Abstract
Consensus case definitions for childhood tuberculosis have been proposed by an international expert panel, aiming to standardize the reporting of cases in research focusing on the diagnosis of intrathoracic tuberculosis in children. These definitions are intended for tuberculosis diagnostic evaluation studies of symptomatic children with clinical suspicion of intrathoracic tuberculosis, and were not intended to predefine inclusion criteria into such studies. Feedback from researchers suggested that further clarification was required and that these case definitions could be further improved. Particular concerns were the perceived complexity and overlap of some case definitions, as well as the potential exclusion of children with acute onset of symptoms or less severe disease. The updated case definitions proposed here incorporate a number of key changes that aim to reduce complexity and improve research performance, while maintaining the original focus on symptomatic children suspected of having intrathoracic tuberculosis. The changes proposed should enhance harmonized classification for intrathoracic tuberculosis disease in children across studies, resulting in greater comparability and the much-needed ability to pool study results.
Keywords: childhood tuberculosis, tuberculosis classification, tuberculosis case definitions, tuberculosis diagnosis
Tuberculosis is an important cause of morbidity and mortality in children in tuberculosis-endemic settings [1]. The World Health Organization (WHO) estimated that there were a total of 550 000 childhood tuberculosis cases globally in 2013 [2]. Due to acknowledged limitations of case detection and underreporting, these figures likely underestimate the true burden of childhood tuberculosis [3]. The WHO estimated that there were 80 000 deaths in children due to tuberculosis in 2013, an estimate that only included human immunodeficiency virus (HIV)–uninfected children [4]. Although there are no data of the numbers of tuberculosis-related deaths in HIV-infected children, the greatly increased risks of tuberculosis and of tuberculosis-related mortality in HIV-infected children compared with HIV-uninfected children are well established [4–6].
The diagnosis of tuberculosis in children is challenging, especially in infants and young children (<5 years), who are at particular risk for disease and adverse outcomes from tuberculosis [7]. Diagnostic and management challenges in children are due to the paucibacillary nature of tuberculosis disease, challenges of obtaining respiratory samples, and the wide spectrum of disease manifestations and severity that often overlap with other common childhood conditions such as pneumonia, HIV-associated lung disease, and malnutrition [8–11]. Depending on the study setting and resources, microbiological confirmation is established by culture in only 15%–50% of pediatric cases. Although the recently endorsed Xpert MTB/RIF assay is more sensitive and specific than smear microscopy in children with tuberculosis, it only has a sensitivity of approximately 66% on respiratory specimens compared with culture [8, 9, 12]. For these reasons, the diagnosis of intrathoracic tuberculosis in children frequently is based on nonspecific symptoms and signs, supported by evidence of exposure to a tuberculosis case or infection with Mycobacterium tuberculosis, and on chest radiography [13]. The limitations of this diagnostic approach are well recognized. It is universally acknowledged that improving the diagnosis of tuberculosis in children both through the development of novel diagnostics and by optimizing the use of current tools are major research priorities [14, 15].
Research aiming to improve tools for the diagnosis of intrathoracic childhood tuberculosis has utilized varying case definitions for the reporting of study findings from several settings, particularly for cases that are clinically diagnosed but not microbiologically confirmed. This has made it difficult to compare findings between studies or to conduct meta-analyses of performance of specific diagnostic tools that might provide an evidence base to inform policy recommendations [11, 12]. Additional methodological challenges for comparison between studies include the need for standardization of diagnostic procedures as well as the definition of the epidemiological setting and study population. In recognition of the importance of developing improved diagnostics and the need for standardized evaluation, an international expert panel developed consensus recommendations for the conduct of research focusing on intrathoracic tuberculosis in children and promoting the use of uniform, standardized case definitions when evaluating novel diagnostic tools [16, 17].
Since the publication of these definitions in 2012 and their subsequent uptake in clinical research, evaluation and validation and clinical observations from their application in the field have identified a need for further clarification and revision. Following a process of consultation, dissemination, and review of recent relevant literature, we present updated case definitions, discuss the rationale behind these changes, and suggest areas for future research.
