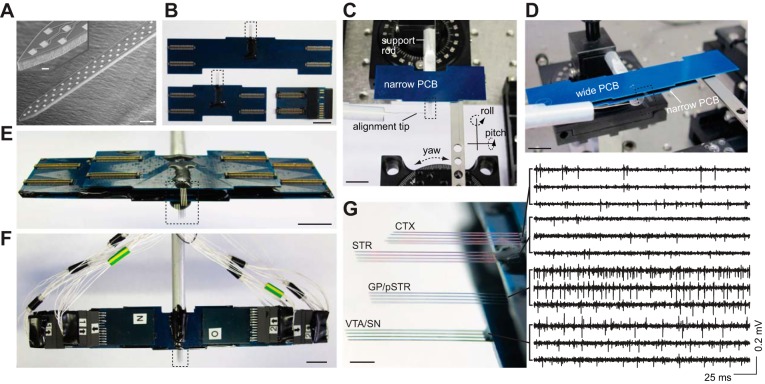
Fig. 1.
Three-dimensional (3D) silicon microprobe development. A: scanning electron micrograph of a microfabricated silicon prong containing a high-density 64-electrode array. The electrode array spans 1 mm from end to end. Scale bar represents 50 μm. Inset shows a higher magnification view of the tip (10-μm scale bar). B: wide-winged (top) and narrow (bottom left) printed circuit boards (PCBs) used to create a stacked multilayer 3D microprobe. Each PCB is attached to a multipronged 256-electrode silicon probe (boxed areas). B, bottom right: one of eight 128-channel head stages used to read out electrophysiological signals. Scale bars in B–F represent 10 mm. C: alignment of the first layer consisting of a narrow PCB to an aluminum support rod. The alignment tip is used to orient the silicon prongs parallel to the insertion axis. D: alignment of the second layer consisting of a wide PCB to the underlying narrow PCB. E: fully assembled 1,024-electrode 3D microprobe combining 2 narrow and 2 wide-winged PCB layers each containing a 256-electrodes array. F: fully connected ready-to-use 3D microprobe with 1,024 electrodes. The 3D microprobe is imaged with all head stages and wires attached, in preparation for recording. Note the significant reduction in external wires relative to the number of electrodes due to multiplexing circuitry on the head stages. There are a total of 8 head stages, with half not visible as they are plugged into the bottom side of the structure. G, left: high-magnification view of the boxed area in E showing the exact 3D configuration. G, right: representative band-pass filtered signals (600-6,500 Hz) from 3 electrode recording sites in each layer. Scale bar represents 2 mm. CTX, orbitofrontal cortex; STR, anterior striatum; pSTR, posterior striatum; GP, globus pallidus; VTA, ventral tegmental area; SN, substantia nigra.
The minimum device feature size was 0.4 μm, corresponding to the narrowest width of the conducting Au wires. The microprobes consisted of 22-μm-thick Si for mechanical support, 0.5-μm bottom insulating nitride, 0.1-μm Au, and 0.5-μm top nitride layers. To enable high-density recordings from local microcircuits, up to 64 electrodes were patterned on the top face of each silicon prong. The Au recording sites had dimensions of 10 μm × 10 μm and a center-to-center neighbor spacing of 37 μm, except near the tips, where spacing was slightly modified to accommodate the tapered prong profile. The spacing was selected to provide a dense and often redundant sampling of extracellular fields along the depth of the array, and was based on prior experimental measurements and biophysical models (Gold et al. 2007; Henze et al. 2000). Prongs had a maximal width of 86 μm that tapered to a point at the tip, and were separated by 300–400 μm to provide sampling at multiple proximal locations in the same or nearby brain structure. Thus the overall silicon prong dimensions were 7 mm × 86 μm × 23 μm (l × w × t).
Devices containing 256 electrodes were attached and wire bonded to a custom-built printed circuit board (PCBs manufactured at Hughes Circuits; wire bonding at IDAX Microelectronics). PCBs contained four compact 64-pin electrical connectors (Molex, Slimstack 502430-6410) for head stage access (Fig. 1B). The wire bond pads were encapsulated with epoxy (Resinlab EP965 black), which was cured at 50°C. Each recording site was gold plated (Sifco 80535500) with constant-potential pulses (−2.5 V relative to a Pt wire reference, 1–5 s) to reduce impedance below 0.5 MΩ to improve the signal-to-noise ratio (SNR) (Du et al. 2011).
To extend the recording tools into the third dimension for making simultaneous recordings from multiple distinct areas, we developed an assembly method for rigidly combining four separate layers. Each layer consisted of a 256-electrode array bonded to a PCB. To permit electrical access to the interior PCBs, two of the layers, targeting the outermost areas, were electrically connected to narrow PCBs, whereas the two inner layers were connected to wide PCBs (Fig. 1B). During assembly, the position and orientation of silicon prongs in each layer was controlled with micrometer precision using a motorized micromanipulator providing translation in three axes (Sutter Instruments MP-285), as well as optomechanical parts providing roll and pitch (Thor Labs dual axis goniometer GN2), and yaw (Thor Labs rotation platform RP01). The combined positioning system provided six independently controlled degrees of freedom to achieve 3D alignment of four electrode arrays. The first layer in the 3D microprobe was bonded with epoxy to an aluminum rod for mechanical support (Fig. 1C). Each successive layer was aligned and then rigidly and permanently bonded together for mechanical support before adding the next layer (Fig. 1D).
Here we demonstrate this technology with a 3D configuration targeting several interconnected nuclei in the basal ganglia including the striatum, globus pallidus (GP), ventral tegmental area (VTA), substantia nigra (SN), and the orbitofrontal cortex, which projects to the striatum (Alexander et al. 1986). To specifically target these structures in the mouse, we spaced successive silicon layers by ∼1 to 3 mm. Collectively, this 3D microprobe contained 1,024 recording sites with precisely known spatial positions (Fig. 1E).
