Abstract
Background. Novel diagnostics have been widely applied across human immunodeficiency virus (HIV) and tuberculosis prevention and treatment programs. To achieve the greatest impact, HIV and tuberculosis diagnostic programs must carefully plan and implement within the context of a specific healthcare system and the laboratory capacity.
Methods. A workshop was convened in Cape Town in September 2014. Participants included experts from laboratory and clinical practices, officials from ministries of health, and representatives from industry.
Results. The article summarizes best practices, challenges, and lessons learned from implementation experiences across sub-Saharan Africa for (1) building laboratory programs within the context of a healthcare system; (2) utilizing experience of clinicians and healthcare partners in planning and implementing the right diagnostic; and (3) evaluating the effects of new diagnostics on the healthcare system and on patient health outcomes.
Conclusions. The successful implementation of HIV and tuberculosis diagnostics in resource-limited settings relies on careful consideration of each specific context.
Keywords: diagnostics, HIV, tuberculosis, diagnostics’ implementation, point-of-care
Diagnostics are essential for optimal patient healthcare and for monitoring and meeting public health objectives. Novel diagnostics have been widely applied across human immunodeficiency virus (HIV) and tuberculosis prevention and control programs. However, diagnostic technologies often deliver less than their full potential due to issues inherent in the larger healthcare system. It is not always easy to have the right test, at the right time, and in the right place, with timely communication, action, and follow-up for optimal patient healthcare.
The ideal diagnostic should not only be fast, sensitive, specific, accessible, and cost effective, but it must also respond to the local healthcare context [1]. There are new HIV and tuberculosis diagnostics available, as well as in the development pipeline, that are designed to be versatile, robust, and applicable in primary healthcare settings [2, 3].
To achieve the greatest impact for diagnostic technologies, HIV and tuberculosis programs must evaluate implementation within the context of the healthcare system and laboratory capacity. To ensure diagnostics are maintained and utilized to their potential, healthcare programs must endeavor to (1) identify improved diagnostics and more effective uses for existing ones; (2) evaluate the potential effectiveness of new diagnostics in program operations; (3) plan for implementation of new diagnostics; (4) implement new diagnostics with the expectation of optimizing patient health outcomes; (5) evaluate the effect of new diagnostics after implementation; and (6) develop sustainability plans [4].
BACKGROUND
To discuss implementation of HIV and tuberculosis diagnostics from the perspectives of the healthcare system, the laboratory, and the clinic, a workshop was organized by the US National Institutes of Health and the US Centers for Disease Control and Prevention, with support from the US President's Emergency Plan for AIDS Relief. The workshop, “Implementing HIV and Tuberculosis Diagnostics in Resource-Limited Settings,” was held in Cape Town, South Africa, on 22–23 September 2014. Participants were implementation experts from laboratory and clinical practices, officials from ministries of health, and representatives from industry, from Botswana, Kenya, Malawi, South Africa, Switzerland, Tanzania, Uganda, the United Kingdom, and the United States.
DISCUSSION
Why Is Context Important?
Context must be considered when deciding if a new diagnostic is more appropriate than alternative technologies. In some settings, challenges in infrastructure and resources restrict the success of diagnostics’ implementation at both the centralized laboratory and the point of care (POC). For settings where the costs of replacing or establishing centralized and highly technical laboratory systems may be prohibitive, POC diagnostics that are well deployed with high-quality standards may be the appropriate choice [5]. For example, POC testing may be a good solution for early infant HIV diagnosis in remote settings [6]. Regardless of the diagnostic being implemented and before scaling up, field studies should evaluate whether a test would improve patient health outcomes and be cost effective [7]. Optimally, these studies would provide data on the performance characteristics of the test on patient populations and healthcare settings similar to those being considered.
Ministries of health and country programs must determine the importance of implementing any new diagnostic for their healthcare systems. This government-led strategy with the participation of patients, clinicians, and other stakeholders is highly desirable [8–10], and could be used to address decisions for new instrumentation, including country-wide negotiation for pricing, maintenance agreements, and technical assistance [11]. The World Health Organization (WHO) prequalification program for new diagnostics has been shown to help governments establish a new diagnostic within a country-specific strategy [12]. Consequently, a diagnostic that addresses these insights will be more effectively implemented and fit better within healthcare systems and national programs [13, 14].