REVISION PROCESS
An updated review of the case definitions, including recent evaluations, was presented annually (in 2012 and 2013) for discussion and feedback to a wide range of child tuberculosis researchers at symposia of the annual Global Lung Health conference of the International Union Against Tuberculosis and Lung Disease (The Union). Motivated by these discussions, a subcommittee of the original expert panel met in 2014 to discuss revisions of the case definitions. In preparation for this meeting, a literature search was conducted in PubMed and Embase databases covering the period since initial publication from 1 April 2012 through 1 February 2015. Search terms included childhood tuberculosis, tuberculosis classification, and tuberculosis diagnostics. Unpublished literature (conference presentations or abstracts, unpublished manuscripts) was also reviewed. A list of elements for consideration for change was prepared as well as suggestions for changes, and these were circulated to the subcommittee for further review and comment. A revised draft was prepared for discussion at the meeting and finalized after further face-to-face discussions.
RESULTS
Consensus agreement was reached by participants of the meeting on the following:
The scope of the clinical case definitions should continue to focus on intrathoracic tuberculosis in children presenting with symptoms. However, there was agreement for the need to develop in the future a similar classification for extrathoracic tuberculosis, especially for young children, as has previously been done for tuberculous meningitis [18].
Entry criteria into tuberculosis diagnostic studies can include children with compatible symptoms and signs of any duration. As initially stated, the standardized definitions of the common presenting clinical features are “for purposes of reporting” and “not to define criteria for enrollment.” There is a need to further emphasize that the symptoms and signs used for purposes of classifying a child, previously listed under “Clinical signs/symptoms suggestive of tuberculosis” [16], are not intended to be used as study entry criteria. There has been confusion and misinterpretation since initial publication that only those with “clinical signs/symptoms suggestive of tuberculosis” could be enrolled. The committee did not consider it necessary to redefine the entry criteria given the wide variation in clinical research settings, but agreed that clearly defined inclusion criteria with careful documentation of the symptoms and signs at entry to tuberculosis diagnostic evaluation studies were essential.
The “clinical signs/symptoms suggestive of tuberculosis” and “interpretation of chest X-ray” for classification of children in tuberculosis diagnostic studies remain unchanged. Children with suspected tuberculosis are followed and reviewed following enrollment in diagnostic evaluation studies, and so the duration and resolution of clinical features can be determined and well defined for purposes of categorization within clinical case definitions.
Tuberculosis exposure: The period of time used to document tuberculosis exposure prior to enrollment has been shortened from 24 months to 12 months,
The case definition of “confirmed tuberculosis” now includes a positive WHO-endorsed nucleic acid amplification test (eg, Xpert MTB/RIF) in addition to culture confirmation using a valid respiratory specimen for microbiologic examination, irrespective of the culture result.
- The case definitions for reporting were narrowed from 5 to 3 groups:
- The case definitions “probable tuberculosis” and “possible tuberculosis” are merged into a single case definition of “unconfirmed tuberculosis.”
- The case definitions of “unlikely tuberculosis” and “not tuberculosis” are merged into a single case definition of “unlikely tuberculosis.”
- Symptomatic children suspected of tuberculosis whose symptoms spontaneously improved in the absence of antituberculosis treatment are included in the “unlikely tuberculosis” classification even if they had initially met other criteria for “unconfirmed tuberculosis.”
“Unconfirmed tuberculosis” and “unlikely tuberculosis” case definitions should be subdivided to distinguish those with and without immunologic evidence of infection with M. tuberculosis (ie, tuberculin skin test or interferon-γ release assay positive).
The limitations of “response to antituberculosis treatment” are noted.
All children who die following study entry need to be reported, including those who are unclassified due to the timing of the death. Death in children suspected of having tuberculosis, including those who are later confirmed not to have tuberculosis, often occurs early following presentation for diagnosis and management. Additionally, sampling for microbiological investigations may not be appropriate or ethical in a child with very severe disease, and the decision to treat for tuberculosis may be made without invasive sampling. Therefore, death may occur before a full diagnostic workup for microbiological confirmation is performed, or before sufficient time elapses to classify a child. It is suggested that the allocation of the child to one of the diagnostic groups be considered postmortem by an expert panel.