We used a multiplexed signal readout method to minimize the recording system's external wiring requirements (Du et al. 2011), which reduced the external cable to just 65 wires (Fig. 1F). Readout was achieved via eight custom-built 128-channel detachable head stage modules (Fig. 1B). Head stages contained commercial integrated electronic circuits (Intan Technologies RHA-2164B) (Harrison and Charles 2003) providing signal multiplexing (32 electrodes per multiplexed output wire), amplification (gain=200), and filtering (0.1–8000 Hz) functions. Each head stage contained two 64-pin connectors (Molex, Slimstack 502426-6410) connecting to the PCBs bonded to the silicon microprobes. Analog signals were transmitted through thin flexible cables and subsequently digitized on four 16-bit analog-to-digital conversion (ADC) cards (USB-6356, National Instruments), each card supporting 256 channels, and each channel representing one recording electrode. Signals from all 1,024 channels were simultaneously and continuously sampled at a rate of 25 kHz per channel, with some representative signals that were filtered offline shown in Fig. 1G. There was a sampling delay of 40 μs between the first and last channel corresponding to the multiplexing switching time, but since this delay is significantly less than electrophysiological timescales of action potentials it does not degrade the overall system performance. Mouse behavioral parameters including licking activity and spherical treadmill rotation were monitored at a rate of 10 kHz with an additional ADC card (USB-6351, National Instruments). All ADC cards were synchronized via a shared internal clock. Local field potential (LFP) signals were downsampled offline to 1 kHz. All data acquisition, as well as control of stimulus timing, was performed with custom LabVIEW scripts. All data analysis was carried out with custom Matlab scripts.
Animal surgical, behavioral, and recording procedures.
All procedures were approved by the University of California, Los Angeles Chancellor's Animal Research Committee. Singly housed male C57Bl/6J mice (n = 6, 12–16 wk old, The Jackson Laboratory) were used in the experiments. Animals underwent an initial surgery under isoflurane anesthesia in a stereotaxic apparatus to bilaterally fix stainless steel head restraint bars on the skull (10 mm × 7.5 mm, 0.6 g, laser cut at Fab2Order). Animals were anesthetized with isoflurane for a second surgery on the recording session day to make three rectangular craniotomies for microprobe insertion. The dura mater was opened to facilitate insertion. An additional craniotomy was made over the posterior cerebellum for placement of an electrical reference wire.
After recovery from the first surgery, animals were food restricted and fed daily after each training session to maintain ∼90% of their baseline weight. They received water ad libitum. During daily training sessions, animals were mounted with the head bar bracket on a behavioral testing rig and on top of a rotatable spherical treadmill (200 mm diameter, Graham Sweet Studios, free to rotate forward and backward when animals walk or run). The treadmill's linear velocity was monitored with an optical mouse (Niell and Stryker 2010). A reward solution (5 μl, 10% sweetened condensed milk) was dispensed from a tube positioned between an infrared beam lick meter (Island Motion), and its delivery was signaled by an audible solenoid valve actuation (Neptune Research 161T010). Initially, animals were habituated to head fixation and trained to respond to reward. During these sessions mice received rewards alone (lick tube; maximum 100 rewards per session, 13–21 s intertrial interval, ITI), and a constant flow of odorless air (1.5 l/min) through an air tube. Thus mice learned to associate the solenoid actuation sound with a reward, and after consuming at least 90% delivered rewards for 2 consecutive days, they began Pavlovian conditioning with olfactory cues. Odorants were introduced via an olfactometer by bubbling air (0.15 l/min) through aromatic liquids diluted 1:10 in mineral oil (Sigma-Aldrich), and mixing this product with the 1.5 l/min main stream of air. Odors were presented in pseudorandom order (1 s duration, 17–29 s ITI, 100 rewarded CS+ and 100 unrewarded CS− trials). The odor corresponding to CS+ trials was amyl acetate, and citral for CS− trials. During training mice began to lick in anticipation of the reward (in the interval between odor and reward; 0–2.5 s). In the recording session animals received 100 CS+ with 85% reward probability and 100 CS− stimuli with no reward. To quantify performance, a correct CS+ trial was defined as initiation of licking 0–2.5 s post cue onset. A correct CS− trial was defined as withholding of licking 0–5 s post cue onset.
After the craniotomy surgery, animals recovered from anesthesia in their home cage for ∼2 h before being placed on the testing rig. An Ag/AgCl reference wire was placed on the cerebellar surface, covered in ACSF-saturated water absorbing foam (Gelfoam, Pfizer) to improve electrical contact, and sealed with silicone elastomer (Kwik-Cast, WPI). The silicon microprobes were coated with fluorescent dye (Invitrogen DiD), and slowly lowered to stereotaxically defined coordinates with a micromanipulator (MP-285, Sutter Instruments). The insertion was monitored with a surgical microscope (Zeiss OPMI pico). After reaching the target depth a drop of mineral oil was placed on the exposed cortical surface, and a 45 min settling period elapsed before beginning the behavioral task and data acquisition. Mice were given occasional rewards (every 2–5 min) during the settling period to help maintain a high level of motivation. Following each recording probes were cleaned in trypsin solution (Invitrogen), rinsed with deionized water and ethanol, and stored for reuse.
Histology and electrode position determination.
Following each experiment the brain was coronally sectioned at 100 μm on a −20°C cryostat and individual sections were placed in order into a 24-well plate containing cold cryoprotectant solution. We then performed immunohistochemistry using standard procedures on slices in the regions of interest to determine the exact placement of the silicon prongs in each region. Sections were stained for neuronal nuclei with rabbit α NeuN (Cell Signaling Technology D3S3I) and for dopamine terminals with sheep α tyrosine hydroxylase (Millipore AB1542), in addition to the DiD which diffusively labeled tissue near the probe insertion sites. Each of the 1,024 recording sites was assigned a coordinate in 3D Cartesian space based on the expected location relative to bregma, and assigned to a brain region based on brain slice confocal imaging (Fig. 2A). The following possible region assignments were used: orbitofrontal cortex (CTX); anterior striatum (STR); posterior striatum (pSTR); globus pallidus (GP); VTA; SN; other midbrain areas; and other unspecified areas. The single-unit analysis did not use signals from other midbrain areas outside the VTA and SN, or other unspecified areas. Furthermore, data from the VTA and SN were combined. Local field potential (LFP) coherence maps were constructed using all recording sites.