Clinicians and healthcare workers should be included in discussions and decisions on the use of new diagnostics. Clinicians in the field have a unique perspective on the utility of tests for diagnosis and disease management, as well as on optimal utilization of diagnostics. Furthermore, healthcare workers may also provide insights into issues such as specimen transport and communication of results to patients. Nontraditional partners should also be considered in resource-limited settings to develop creative and innovative solutions to the challenges of deploying diagnostics. These partners may include those with expertise in costing and modeling, engineering, specimen transport, and software development. An example is Uganda's Stop TB Network, which includes the post office as an integral part of the specimen referral system to ensure the reliable and safe transport of specimens [15].
How Do Healthcare Systems Support Diagnostics?
Implementation planning must consider all aspects of the healthcare system, including the potential need for its improvement. Strong healthcare systems are essential for successful implementation of diagnostics. To extend the impact of diagnostics, healthcare system strengthening should include (1) appropriate staffing and training; (2) good communications between clinicians and laboratories and between clinicians and patients; (3) suitable regulatory systems; (4) strong supply chains for laboratories; (5) appropriate community engagement for testing and linkage to healthcare; (6) appropriate cost-effectiveness analyses; and (7) good management practices.
In recent years, several diagnostics for the detection of various infectious diseases have been successfully launched. While these successes are encouraging, it is important to note that the successful implementation of any new diagnostic would always require a strong healthcare system [13]. Without a strong healthcare system, a more sensitive and specific diagnostic may fail to have a positive impact on healthcare outcomes. Such was the case when evaluating the implementation of rapid malaria diagnostic tests in Tanzania. This study found that the implementation of these tests, with only basic training to clinicians on their utilization, provided quick and reliable malaria diagnoses, but had little impact on stopping the common healthcare practice of overprescribing antimalarial drugs and antibiotics [16]. In December 2010, the WHO recommended the use of Xpert MTB/RIF, an automated, cartridge-based nucleic acid amplification assay, for the detection of tuberculosis and rifampicin drug resistance. The WHO has also been monitoring its global rollout to promote coordination [17]. In South Africa, the rollout of this technology highlights the need to strengthen healthcare systems when implementing a new diagnostic. Thus far, in South Africa, the use of Xpert MTB/RIF has resulted in accurate tuberculosis diagnoses of not only patients, but also household members who come to the clinic with the patients, and improved detection of rifampicin-resistant tuberculosis. It also has helped in decongesting clinics as fewer patient visits are required [18].
Healthcare systems should provide essential support for diagnostics to be fully effective; however, this practice is often neglected in resource-limited settings [19]. Data management is a critical aspect of healthcare systems. Some data collection and use gaps present in many resource-limited settings include (1) lack of data analysis for already collected data; (2) absence of updated registers for currently available tests; (3) inappropriate metrics for study design and implementation; (4) lack of education of clinicians on interpretation of data and test results; (5) poor dissemination of negative results; (6) absence of alert systems within clinics for abnormal results; (7) lack of data-driven forecasting of diagnostic testing needs; (8) insufficient data for accurate costing and procurement figures; and (9) lack of unique patient identifiers. One of the reasons these gaps persist is a lack of routine communication among laboratories, clinicians, governments, donors, and other stakeholders [20, 21].
No country represented at the workshop has yet to fully implement a country-wide unique patient identifier; however, deployment of a unique patient identifier for healthcare would have a significant positive impact on patient health outcomes and laboratory systems. It would also facilitate the linkage of test results with the appropriate patient, thereby reducing duplicate tests and lost test results and improving the accuracy of public health data [22].
How Do Laboratory Systems Support Diagnostics?
To improve the effectiveness of diagnostics, laboratory systems should focus on (1) quality management; (2) proper equipment care and maintenance; (3) strong supply chains and appropriate facilities; (4) reliable specimen referral networks; (5) adequate laboratory information systems; and (6) suitable laboratory training. In many resource-limited settings, there are gaps in these essential elements of the laboratory system [23–25].