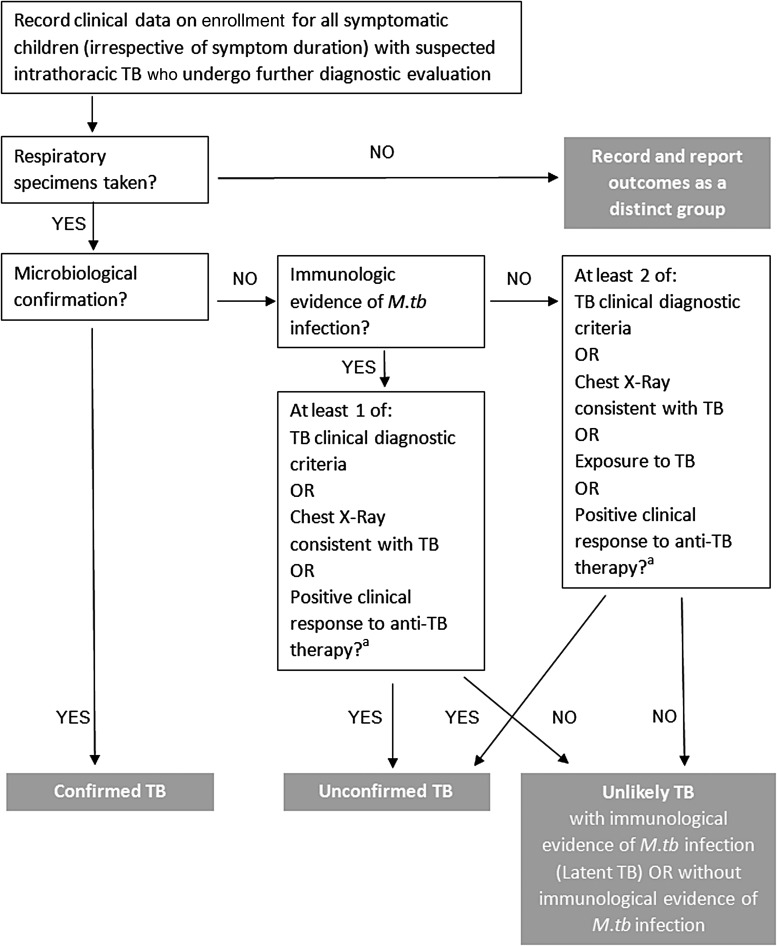
Table 1 summarizes the changes proposed to the criteria definitions. Table 2 presents a revised tuberculosis classification, Table 3 presents the revised case definitions, and Figure 1 presents a revised case definition algorithm.
Table 1.
Summary of Changes to Previously Proposed Consensus Case Definitions
Scope (unchanged)
|
Entry criteria (unchanged)
|
Intrathoracic tuberculosis diagnostic criteria
|
Clinical case definitions for intrathoracic tuberculosis
|
Other
|
Source: Adapted from Graham et al [16].
Abbreviations: IGRA, interferon-γ release assay; NAAT, nucleic acid amplification test; TST, tuberculin skin test; WHO, World Health Organization.
Table 2.
Revised Classification of Intrathoracic Tuberculosis Case Definitions for Diagnostic Evaluation Studies in Children
| Case Definition | Refined Criteriaa |
|---|---|
| Confirmed tuberculosis | Bacteriological confirmation obtained Requires Mycobacterium tuberculosis to be confirmed (culture or Xpert MTB/RIF assay) from at least 1 respiratory specimen |
| Unconfirmed tuberculosis | Bacteriological confirmation NOT obtained AND at least 2 of the following:
|
| Unlikely tuberculosis | Bacteriological confirmation NOT obtained AND Criteria for “unconfirmed tuberculosis” NOT met
|
Abbreviations: IGRA, interferon-γ release assay; TST, tuberculin skin test.
a All children should have symptoms compatible with tuberculosis as determined by the treating clinician.