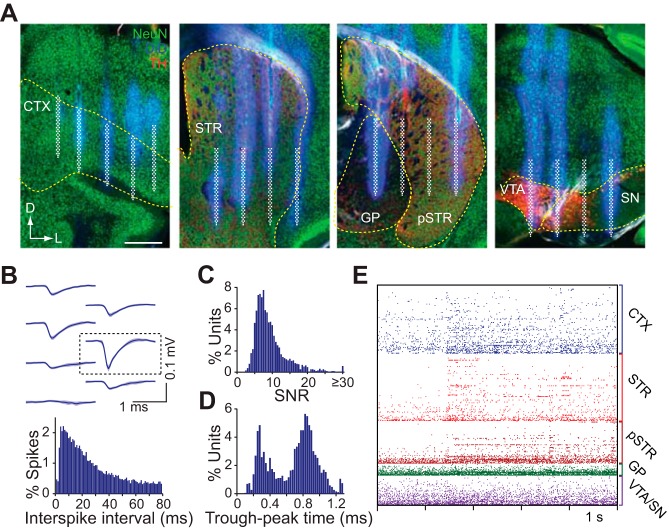
Fig. 2.
Histology and single-unit characteristics. A: confocal microscope images of the coronal brain sections from four simultaneously targeted areas. The DiD fluorescence signals indicate the silicon prong insertion sites, from which the electrode positions were reconstructed. The approximate location of the electrode arrays are superimposed in white. Sections are colabeled with antibodies against NeuN and tyrosine hydroxylase. Dashed lines indicate boundaries of the regions of interest. Scale bar represents 0.5 mm. B, top: representative mean spike waveform of a putative cortical unit on a local cluster of electrodes. The boxed area corresponds to the electrode where the unit is approximately located. B, bottom: interspike interval distribution for the above unit. C: signal-to-noise (SNR) distribution of all putative single units from 6 recording sessions. D: bimodal spike width (trough-to-peak time) distribution of all putative single-units. E: activity raster of 308 (95 orbitofrontal, 155 striatal, 17 pallidal, and 41 VTA/SN) simultaneously recorded units from 1 animal during an olfactory stimulus presentation.
Spike sorting and single-unit position determination.
The 1,024 electrode array was first subdivided into multiple sets of 2–5 local electrodes. Due to the proximity of neighboring recording sites, units were often recorded on multiple electrodes (Fig. 2B). To minimize globally correlated signal artifacts, the mean background signal was removed from each electrode by subtracting the mean voltage of all electrodes on the corresponding silicon prong. Signals were then filtered in the 600–6,500 Hz band, and spikes were detected on the spike trough with an SNR threshold of ∼3 (Fig. 2C). Putative units were then isolated on this local set of electrodes. We isolated units by automatically generating waveform-based templates using data from the first 5 min of the recording session, and then matching spikes from the entire session to those templates. Possible duplicate units occurring on more than one local set of electrodes were automatically identified by comparing mean spike waveform, and discarded. Time stamps from the remaining units were used to repeat waveform collection under a wider frequency band (300–6,500 Hz) for visualization and manual scoring. Finally, all units were manually inspected and discarded if their waveforms appeared to represent a signal artifact, or if they did not meet minimum waveform amplitude (50 μV) or spike number criteria (at least 150 across the recording session). Based on the targeted electrode positions, single units were assigned an estimated 3D coordinate as well as a histologically determined brain region. The estimated position coincided with the recording site exhibiting the highest spike amplitude for that unit (Fig. 2B).
Signal and resting state neural correlations.
For signal correlations (rsignal) we calculated the Pearson correlation coefficient from the mean cue-triggered firing rate during correct CS+ trials 0–2.5 s after cue onset, in 50-ms bins. For resting state correlations (rrest) we defined resting periods as intervals of at least 2 s during which animals did not make any movements (running, licking) and were not presented with any external stimuli (cues and reward). We serially concatenated spiking activity occurring within these epochs to create a continuous time series vector (maximum 500 s, 50-ms bins) representing the resting state activity. We calculated resting state activity for each individual unit, and then obtained the pairwise Pearson correlation coefficient corresponding to rrest using these vectors. To identify significant resting state correlations, we performed a permutation test for correlations (see Statistical tests). We defined a pair of units as significantly correlated, hence functionally connected (FC), if the test showed a P value of <0.01.
K-means clustering.
The signal correlation of evoked unit activity during correct CS+ trials was clustered using a custom k-means algorithm. K-means clustering was performed separately in five regions (cortex, striatum, posterior striatum, GP, and VTA/SN). Based on a qualitative assessment of cluster quality, the number of clusters k into which cells were grouped was set to three in the cortex and anterior striatum and two in the posterior striatum, GP, and VTA/SN nuclei. Results were compiled into a single correlation matrix containing five nested matrices representing the independently calculated intraregional k-means clusters, as well as interregional signal correlation values which were not explicitly clustered.
Statistical tests.
We used custom Matlab scripts to perform nonparametric permutation tests to determine the significance of paired group comparisons, unpaired group comparisons, and pairwise correlations. For very large sample sizes the permutation tests are nearly indistinguishable from standard parametric tests (e.g., the unpaired t-test is comparable to the unpaired group permutation test); however, as sample size is often small (n < 10), permutation tests provide a more reliable estimate of significance as there are no assumptions about the underlying distribution of data values (Narayanan and Laubach 2009). All statistical test functions used in this work are provided as a Data Supplement, available with the online version of this article, and are briefly described below.
For unpaired group comparisons (unpaired group permutation test), we calculated the observed difference in means between the two groups, pooled all of the data, and randomly assigned data points into two shuffled groups. We then calculated the difference in means between the shuffled groups, repeating this step 10,000 times. This resulted in a distribution of possible between-group differences in means that could be expected by chance. The fraction of difference values from the random distribution that was greater than the observed mean over the total number of iterations was reported as the P value for the test. For paired group comparisons (paired group permutation test), paired values from the same subject were never uncoupled during the randomization; otherwise the algorithm was identical to the unpaired test.