Decisions to invest in and implement new diagnostics are often influenced by a wide array of internal and external forces; however, it is critical that well-planned evaluations, including cost-effectiveness analyses, drive these decisions [26]. The successful implementation of a new diagnostic test requires research on the disease burden, as well as on the best placement strategies for that new test. For example, prior to the country-wide implementation of the Xpert MTB/RIF assay, Uganda tested different diagnostic placement models. This research determined that the best placement strategy was to prioritize sites based on high tuberculosis prevalence [27]. In other settings, the identification of testing volume needs at country and site levels, and the execution of a gap analysis to identify weaknesses in supportive systems, may be critical elements for successful implementation of diagnostics [28].
The diagnostics landscape is further compromised by the decision to adopt technologies in the absence of sufficient in-country data, or when scale-up responds to external decisions taken by donors or partners to provide funds for the implementation of a test they have selected [10].
Most country laboratory systems include public, private, and nongovernmental laboratories. Some common challenges include quality control and assurance, connectivity, specimen transport, results dissemination, and clinical filing of medical records [19]. For example, in laboratory settings, there may be a negative impact on the quality of testing when equipment is not maintained, stockouts are frequent, technicians are not trained, and the quality of laboratory information systems is not adequate. An example of a best practices model that addresses some of these challenges is the Ugandan integrated tuberculosis laboratory network. This system links all levels of laboratories to improve access to tuberculosis diagnostics while maintaining quality. Uganda has defined 8 levels of tuberculosis diagnostic testing, from microscopy at the primary level to culture and resistance testing at the reference level. All sites are mapped and linked by a toll-free number for specimen transport information and general communications. Additionally, the post office is integrated into this system to ensure timely and safe specimen transport. This integration, for example, in 2011, resulted in >94% of specimens received in the testing laboratory by 3 days and most results dispatched electronically [29]. This example demonstrates the value of linking public and private laboratories within a laboratory system. The Ugandan government credits this laboratory system with transforming the paradigm of laboratory services and improving outcomes across the healthcare system [30].
Addressing another one of these challenges, connectivity, may lead to improved communication of results among laboratories and clinicians, and potentially between clinicians and patients. For example, the use of Short Messaging System (SMS) by clinicians to communicate test results to patients may eliminate the need for unnecessary clinic visits [31]. Literacy and experience with technology have been shown to be critical to mobile health (m-health) success. A previous study discusses the predictors of success when using an SMS-text application to improve linkage to healthcare for HIV patients in rural Uganda. This study found that participants who were capable of reading a complete sentence and able to open an SMS-text, at study entry, opened and successfully interpreted the SMS-text and returned to clinic at the appropriate time, when notified of test results [32]. Improving connectivity, when combined with a unique patient identifier system, may lead to a better integration of information on multiple patient test results.
How Do Clinical Considerations Support Diagnostics?
Before considering the widespread implementation of any new diagnostic, the level of impact on patient healthcare must be evaluated by comparing the new diagnostic with current technologies within the setting's context. These evaluations could include field experiments, research studies, and mathematical modeling, when appropriate [33]. It cannot be assumed that faster and more sensitive diagnostics, including POC technologies and rapid tests, would translate into better patient healthcare or the improvement of patient health outcomes [34, 35].
Once a new diagnostic is implemented, additional evaluations may identify opportunities to extend its impact on healthcare systems. For example, the current use of Xpert MTB/RIF in South Africa could be evaluated to determine the impact on patient clinical outcomes [36–38]. In the case of HIV, the WHO HIV treatment guidelines call for monitoring an individual's response to antiretroviral (ARV) treatment and HIV disease progression [39]. The WHO has also issued guidance for implementing HIV viral load (VL) testing, which addresses planning, scale-up, and sustainability [8]. Using these tools, any setting considering scaling up VL testing for monitoring disease progression can analyze the country-specific context and carefully plan a phase-in strategy. In addition, patient education, training of healthcare workers, and integration of healthcare services can be evaluated to determine their impact in this rollout [40].