Table 3.
Revised Details and Definitions for Research Evaluation and Reporting Purposes
| Entry criteria: Any current compatible symptom irrespective of duration in a child suspected to have tuberculosis; use the clinical features for clinical diagnosis or disease classification. Clearly define inclusion criteria for research with careful documentation of presenting symptoms, contact history, and signs. |
|
|
|
|
|
|
Abbreviations: 2TU, 2 tuberculin units; 5TU, 5 tuberculin units; CXR, chest radiograph; HIV, human immunodeficiency virus; IGRA, interferon-γ release assay; NAAT, nucleic acid amplification test; PPD, purified protein derivative; WHO, World Health Organization.
a Previous symptoms are all relevant.
b If other causes are excluded or not responding to appropriate treatment thereof.
Figure 1.
Proposed revised algorithm for classification of case definitions for research reporting from diagnostic evaluation studies of intrathoracic tuberculosis in children. Response or no response to antituberculosis therapy is only relevant if antituberculosis therapy was given as determined by clinical indications. A trial of treatment should NOT be used as a diagnostic tool. aNote that this does not refer to a positive clinical response without anti-TB therapy, which would be a negative feature for this category. Abbreviations: M.tb, Mycobacterium tuberculosis; TB, tuberculosis.
DISCUSSION
The development and refinement of standardized methodological approaches for the evaluation of tuberculosis diagnostics and other studies in pediatric populations remains a critical challenge, including for the standardization of specimen collection, laboratory procedures, and reporting of data. The initial efforts by an international consensus panel to address the challenge were designed as evolving tools, to be refined as new evidence accumulates [16, 17]. Consistent with these aims, the 2015 revised consensus case definitions are informed by observations, feedback, new developments, and emerging evidence since their initial publication, including data from studies specifically aimed to validate aspects of the case definitions. The original consensus definitions did not predefine or restrict entry criteria, but rather aimed to standardize clinical definitions for reporting purposes [16]. However, this intent has been clarified and further emphasized in the current update.
In recent years, diagnostic evaluation studies undertaken in children with suspected tuberculosis in a range of settings and study populations have provided an evidence base for WHO to recommend the use of Xpert MTB/RIF in children [12]. The specificity of Xpert MTB/RIF for identification of M. tuberculosis is consistently very high, and the global rollout of Xpert technology means that resource-limited settings will increasingly have access for diagnosis, including where culture is not readily available. Therefore, a positive Xpert MTB/RIF result in a respiratory specimen is considered to be bacteriological confirmation of intrathoracic tuberculosis, irrespective of the culture result. “Valid specimens” for the detection of DNA of M. tuberculosis with Xpert include respiratory specimens from sputum sampled by expectoration, sputum induction, gastric aspirates, or nasopharyngeal aspirates and can include the use of a stool sample [19, 20]. However, the sensitivity of the currently available Xpert MTB/RIF assay is lower than that of culture in children [21], and therefore culture should remain the preferred method for microbiological confirmation, where available.
The major ongoing challenge in classification of tuberculosis in children remains with those who are not bacteriologically confirmed to have tuberculosis, but in whom the diagnosis relies on clinical parameters and investigations with known limitations. A number of studies have recently evaluated the clinical definitions originally proposed for purposes of classifying unconfirmed cases. Zar et al reported hospitalized children with confirmed tuberculosis and unconfirmed tuberculosis who presented with acute symptoms [22], as found in previous studies of children hospitalized with pneumonia [11]. Using the current clinical definitions for classifying unconfirmed tuberculosis cases, the lack of persistence of symptoms until the time of clinical diagnosis would affect how such children are finally categorized. These studies [11, 22] highlight the need for broad, symptomatic entry criteria that are compatible with tuberculosis, yet minimally restrictive and not duration dependent.