The permutation test for correlations involved calculating the observed correlation coefficient (robserved) between two array values being compared, then shuffling the values in one array relative to the other and recalculating the shuffled correlation coefficient (rshuffled, 1,000–10,000 iterations). This resulted in a distribution of possible correlation coefficients that could be expected by chance. The fraction of rshuffled values with absolute value greater than robserved was reported as a P value for the permutation test for correlations.
RESULTS
Recording system demonstration.
To demonstrate our recording system's capabilities, we conducted acute recordings with the device in six cognitively alert head-fixed mice (one recording session per animal, duration of ∼90 min). The anatomical placement of each individual silicon prong was verified histologically after every experiment. This allowed us to accurately estimate the location of each recording site in the brain (Fig. 2A). We restricted our single-unit analysis to cells from histologically identifiable regions of interest (orbitofrontal cortex, striatum, GP, and VTA/SN, hereafter referred to collectively as the recording field). These regions were chosen because of their interconnectivity (Alexander et al. 1986), their involvement in reward-guided actions (Schultz 2000) and because such large-scale recordings have never been carried out in these areas at the same time. We used a semiautomated spike sorting algorithm for high density electrode array recordings to extract putative single unit spiking information (Fig. 2B). Each recording session yielded an average of 315 ± 80 (mean ± 1 SD; range: 222–418) simultaneously measured units across all electrodes on the 3D microprobe. The extracellular spike characteristics of these units were of high quality as measured by their SNR (median = 8.4; Fig. 2C) and were broadly separable into two categories corresponding to narrow (mean ± 1 SD: 0.3 ± 0.1 ms) and wide (0.8 ± 0.2 ms) waveforms (Fig. 2D). This dissociation is consistent with recordings from a heterogeneous population containing broad and narrow spiking cells (Barthó et al. 2004; Mallet et al. 2005; McCormick et al. 1985). Here we analyzed the dynamics of the combined population. We tested the power of the 3D silicon microprobe device to reveal widespread modulation and correlation of neural activity by rewarding stimuli and cues that predict them (Fig. 2E). The results we present here focus on analytical approaches facilitated by this technology's ability to record at high throughput and large scale.
Mapping neural dynamics at multiple scales.
To explore behaviorally modulated network dynamics across the orbitofrontal cortex and basal ganglia, we trained mice to perform olfactory discrimination with a Pavlovian reward conditioning task (Cohen et al. 2012). Our experimental setup allowed us to deliver external olfactory and reward stimuli, and monitor licking to assess behavioral performance (Fig. 3A). Over several sessions, animals were repeatedly presented with a conditioned stimulus paired with a reward (CS+ trials), and a second neutral stimulus that was delivered without reward (CS− trials). During training mice acquired an anticipatory licking response that was selective to CS+ over CS− cues, indicating mice had learned to discriminate the two olfactory stimuli (Fig. 3B). Recordings were carried out in well-trained animals following 5–7 daily training sessions.
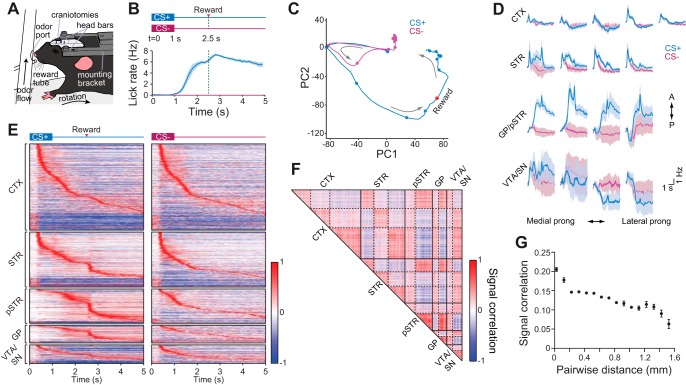
Fig. 3.
Mapping of network dynamics at multiple scales. A: behavioral testing and recording setup for a head fixed mouse mounted on top of a spherical treadmill. B, top: reward conditioning scheme showing the two types of stimulus conditions: CS+ odor (1 s duration) paired with a reward at 2.5 s, and unpaired CS− odor (1 s duration). B, bottom: mean lick rate of all animals triggered on correct CS+ (anticipatory licking) and CS− (withheld licking) trials. Dashed green line represents time of reward delivery for CS+ trials. Shading represents ± SE. C: principal component analysis (PCA) trajectory of the mean activity of 308 simultaneously recorded units from 1 animal under different stimulus conditions. The trajectories start near the origin. Dots denote 500-ms time intervals. The red dot indicates the reward delivery time. Arrows indicate the trajectory direction. D: mean change in population firing rate of units recorded on the 17 silicon prongs comprising the 3D microprobe. Activity is plotted from t = 0 to t = 5 s post stimulus onset. Data represent combined recordings from 6 animals. Shading represents ± SE. E: mean evoked activity of units in different regions triggered on correct CS+ (left) and CS− (right) trials. Data represent 1,609 units combined from 6 animals. Activity is plotted as the normalized difference in firing rate relative to a baseline period prior to cue onset. Units are ordered according to their latency to peak firing separately within each region and stimulus condition. F: signal correlation matrix for mean task-evoked activity during CS+ trials. Data correspond to the units displayed in E combined from 6 animals. Matrix elements were arranged by independent k-means clustering in each of the anatomical areas indicated. Three clusters were assigned to the cortex and striatum, and the other areas were assigned two clusters. Solid lines demarcate the brain areas where k-means clustering took place separately. Dashed lines indicate the k-means cluster boundaries within each area. G: mean signal correlation vs. pairwise distance between units located in the same brain region (CTX, STR, pSTR, GP, or VTA/SN). Data are combined from all simultaneously recorded cell pairs in 6 animals. Points denote means ± SE binned in increments of 0.1 mm.