As new diagnostics are implemented, healthcare workers should educate patients regarding their tests and results. This education is essential when the healthcare paradigm changes with the implementation of new diagnostics [41]. For example, CD4 cell counts have been used not only to determine when an HIV-infected individual is eligible to start ARV treatment, but also to determine treatment success [42]. In resource-limited settings, VL testing is increasingly being accepted as the surrogate marker for determining HIV treatment success or for determining success or failure of a given ARV regimen in a particular individual [43]. With the implementation of this new paradigm, more people are being started on ARV treatment without CD4 cell counts, such as pregnant women. Thus, healthcare workers must now educate HIV-infected patients regarding VL testing and its newer importance to monitoring disease, as well as on issues such as (1) why the test is being requested; (2) what the test measures; (3) what values or ranges are desirable; (4) what the advantages over the old test are; (5) how often to be tested and when to be tested; (6) the impact on disease progression; and (7) the significance of resistance patterns [40].
The integration of HIV and tuberculosis diagnosis and treatment, at the individual patient and the community levels, is crucial to achieve measurable and significant success in controlling these 2 diseases [44]. The lack of coordination and integration of HIV and tuberculosis diagnostics with healthcare services has a noticeable and negative impact on public health, such as an increased number of patients who return to their communities untreated, impacting patients and promoting transmission [45]. Data from the HIV treatment cascade for adults aged ≥15 years in sub-Saharan Africa show that the HIV care cascade continues to lose patients to healthcare at all points. Similarly, the tuberculosis care cascade continues to lose patients at every step from diagnosis to treatment [44]. Some factors for these low follow-up rates are (1) congested clinics; (2) high costs of travel expenses; and (3) inability to be absent from work [46].
CONCLUSIONS AND RECOMMENDATIONS
HIV and tuberculosis diagnostics are the entry points to care and treatment. Given the diversity of healthcare settings, laboratory infrastructures, and supportive systems where diagnostics are deployed, implementation adapted to the context is essential for optimal patient health outcomes. However, one size does not fit all. Technology selection should not be universally applied across different settings. Context-specific improvements in laboratory operations and clinical practices, as well as healthcare systems, may be needed to maximize the impact of a novel diagnostic in each setting. A diagnostic can only perform to its potential when adopted under context-specific conditions and with the maximization of linkage to healthcare.
Integration of HIV and tuberculosis diagnostics with linkage to healthcare is essential, particularly in resource-limited settings. Some of the practices that can improve this integration are (1) the evaluation of diagnostic platforms and their impact on linkage to healthcare; (2) the improvement of communications between laboratories and clinical care settings; (3) the establishment of automated processes for prescriptions; (4) the improvement of healthcare staff service attitude; (5) the use of communication technologies to notify patients of test results; (6) the establishment of off-site pharmacies; (7) the creation of specimen referral networks integrated within laboratory systems; and (8) the standardization of training for laboratory personnel, clinicians, and community representatives.
Researchers, funders, and public health officials should continue to promote sharing of data through scientific publications, regional professional societies, government-to-government collaborations, and other mechanisms that would support the dissemination of lessons learned. In addition, best practices from previously deployed diagnostics should be considered. Errors may be avoided by learning from both successful and unsuccessful attempts to implement new diagnostics.
Additional research is necessary to understand laboratory, clinical, and healthcare system factors that impact effectiveness, and how to maximize the potential for novel diagnostics (Figures 1 and 2). Research to provide stronger evidence for best practices, in particular to show a link between management practices and quality of testing, is lacking. Research is needed to understand the potential impact of innovations such as electronic health (e-health), m-health, geographic information system mapping, and other technologies in linking test results with epidemiologic information to help guide HIV and tuberculosis policy priorities. Access to electronic media, such as email or Internet-based communications, would also allow clinics to interface with laboratories efficiently when accessing results and requesting further analyses.
Figure 1.