The intent of the original consensus was to support the diagnosis of tuberculosis identified through passive case finding [16], that is, children presenting with symptoms, being the target population typically included in diagnostic evaluation studies. Definitions will need to be developed for different scenarios such as involving active case finding, for example, during household contact studies, but this is outside the present scope of work. Evaluation of data from active case-finding studies, one as a cohort study with active follow-up [23] and the other as a community-based tuberculosis contact investigation study [24], have reported that 12% and 65%, respectively, of children with confirmed tuberculosis did not have clinical features consistent with the standardized “clinical signs/symptoms suggestive of tuberculosis” used to categorize clinical, unconfirmed cases. Active case finding is likely to identify cases at an earlier stage of disease and a much shorter duration of symptoms compared with children investigated for tuberculosis at the referral level. As the entry point for contact studies is, by definition, a positive history of exposure, it compromises one of the definitions used for clinical classification in the original proposed definitions. These studies emphasize the challenges and importance of employing case definitions that capture disease across a wide range of clinical and epidemiological settings.
In the original definitions, any exposure to a tuberculosis case within 24 months prior to presentation was listed, but the revised definitions have shortened this to 12 months to increase its relevance and specificity. In natural history studies from the prechemotherapy literature, >90% of tuberculosis disease progression in young children occurred within 12 months of primary M. tuberculosis infection [7]. In a meta-analysis of 25 studies of tuberculosis incidence among contacts of tuberculosis cases in low-and-middle income countries, the incidence of tuberculosis was highest in the first year (1.5 per 100 person-years of observation) and declined to about 0.5 after the third year of follow-up [25]. A recent cohort study in Uganda followed children with recent household tuberculosis exposure for 24 months, and almost all tuberculosis cases identified occurred within the first 6 months after enrollment [26].
Certain definitions were left unchanged due to a lack of interim evidence for modification. Given the recognized limitations of chest radiography for the diagnosis of tuberculosis in children and often poor interobserver agreement [27–29], a multistep process is still recommended that includes assessment of chest radiograph quality and independent assessment of radiologic lesion and location by at least 2 separate readers who are blinded to clinical information. There is a need to evaluate potential improved imaging techniques for the diagnosis of intrathoracic tuberculosis in children [30, 31].
The inclusion of treatment response to antituberculosis treatment in the clinical classification has a number of recognized limitations, including a lack of empirical evidence. It is difficult to assess treatment response in children with clinical, unconfirmed tuberculosis due to the inherent uncertainty of the initial diagnosis. It may also be that the child is infected with a strain that is drug resistant. Alternatively, clinical improvement with treatment for tuberculosis does not necessarily confirm tuberculosis, as the drugs used for tuberculosis may also be effective for infections with other pathogens and the role of coinfections of tuberculosis with other bacteria, such as pneumococci, is increasingly recognized [11, 32]. For example, South African children with culture-confirmed tuberculosis had marked initial improvement to broad-spectrum antibiotics used to treat community-acquired pneumonia prior to receiving the culture result and initiating specific treatment for tuberculosis [32]. Evidence from the prechemotherapy era also suggests that spontaneous clinical resolution may occur in some children with confirmed tuberculosis [7]. In a more recent report, 8 of 17 children with bacteriologically confirmed tuberculosis who had not received treatment for tuberculosis were asymptomatic and in good health in the absence of treatment at a median of 73 days after initial presentation [27]. In many of these cases, however, this phenomenon may represent excretion of the bacilli shortly after the primary infection rather than overt disease, highlighting that diagnostic evaluations in children need to consider the natural course of the disease after infection.
A recent vaccine study with active surveillance for tuberculosis cases provided data for retrospective analysis of treatment response [33]. All children had at least 1 baseline symptom compatible with tuberculosis and were treated for tuberculosis with first-line combination therapy. Children considered to have definite or probable tuberculosis at post hoc analysis on the basis of culture confirmation or radiological features were compared to those considered not to have had tuberculosis. The time taken for complete resolution of baseline symptoms was >60 days following tuberculosis treatment in both groups, without difference between the groups [33]. However, the diagnostic value of symptom resolution or persistence in children with definite tuberculosis is the more relevant question here. It could be argued that a measure of symptomatic improvement, rather than complete resolution of symptoms, might be a more useful indicator given that it happens earlier. These uncertainties should be addressed by further research. There was renewed consensus that follow-up to prospectively collect treatment response data is required. Final outcome data provide evidence of clinical impact, an increasingly important aspect of tuberculosis diagnostics evaluations [34].