To visualize the task-evoked dynamics of the entire simultaneously recorded ensemble from an individual animal (308 units), we first performed dimensionality reduction using principal component analysis (PCA) on mean stimulus-triggered firing rate (Fig. 3C) (Cunningham and Yu 2014). The CS+ and CS− trajectories overlapped during the first second of the trial which corresponded to time during odor presentation. However, shortly after odor offset, CS− activity diverged and returned to its original state. In contrast the CS+ trajectory followed a longer path before its return. This divergence likely reflects the differential neural representation between CS+ and CS− odors, with the longer trajectory of CS+ trials encoding the ensuing anticipatory behavior as well as reward consumption.
To map the spatiotemporal organization of these activity patterns, we first subdivided the mean population firing rate at the level of individual silicon prongs. The mean population activity from all recording sessions qualitatively showed regional and subregional variations in both the evoked response strength and timing to different olfactory cues, as well some marked similarities (Fig. 3D). Next we examined regional population dynamics by visualizing the mean normalized response of individual cells combined from all animals (1,609 units; Fig. 3E). A prominent feature is the appearance of continuously modulated activity patterns that persist longer for CS+ than CS− trials.
To examine the spatiotemporal relationship of task-modulated activity, we obtained signal correlation coefficients, calculated in correct CS+ trials. We then performed k-means clustering on rsignal values from each region separately (Adler et al. 2012; Dombeck et al. 2009; Ponzi and Wickens 2012), and used the resulting parameters to compile a correlation matrix representing the entire recording field (Fig. 3F). Clusters within each region represented a population of similarly tuned cells (positive rsignal). Intriguingly, the quilt-like pattern of highly correlated clusters across the matrix implies that the organization of modules of functionally related cells is preserved across different brain structures, suggesting a substantial amount of similarity in the dynamics of ensembles in different regions. Overall, the microcircuitry in each region was organized such that closely spaced cells were more similarly tuned during behavioral response than distally distributed cells (Fig. 3G), consistent with other studies showing a higher connectivity among local microcircuits (Smith and Kohn 2008). The visualization of these high-throughput recordings using different analytical approaches demonstrates the high-throughput activity mapping capabilities of the 3D microprobe.
Mapping task-evoked and spontaneous network correlations.
The similar tuning properties of many neurons across the recording field suggests specific cells are predisposed to fire together through common wiring (Ko et al. 2011). Such shared connectivity should influence spontaneously generated synchronous activity, outside of overt behavioral events (McCormick 1999; Ringach 2009; Skaggs and McNaughton 1996). To examine these intrinsic dynamics, we took advantage of the large scale of the recordings to calculate pairwise spike count correlations, rrest, coinciding with the animal's behavioral resting state. Resting intervals corresponded to periods of immobility (no treadmill rotation and no licking) in the absence of explicitly presented stimuli (Fig. 4A). To examine the relationship between spontaneous and task-evoked neural activity, we compared resting and signal correlations, and found a significant correlation between rrest and rsignal values. The large scale of our recordings made this effect evident within an individual animal (r = 0.1, P < 0.0001, permutation test for correlations; Fig. 4B), as well as in the combined population from all animals (r = 0.1, P < 0.0001 permutation test for correlations; Fig. 4C), implying this is a robust phenomenon. Moreover, the distribution of rrest values within each region exhibited a distance-dependent decrease that was qualitatively similar to that for rsignal (Fig. 4D), further supporting a link between resting and signal correlations.
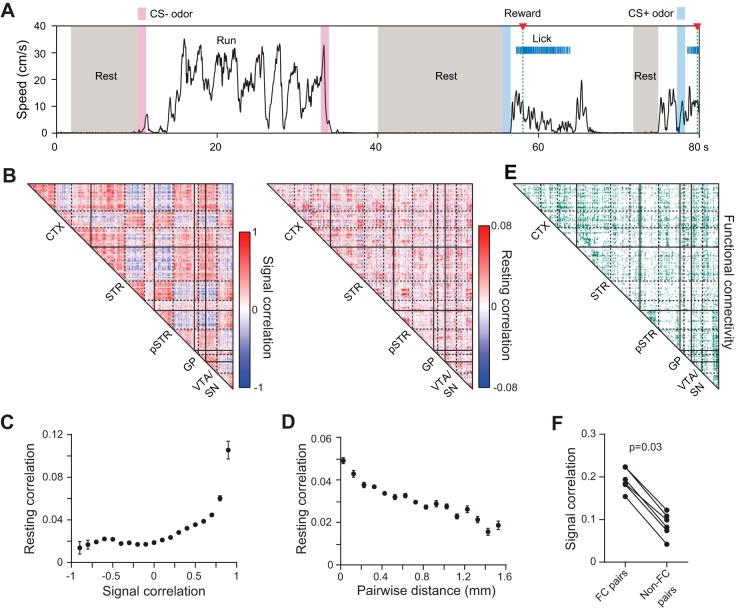
Fig. 4.
Large-scale analysis of network correlations. A: example of resting state intervals corresponding to idle periods during behavioral testing. B, left: signal correlation (rsignal) matrix for mean task-evoked activity during CS+ trials. Data represent simultaneously recorded units from 1 animal. Matrix elements were arranged by independent k-means clustering in each of the anatomical areas indicated. Three clusters were assigned to the cortex and striatum, and the other areas were assigned two clusters. Solid lines demarcate the brain areas where k-means clustering took place separately. Dashed lines indicate the cluster boundaries within each area. B, right: resting correlation (rrest) matrix using the cell ordering identified from k-means clustering of the signal correlations. The matrices are correlated (r = 0.1, P < 0.0001, permutation test for correlations). C: mean resting state correlation coefficient as a function of signal correlation during correct CS+ trials. Individual rrest and rsignal values are correlated (r = 0.1, P < 0.0001, permutation test for correlations). Data are combined from all simultaneously recorded cell pairs in 6 animals. Points denote binned means ± SE. D: mean resting state correlation coefficient as a function of pairwise distance between units located in the same brain region. Data are combined from all simultaneously recorded cell pairs in 6 animals. Points denote means ± SE binned in increments of 0.1 mm. E: matrix identifying pairs of simultaneously recorded cells with a significant resting state correlation, considered a functional connection. The cell ordering was identified from k-means clustering of the corresponding signal correlations in B. F: the signal correlation during correct CS+ trials is higher between functionally connected (FC) cells. Points denote the mean signal correlation coefficient between all functionally connected or unconnected pairs per animal.