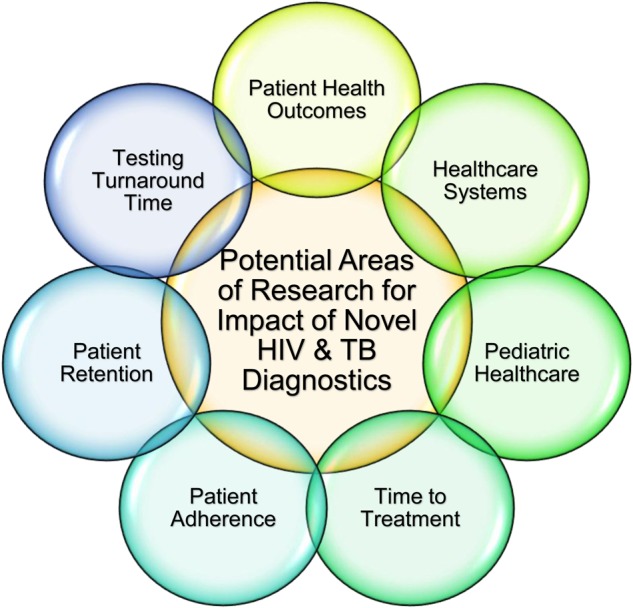
Diagram depicting potential research areas to understand the impact of the implementation of novel human immunodeficiency virus (HIV) and tuberculosis (TB) diagnostics on patient health outcomes, healthcare systems, pediatric healthcare, time to treatment, patient adherence, patient retention, and testing turnaround time.
Figure 2.
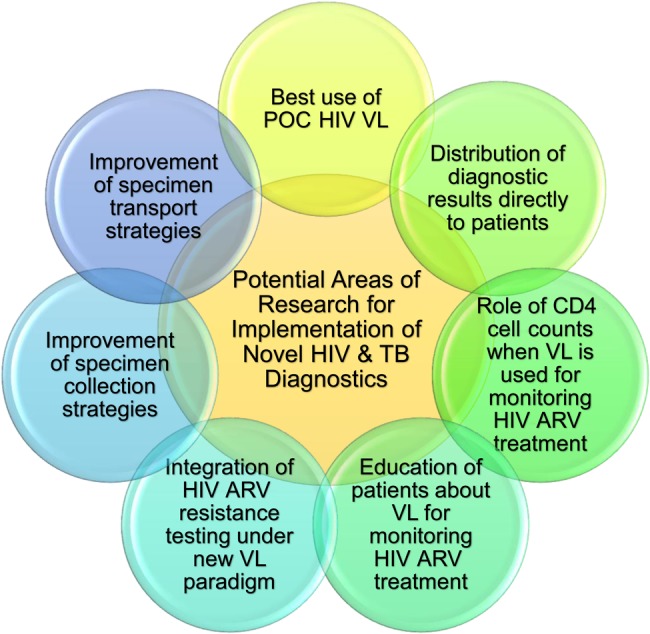
Diagram depicting potential research areas to understand some implementation challenges of novel human immunodeficiency virus (HIV) and tuberculosis (TB) diagnostics, such as the best use of point-of-care (POC) HIV viral load (VL), the distribution of diagnostic results directly to patients, the role of CD4 cell counts when VL is used for monitoring HIV antiretroviral (ARV) treatment, the education of patients about VL for monitoring HIV ARV treatment, the integration of HIV resistance under the new VL paradigm, and the improvement of specimen collection and transport strategies.
In resource-limited settings, to improve the probability for successful implementation of HIV and tuberculosis novel diagnostics, context-specific solutions should address the following persistent gaps: (1) lack of country laboratory strategic plans; (2) absence of routine assessments on impact and cost effectiveness of patient healthcare; (3) avoidance of implementation based on quantity instead of quality; (4) inappropriate communication and distribution of results from postmarketing surveillance studies; and (5) lack of comprehensive costs forecasting for deployment and maintenance.
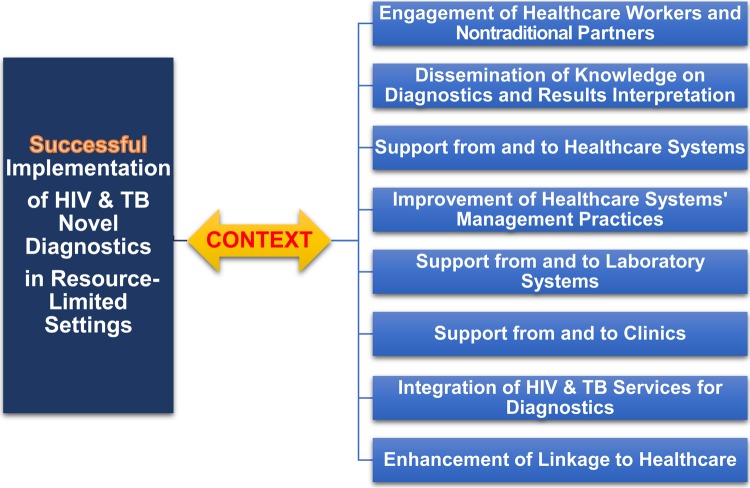
In closing, the successful implementation of new HIV and tuberculosis diagnostics in resource-limited settings should respond to careful considerations of the specific context of each setting, in particular its healthcare system, and should reflect input from traditional and nontraditional partners (Figure 3).