The committee recommended consolidation of the previous “probable” and “possible” tuberculosis case definitions under a single new case definition to be named “unconfirmed tuberculosis.” Although one disadvantage of this approach may be the loss of distinction between diagnostic certainty tiers, the panel placed less emphasis on the need to delineate tiers with uncertain relevance, and more weight on accurate data collection to allow for classification and reclassification based on future evidence. Furthermore, when combined with harmonized data collection, this approach may allow greater flexibility for reclassification across studies and could afford greater ability to assess correlations of parameters and/or combinations thereof with the presence or absence of tuberculosis disease.
The expert committee also recommended consolidating the “unlikely” and “not tuberculosis” case definitions under a single classification of “unlikely tuberculosis.” This will reduce potential confusion without affecting interpretation of study findings. There is no diagnostic test that can completely exclude tuberculosis as a possible diagnosis, and even the confirmation of another etiological diagnosis does not necessarily exclude coinfection with tuberculosis [11]. The case definitions of “unconfirmed tuberculosis” and “unlikely tuberculosis” should be subdivided to distinguish between those with or without immunologic evidence of infection with M. tuberculosis (Table 2), as this distinction may be particularly relevant for biomarker research.
It is important to highlight that although response to antituberculosis treatment is an important feature to document and consider in the final classification of cases for reporting as “unconfirmed” or “unlikely” tuberculosis, these classifications are not intended to inform the clinical decision to start antituberculosis treatment. Therefore, the case definitions are not treatment guidelines. The decision for and timing of tuberculosis treatment initiation are made by the treating clinician and will often occur before all data are available to inform classification for reporting for research purposes. Therefore, a child may commence treatment for tuberculosis and yet may ultimately be classified as having “unlikely tuberculosis.”
Finally, feedback was provided that the original classification did not readily allow for categorization of children enrolled with suspected tuberculosis who died before the required criteria could be established for categorization [35]. Clearly, this is an important group that must be included in reporting, and the consensus is therefore to use an expert panel to classify all children who die after study enrolment.
There remains a need to evaluate the prospective performance of the case definitions in a range of settings. Consistent with the original intent of the consensus group, the revised case definition is an evolving document to be refined as future evidence provides information to improve the accuracy and utility of these definitions. Frequent changes, however, would not be practical or helpful, as a major benefit of the proposed approach is to improve comparability between studies. The timelines of future updates will need to be balanced against the importance of using a homogeneous classification across studies for the evaluation of novel and available diagnostic tools.
Notes
Disclaimer. The views expressed in written conference materials or publications and by speakers and moderators at Department of Health and Human Services (DHHS)–sponsored conferences do not necessarily reflect the official policies of the DHHS; nor does mention of trade names, commercial practices, or organizations imply endorsement by the US government.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract number HHSN272200800014C).