We next determined the proportion of statistically significant pairwise correlations in the resting state, which we refer to as the network's functional connectivity (Fig. 4E). We reasoned that since shared synaptic inputs to a subset of neurons is likely to promote a similar activation pattern during the behavioral task, signal correlations should be higher among functionally connected cells. As predicted, cell pairs with significant resting correlations (i.e., functionally connected pairs) had higher rsignal values during CS+ trials than uncorrelated pairs (P = 0.03, paired group permutation test; Fig. 4F), consistent with a network architecture in which cells with overlapping inputs are more likely to display similar task-evoked activity patterns. Together, these results demonstrate our novel recording technology's ability to map correlated activity within and across multiple regions, enabling functional connectivity analysis at a large scale in the mouse brain in vivo. In mammals, this multiregional analysis is usually confined to neuroimaging methods which provide a large-scale view of correlated brain network activity (Bullmore and Sporns 2009; Vincent et al. 2007) but without cellular resolution. Thus the 3D microprobe technology helps to link together these macroscopic measurements with single-cell studies of brain circuit function.
Mapping state-dependent local field potential coupling.
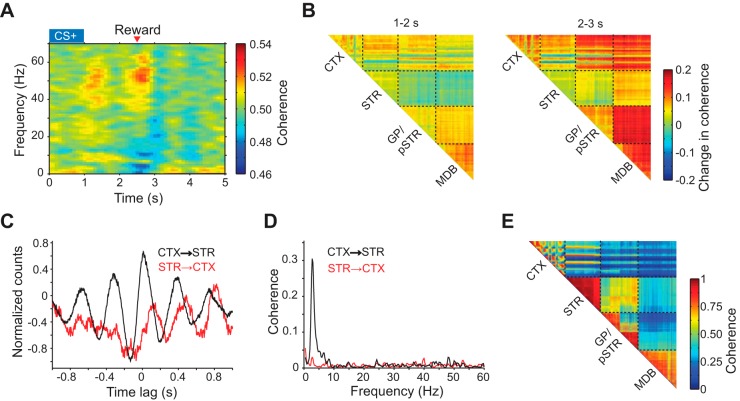
Coherent oscillatory activity is another common hallmark of synchronized neural activity in both cortical (Gray and Singer 1989) and basal ganglia (Berke et al. 2004; Courtemanche et al. 2003) circuits. Since local field potential (LFP) signals are related to the state of local synaptic inputs (Buzsáki et al. 2012; Einevoll et al. 2013), a coherent signal between spike and LFP activity is thought to indicate an interaction between two recording locations (Buzsáki and Schomburg 2015). To understand the functional relationship between distally distributed circuits, simultaneous multiregional recordings of unit and LFP activity are necessary. To explore the application of the recording technology to studying oscillatory interaction patterns, we first characterized the task-evoked fluctuations in spike-LFP coherence between the orbitofrontal cortex and striatum. We found a transient increase in gamma (45–65 Hz) frequency coherence during CS+ trials (Fig. 5A), consistent with the putative involvement of gamma rhythms in coupling distally connected circuits during behavior (Buzsáki and Wang 2012). To visualize how these oscillatory patterns interact across the recording field, we constructed matrices of the mean change in gamma LFP coherence in discrete time epochs of the behavioral task (Fig. 5B). These high-resolution coherence maps, captured in a single animal and recording session, show substantial heterogeneity in functional circuit coupling at both intra- and interregional levels likely reflecting anatomical differences in connectivity and microcircuits that drive gamma oscillations.
Fig. 5.
Mapping of local field potential (LFP) activity. A: mean corticostriatal spike-LFP coherence spectrogram during correct CS+ trials. Data represent 1 animal. B: two time-lapse matrices of mean 45–65 Hz LFP coherence stepped in 1-s intervals during correct CS+ trials. Coherence values are measured relative to a baseline period 1 s before the cue onset. Data represent simultaneous recordings from 1 animal. Dashed lines indicate the boundary between different layers in the 3D microprobe each containing 256 electrodes. MDB represents the midbrain, which includes the VTA/SN nuclei. C: spike-LFP cross correlogram (CCG) during spontaneous resting state activity. Black line denotes the CCG between spikes of one unit in the orbitofrontal cortex and LFP from one electrode in the striatum. Red line denotes the CCG with the spikes and LFP selected from the opposite locations. D: spike-LFP coherence spectrum of the data used to calculate the results in C. E: matrix of 2–4 Hz LFP coherence in the resting state across the four separate regions targeted by the 1,024 electrode microprobe. MDB: midbrain, which includes the VTA, SN, and other nearby midbrain nuclei. Data represent simultaneous recordings from 1 animal. Each pixel in the matrix corresponds to 1 electrode pair.
We next analyzed spike-LFP coherence in the resting state. Specifically, we examined the corticostriatal spike-triggered average LFP signal to identify any preferred oscillatory modes of synchrony. The cross correlation between the activity of a cortical unit and striatal LFP signal displayed an oscillatory relationship with a period of 300 ms and hence frequency of ∼3 Hz, which is consistent with the delta frequency band (Fig. 5C). Furthermore, coupling in the opposite direction (i.e., striatal unit activity and cortical LFP signal) showed a weaker response, demonstrating that interregional synchronization preferentially occurs from the cortex to striatum (Nakhnikian et al. 2014), consistent with corticostriatal wiring. To further elucidate the spectral properties of this oscillatory coupling, we calculated the spike-LFP coherence in the resting state and found that the strongest frequency mode indeed occurred in the delta band (mean ± SD: 2.8 ± 0.5 Hz; Fig. 5D). In agreement with a directionally selective coupling from the cortex to striatum, the spike-LFP coherence in the delta band was asymmetric (P < 0.0001, unpaired group permutation test). Finally, we mapped resting state delta band LFP coherence across the 3D microprobe (Fig. 5E). Cortical delta oscillations are frequently reported during periods of quiet wakefulness (Petersen et al. 2003; Vinck et al. 2015). Here we found that this rhythm is also highly coherent within the anterior striatum (Berke et al. 2004; Courtemanche et al. 2003), and that delta band coherence is heterogeneously organized across cortical and basal ganglia structures (Fujisawa and Buzsáki 2011). Together, these maps demonstrate our ability to resolve behavioral state and frequency-dependent variations in oscillatory network coupling with hundreds of simultaneously recorded units and LFP signals.