Figure 3.
A flow diagram on the successful implementation of human immunodeficiency virus (HIV) and tuberculosis (TB) novel diagnostics in resource-limited settings and the required consideration of a number of factors within specific context. Factors include the engagement of all stakeholders, the dissemination of knowledge on results interpretation, the improvement of healthcare systems’ management practices, the support from and to laboratories and clinics, the integration of HIV and TB diagnostics, and the enhancement of linkage to healthcare.
Notes
Acknowledgments. The authors acknowledge the participants of the workshop and the members of the Scientific Planning Committee: H. Albert, H. Alexander, D. Allen, L. Berrie, T. Boyles, J. Brand, L. Channing, N. Chegou, G. Chipungu, W. Coggin, F. Conradie, M. de Vos, V. Deyde, T. Ellman, J. Estill, E. Fajardo, N. Foster, L. Gonzalez, R. Gordon, N. Gqwaru, G. Handley, N. Ismail, M. Joloba, H. Lee, T. Lindsay, G. Loots, J. Lusike, L. Malinga, J. Markby, C. Masiku, M. Maskew, A. Matteelli, L. Mazzola, K. McCarthy, R. McNerney, J. Meegan, S. Mohammed, S. Molapo, N. Mpofu, K. Muldoon, P. Naidoo, N. Ndjeka, L. Nelson, S. Ngcobo, A. Ntilivamunda, C. Odhiambo, A. Piatek, S. Reed-Allen, A. Reid, I. Sanne, M. Schito, L. Scott, V. Singh, P. Smith, W. Stevens, G. Theron, M. van der Walt, A. Van Rie, C. van Vuuren, W. Venter, L. Vojnov, C. Wallis, G. Walther, C. Werely, R. Wilkinson, and J. Williams. The authors also thank Arthur Stone, Contractor, Science Writer, Henry M. Jackson Foundation, Division of AIDS, National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), for proofreading and editing suggestions.
Disclaimer. The views expressed in this paper and its authors do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention of trade names, commercial practices, or organizations imply endorsement by the US government.
Financial support. This work was supported, in part, by the NIAID, NIH (contract number HHSN272200800014C).
Supplement sponsorship. This article appears as part of the supplement “Advances in Tuberculosis Research: A Blueprint for Opportunities.” This article was sponsored by the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.TDR Diagnostics Evaluation Expert Panel; Banoo S, Bell D, Bossuyt P, et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol 2010; 8(12 suppl):S17–29. [PubMed] [Google Scholar]
- 2.UNITAID. HIV/AIDS. Diagnostics technology landscape. 4th ed, 2014. Available at: http://www.unitaid.org/images/marketdynamics/publications/UNITAID-HIV_Diagnostic_Landscape-4th_edition.pdf Accessed 7 May 2015.
- 3.UNITAID. Tuberculosis diagnostic. Technology and market landscape. Semi-annual update, 2013. Available at: http://www.unitaid.org/images/marketdynamics/publications/UNITAID-TB_Dx_Landscape-Update_Dec%202013.pdf Accessed 7 May 2015.
- 4.National Academy of Sciences. Appendix A—learning what works best: the nation's need for evidence on comparative effectiveness in health care: an issue overview. Learning what works: infrastructure required for comparative effectiveness research, 2009. Available at: http://www.ncbi.nlm.nih.gov/books/NBK64787/pdf/TOC.pdf Accessed 7 May 2015.