DISCUSSION
The 3D microprobe technology presented here uniquely combines several innovative advances in electrode-based sensing: 1) silicon-based probes with submicron minimum feature size leading to narrower structures than other microfabricated devices with a comparable number of electrodes, 2) multiplexed readout of electrical signals to reduce external wire bundle sizes 15-fold relative to standard readout methods, and 3) compact three-dimensional assembly, to provide precise, reproducible, and easily customizable anatomical targeting of multiple separate regions of the mouse brain. Leveraging the substantial gains in device miniaturization and scalability in the number of electrodes that these improvements provide, we carried out simultaneous in vivo measurements with 1,024 electrodes, the largest number reported so far in any rodent experiment. This improvement is of particular benefit for mouse studies where electrode-based tools require the highest degree of miniaturization. These methods are further expandable to thousands of electrodes for the mouse brain, and by extrapolation, potentially tens of thousands of electrodes for larger rodent and primate brain activity mapping in any 3D configuration.
Components of this technology are broadly accessible thanks to a number of commercially available services. Planar silicon probes were fabricated entirely at a MEMS foundry specializing in silicon etching and patterning of metals and insulators with submicron resolution. The production process yields hundreds of assorted functional probes per wafer batch with 64, 128, and 256 electrodes, lowering the effective cost per device. Other recording system components are available from third-party manufacturers at relatively low cost to build a complete 1,024 electrode probe. Moreover, an advantage of recording acutely in head-fixed animals is that the device is indefinitely reusable, thereby reducing assembly time and expense. All electronic components comprising the head stage are available commercially, and as a consequence of newly available multiplexed amplifier systems and open-source approaches in electrophysiology (Siegle et al. 2014), the cost of measuring signals from thousands of electronic channels has also dropped dramatically in the past few years. Finally, with parallel advances in surgical automation for making craniotomies (Pak et al. 2015), the preparation time prior to carrying out recordings in mice could be further reduced, and the recording procedures themselves eventually automated.
The assembly of 3D structures has been greatly simplified with our multilayer stacking and alignment method, which, unlike previous approaches involving mechanical scaffolds (Hoogerwerf and Wise 1994), does not require any customized components to create a specific configuration, thus can be rapidly adapted to changing experimental needs. A set of wide and narrow PCBs allowed us to space consecutive PCB layers by as little as 0.8 mm, with a further reduction to ∼0.4 mm possible using thinner PCBs. In principle, the multilayer assembly approach can be expanded to more than four layers and is ultimately only limited by available space. The probe assembly system is built with standard optomechanical parts, motorized micromanipulators, and inexpensive machined components. A potential limitation of this assembly method is that layers targeting individual regions are rigidly bonded together and their depth cannot be independently adjusted. On the other hand, the millimeter-scale span of the electrode array on the silicon prongs helps compensate for small targeting errors, increasing the likelihood that at least some electrodes will reach the area of interest.
The advantage of monitoring neural activity across multiple scales is evident by our recordings in the mouse orbitofrontal cortex and several basal ganglia nuclei, which revealed information regarding the multiregional organization of circuit dynamics during periods of behavioral responding and rest. The dynamics contained some marked similarities at the interregional level as seen by their response to cues. We also showed that local circuits preferentially perform specialized computations, as seen by subregional variations in activity and spatially decaying correlations. Furthermore, we identified a significant correlation between signal and resting state correlations, similar to phenomena found in human fMRI measurements at a larger albeit coarser scale (Cole et al. 2014). Similar relationships between noise and signal correlations have been found in previous electrophysiological studies (Bair et al. 2001; Smith and Kohn 2008), but at a smaller scale and with a lower throughput than demonstrated by our 3D microprobe. The high throughput of these recordings can greatly accelerate experimentation by enabling functional screening of multiple distal brain areas in parallel and thereby reduce animal usage. We simultaneously recorded hundreds of units in an individual session, and compiled data from over 1,600 units in just 6 sessions. A pitfall of conducting these measurements in head-fixed mice is that they are limited to a single recording session per animal. Thus, studying neural activity as animals learn complex behavioral tasks may require several independent cohorts of animals representing different stages of training. Alternatively, studies could rely on relatively simple tasks that can be acquired within one training session (Komiyama et al. 2010).
This recording technology opens up new possibilities for using mouse models to understand how neurological and psychiatric disorders alter systems-level brain functions (Deco and Kringelbach 2014). For instance, recording across the basal ganglia could reveal abnormal feedback-loop dynamics in addiction or movement disorders. Neuroscience has only recently begun to access the large-scale multiregional regime of brain activity with cellular resolution (Ahrens et al. 2012; Berényi et al. 2014; Lecoq et al. 2014), and the recording capabilities of 3D silicon microprobes offer unique opportunities for mapping network dynamics in the mouse.
GRANTS
This work was supported by National Institute on Drug Abuse Grant R01-DA-034178, National Science Foundation Grant CBET-1263785, the Alfred P. Sloan Foundation, McKnight Foundation, and a Harvey L. Karp Discovery Award to S. C. Masmanidis, and a Ruth L. Kirschstein National Research Service Award (T32-NS058280) to K. I. Bakhurin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.S., L.D.C., and S.C.M. conception and design of research; J.L.S. and L.D.C. performed experiments; J.L.S., L.D.C., S.P., K.I.B., and S.C.M. analyzed data; J.L.S., L.D.C., and S.C.M. interpreted results of experiments; J.L.S., L.D.C., and S.C.M. prepared figures; J.L.S., L.D.C., and S.C.M. drafted manuscript; J.L.S., L.D.C., and S.C.M. edited and revised manuscript; J.L.S., L.D.C., S.P., K.I.B., and S.C.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Jhun, P.O. Polack, W. Babiec, and P. Golshani for technical assistance, S. Sampath and X. Zhang for silicon wafer fabrication, K. Lee for electron microscope imaging, W. Lee for microprobe wire bonding and head stage assembly, T. Heitzman and M. Walsh for olfactometer development, R.R. Harrison for integrated circuit development, and B.S. Huang for comments on the manuscript.
REFERENCES
- Adler A, Katabi S, Finkes I, Israel Z, Prut Y, Bergman H. Temporal convergence of dynamic cell assemblies in the striato-pallidal network. J Neurosci 32: 2473–2484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens MB, Li JM, Orger MB, Robson DN, Schier AF, Engert F, Portugues R. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485: 471–477, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381, 1986. [DOI] [PubMed] [Google Scholar]
- Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci 21: 1676–1697, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004. [DOI] [PubMed] [Google Scholar]
- Berényi A, Somogyvári Z, Nagy AJ, Roux L, Long JD, Fujisawa S, Stark E, Leonardo A, Harris TD, Buzsáki G. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J Neurophysiol 111: 1132–1149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896, 2004. [DOI] [PubMed] [Google Scholar]
- Blanche TJ, Spacek MA, Hetke JF, Swindale NV. Polytrodes: high-density silicon electrode arrays for large-scale multiunit recording. J Neurophysiol 93: 2987–3000, 2005. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198, 2009. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci 7: 446–451, 2004. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13: 407–420, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Schomburg EW. What does gamma coherence tell us about inter-regional neural communication? Nat Neurosci 18: 484–489, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci 35: 203–225, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482: 85–88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron 83: 238–251, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci 23: 11741–11752, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Henze DA, Jamieson B, Harris KD, Sirota A, Barthó P, Wise KD, Buzsáki G. Massively parallel recording of unit and local field potentials with silicon-based electrodes. J Neurophysiol 90: 1314–1323, 2003. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, Yu BM. Dimensionality reduction for large-scale neural recordings. Nat Neurosci 17: 1500–1509, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Kringelbach ML. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron 84: 892–905, 2014. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Graziano MS, Tank DW. Functional clustering of neurons in motor cortex determined by cellular resolution imaging in awake behaving mice. J Neurosci 29: 13751–13760, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56: 43–57, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Reiser MB. Real neuroscience in virtual worlds. Curr Opin Neurobiol 22: 3–10, 2012. [DOI] [PubMed] [Google Scholar]
- Du J, Blanche TJ, Harrison RR, Lester HA, Masmanidis SC. Multiplexed, high density electrophysiology with nanofabricated neural probes. PLoS One 6: e26204, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einevoll GT, Kayser C, Logothetis NK, Panzeri S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci 14: 770–785, 2013. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 72: 153–165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C. Using extracellular action potential recordings to constrain compartmental models. J Comput Neurosci 23: 39–58, 2007. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual-cortex. Proc Natl Acad Sci USA 86: 1698–1702, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RR, Charles C. A low-power low-noise CMOS amplifier for neural recording applications. IEEE J Solid-St Circ 38: 958–965, 2003. [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD, Buzsáki G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84: 390–400, 2000. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf AC, Wise KD. A 3-dimensional microelectrode array for chronic neural recording. IEEE T Bio-Med Eng 41: 1136–1146, 1994. [DOI] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ, Mrsic-Flogel TD. Functional specificity of local synaptic connections in neocortical networks. Nature 473: 87–91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464: 1182–1186, 2010. [DOI] [PubMed] [Google Scholar]
- Lecoq J, Savall J, Vucinic D, Grewe BF, Kim H, Li JZ, Kitch LJ, Schnitzer MJ. Visualizing mammalian brain area interactions by dual-axis two-photon calcium imaging. Nat Neurosci 17: 1825–1829, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci 25: 3857–3869, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Spontaneous activity: signal or noise? Science 285: 541–543, 1999. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54: 782–806, 1985. [DOI] [PubMed] [Google Scholar]
- Nakhnikian A, Rebec GV, Grasse LM, Dwiel LL, Shimono M, Beggs JM. Behavior modulates effective connectivity between cortex and striatum. PLoS One 9: e89443, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Methods for studying functional interactions among neuronal populations. Methods Mol Biol 489: 135–165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65: 472–479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak N, Siegle JH, Kinney JP, Denman DJ, Blanche TJ, Boyden ES. Closed-loop, ultraprecise, automated craniotomies. J Neurophysiol 113: 3943–3953, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Pare D. Measuring correlations and interactions among four simultaneously recorded brain regions during learning. J Neurophysiol 101: 2507–2515, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA 100: 13638–13643, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzi A, Wickens J. Input dependent cell assembly dynamics in a model of the striatal medium spiny neuron network. Front Syst Neurosci 6: 6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach DL. Spontaneous and driven cortical activity: implications for computation. Curr Opin Neurobiol 19: 439–444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci 1: 199–207, 2000. [DOI] [PubMed] [Google Scholar]
- Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, Rajangam S, Subramanian V, Ifft PJ, Li Z, Ramakrishnan A, Tate A, Zhuang KZ, Nicolelis MA. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat Methods 11: 670–676, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JH, Hale GJ, Newman JP, Voigts J. Neural ensemble communities: open-source approaches to hardware for large-scale electrophysiology. Curr Opin Neurobiol 32C: 53–59, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271: 1870–1873, 1996. [DOI] [PubMed] [Google Scholar]
- Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci 28: 12591–12603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson IH, Kording KP. How advances in neural recording affect data analysis. Nature Neurosci 14: 139–142, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86, 2007. [DOI] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86: 740–754, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise KD, Sodagar AM, Yao Y, Gulari MN, Perlin GE, Najafi K. Microelectrodes, microelectronics, and implantable neural microsystems. Proc IEEE 96: 1184–1202, 2008. [Google Scholar]