- 5.Larson BA, Schnippel K, Brennan A, et al. Same-day CD4 testing to improve uptake of HIV care and treatment in South Africa: point-of-care is not enough. AIDS Res Treat 2013; 2013:941493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Adapting WHO normative HIV guidelines for national programmes, 2011. Available at: http://whqlibdoc.who.int/publications/2011/9789241501828_eng.pdf Accessed 7 May 2015.
- 7.Centers for Disease Control and Prevention. Guidelines for appropriate evaluations of HIV testing technologies in Africa, 2002. Available at: http://www.who.int/diagnostics_laboratory/publications/EN_HIVEval_Guide.pdf Accessed 7 May 2015.
- 8.World Health Organization. Technical and operational considerations for implementing HIV viral load testing. Interim technical update, 2014 Available at: comment http://www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/ Accessed 7 May 2015.
- 9.World Health Organization. TB diagnostics and laboratory services: information note, 2009. Available at: http://www.who.int/tb/dots/lab.pdf Accessed 7 May 2015.
- 10.United Nations. Achieving the global public health agenda dialogues at the Economic and Social Council, 2009. Available at: http://www.un.org/en/ecosoc/docs/pdfs/achieving_global_public_health_agenda.pdf Accessed 7 May 2015.
- 11.Peter TF, Shimada Y, Freeman RR, et al. The need for standardization in laboratory networks. Am J Clin Pathol 2009; 131:867–74. [DOI] [PubMed] [Google Scholar]
- 12.Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 2013; 13:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caliendo AM, Gilbert DN, Ginocchio CC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57(suppl 3):S139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.University of Pittsburgh Medical Center—Center for Health Security. Diagnosing infection at the point of care, 2013. Available at: http://www.upmchealthsecurity.org/our-work/pubs_archive/pubs-pdfs/2013/2013-08-28-diagnosing_infection_poc-REV.pdf. Accessed 7 May 2015.
- 15.World Health Organization. Partnering and public health practice. Experience of national TB partnerships, 2013. Available at: www.stoptb.org/assets/documents/countries/partnerships/stop_tb_layout_low_res_final.pdf Accessed 7 May 2015.
- 16.Reyburn H, Mbakilwa H, Mwangi R, et al. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. BMJ 2007; 334:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. TB diagnostics and laboratory strengthening: WHO monitoring of Xpert MTB/RIF roll-out. TB Xpert Project Available at: http://www.who.int/tb/laboratory/mtbrifrollout/en/ Accessed 7 May 2015.
- 18.Clouse K, Page-Shipp L, Dansey H, et al. Implementation of Xpert MTB/RIF for routine point-of-care diagnosis of tuberculosis at the primary care level. S Afr Med J 2012; 102:805–7. [DOI] [PubMed] [Google Scholar]
- 19.Martin R, Barnhart S. Global laboratory systems development: needs and approaches. Infect Dis Clin North Am 2011; 25:677–91, x. [DOI] [PubMed] [Google Scholar]
- 20.Lucas H. Information and communications technology for future health systems in developing countries. Soc Sci Med 2008; 66:2122–32. [DOI] [PubMed] [Google Scholar]
- 21.Yu D, Souteyrand Y, Banda MA, Kaufman J, Perriens JH. Investment in HIV/AIDS programs: does it help strengthen health systems in developing countries? Global Health 2008; 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson BA, Brennan A, McNamara L, et al. Lost opportunities to complete CD4+ lymphocyte testing among patients who tested positive for HIV in South Africa. Bull World Health Organ 2010; 88:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbireer AM, Opio AA, Brough RL, Jackson JB, Manabe YC. Strengthening public laboratory service in sub-Saharan Africa: Uganda case study. Lab Medicine 2011; 42:719–25. [Google Scholar]
- 24.Alemnji G, Fonjungo P, Van Der Pol B, Peter T, Kantor R, Nkengasong J. The centrality of laboratory services in the HIV treatment and prevention cascade: the need for effective linkages and referrals in resource-limited settings. AIDS Patient Care STDS 2014; 28:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peter TF, Rotz PD, Blair DH, et al. Impact of laboratory accreditation on patient care and the health system. Am J Clin Pathol 2010; 134:550–5. [DOI] [PubMed] [Google Scholar]
- 26.Acuna-Villaorduna C, Vassall A, Henostroza G, et al. Cost-effectiveness analysis of introduction of rapid, alternative methods to identify multidrug-resistant tuberculosis in middle-income countries. Clin Infect Dis 2008; 47:487–95. [DOI] [PubMed] [Google Scholar]
- 27.Pho MT, Deo S, Palamountain KM, et al. Optimizing tuberculosis case detection through a novel diagnostic device placement model: the case of Uganda. PLoS One 2015; 10:e0122574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massambu C, Mwangi C. The Tanzania experience: clinical laboratory testing harmonization and equipment standardization at different levels of a tiered health laboratory system. Am J Clin Pathol 2009; 131:861–6. [DOI] [PubMed] [Google Scholar]
- 29.Joloba ML. Activities of Uganda SRL, 2012. Available at: http://www.stoptb.org/wg/gli/assets/html/GLI5/Activities%20of%20Uganda%20SRL.pdf Accessed 11 May 2015.
- 30.Ministry of Health, Republic of Uganda. Three Uganda laboratories receive International Best Practice Awards: “Honourable Mention”, in respect of their innovative Sample Transport System, 2015. Available at: http://health.go.ug/mohweb/node/139 Accessed 7 May 2015.
- 31.Siedner MJ, Haberer JE, Bwana MB, et al. High acceptability for cell phone text messages to improve communication of laboratory results with HIV-infected patients in rural Uganda: a cross-sectional survey study. BMC Med Inform Decis Mak 2012; 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siedner MJ, Santorino D, Haberer JE, et al. Know your audience: predictors of success for a patient-centered texting app to augment linkage to HIV care in rural Uganda. J Med Internet Res 2015; 17:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estill J, Egger M, Blaser N, et al. Cost-effectiveness of point-of-care viral load monitoring of antiretroviral therapy in resource-limited settings: mathematical modelling study. AIDS 2013; 27:1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanrahan CF, Selibas K, Deery CB, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care Xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One 2013; 8:e65421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Handel T, Hampton KH, Sanne I, et al. The impact of Xpert (R) MTB/RIF in sparsely populated rural settings. Int J Tuberc Lung Dis 2015; 19:392–8. [DOI] [PubMed] [Google Scholar]
- 36.Dheda K, Theron G, Welte A. Cost-effectiveness of Xpert MTB/RIF and investing in health care in Africa. Lancet Glob Health 2014; 2:e554–6. [DOI] [PubMed] [Google Scholar]
- 37.Theron G, Peter J, Dowdy D, et al. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis 2014; 14:527–32. [DOI] [PubMed] [Google Scholar]
- 38.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014; 383:424–35. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2013. Available at: http://www.who.int/hiv/pub/guidelines/keypopulations/en/ Accessed 7 May 2015. [PubMed]
- 40.Médecins Sans Frontières. MSF access campaign: putting HIV treatment to the test: a product guide for viral load and point-of-care CD4 diagnostic tools, 2013. Available at: https://www.msf.es/sites/default/files/publicacion/msf_informe_Putting_HIV_treatment_to_the_test.pdf Accessed 7 May 2015.
- 41.Saha S, Beach MC, Cooper LA. Patient centeredness, cultural competence and healthcare quality. J Natl Med Assoc 2008; 100:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calmy A, Balestre E, Bonnet F, et al. Mean CD4 cell count changes in patients failing a first-line antiretroviral therapy in resource-limited settings. BMC Infect Dis 2012; 12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyle T, Smith C, Vitiello P, et al. Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis 2012; 54:724–32. [DOI] [PubMed] [Google Scholar]
- 44.United Nations Joint Programme on HIV/AIDS. The Gap Report, 2013. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf Accessed 7 May 2015.
- 45.World Health Organization. Global tuberculosis report, 2014. Available at: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1 Accessed 7 May 2015.
- 46.Voss De Lima Y, Evans D, Page-Shipp L, et al. Linkage to care and treatment for TB and HIV among people newly diagnosed with TB or HIV-associated TB at a large, inner city South African hospital. PLoS One 2013; 8:e49140. [DOI] [PMC free article] [PubMed] [Google Scholar]