Supplement sponsorship. This article appears as part of the supplement “Advances in Tuberculosis Research: A Blueprint for Opportunities.” This article was sponsored by the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Graham SM, Sismanidis C, Menzies HJ, Marais BJ, Detjen AK, Black RE. Importance of tuberculosis control to address child survival. Lancet 2014; 383:1605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 3.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Global Health 2014; 2:e453–9. [DOI] [PubMed] [Google Scholar]
- 4.Buck WC, Olson D, Kabue MM, et al. Risk factors for mortality in Malawian children with human immunodeficiency virus and tuberculosis co-infection. Int J Tuberc Lung Dis 2013; 17:1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis 2009; 48:108–14. [DOI] [PubMed] [Google Scholar]
- 6.Li N, Manji KP, Spiegelman D, et al. Incident tuberculosis and risk factors among HIV-infected children in Tanzania. AIDS 2013; 27:1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 8.Zar HJ, Connell TG, Nicol M. Diagnosis of pulmonary tuberculosis in children: new advances. Exp Rev Anti-infect Therapy 2010; 8:277–88. [DOI] [PubMed] [Google Scholar]
- 9.Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Resp Rev 2011; 12:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaganath D, Mupere E. Childhood tuberculosis and malnutrition. J Infect Dis 2012; 206:1809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliwa JN KJ, Marais BJ, Madhi SA, Graham SM. Tuberculosis and childhood pneumonia in tuberculosis endemic settings—common, cause or consequence? A review of the evidence. Lancet Resp Med 2015; 3:235–43. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Policy update: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Available at: http://apps.who.int/iris/bitstream/10665/112472/1/9789241506335_eng.pdf?ua=1 Accessed 4 December 2014.
- 13.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children: second edition. Geneva, Switzerland: WHO, 2014. [PubMed] [Google Scholar]
- 14.World Health Organization. An international roadmap for tuberculosis research: towards a world free of tuberculosis. Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 15.WHO/The Union/UNICEF/CDC/USAID/TAG. Roadmap for childhood tuberculosis: towards zero deaths. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 16.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis 2012; 205(suppl 2):S199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuevas LE, Browning R, Bossuyt P, et al. Evaluation of tuberculosis diagnostics in children: 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. Consensus from an expert panel. J Infect Dis 2012; 205(suppl 2):S209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10:803–12. [DOI] [PubMed] [Google Scholar]
- 19.Walters E, Gie RP, Hesseling AC, Friedrich SO, Diacon AH, Gie RP. Rapid diagnosis of pediatric intrathoracic tuberculosis from stool samples using the Xpert MTB/RIF Assay: a pilot study. Pediatr Infect Dis J 2012; 31:1316. [DOI] [PubMed] [Google Scholar]
- 20.Nicol MP, Spiers K, Workman L, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis 2013; 57:e18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detjen AK, Di Nardo AR, Leyden J, et al. Xpert® MTB/RIF for the diagnosis of pulmonary tuberculosis in children—a systematic review and meta-analysis. Lancet Resp Med 2015; 3:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zar HJ, Workman LJ, Little F, Nicol MP. Diagnosis of pulmonary tuberculosis in children: assessment of the 2012 National Institutes of Health expert consensus criteria. Clin Infect Dis 2015; 61(suppl 3):S173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beneri C, Aaron L, Soyeon K, et al. Understanding NIH clinical case definitions for pediatric intrathoracic TB by applying them to a clinical trial. Int J Tuberc Lung Dis 2015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiseman CA, Mandalakas AM, Kirshner HL. Novel application of NIH case definitions in a paediatric tuberculosis contact investigation study. Int J Tuberc Lung Dis 2015; 19:446–53. [DOI] [PubMed] [Google Scholar]
- 25.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Resp J 2013; 41:140–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaganath D, Zalwango S, Okware B, et al. Contact investigation for active tuberculosis among child contacts in Uganda. Clin Infect Dis 2013; 57:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelbrecht AL, Marais BJ, Donald PR, Schaaf HS. A critical look at the diagnostic value of culture-confirmation in childhood tuberculosis. J Infect 2006; 53:364–9. [DOI] [PubMed] [Google Scholar]
- 28.Kaguthi G, Nduba V, Nyokabi J, Onchiri F, Gie R, Borgdorff M. Chest radiographs for pediatric TB diagnosis: interrater agreement and utility. Interdisc Perspect Infect Dis 2014; 2014:291841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swingler GH, du Toit G, Andronikou S, van der Merwe L, Zar HJ. Diagnostic accuracy of chest radiography in detecting mediastinal lymphadenopathy in suspected pulmonary tuberculosis. Arch Dis Child 2005; 90:1153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breuninger M, van Ginneken B, Philipsen RH, et al. Diagnostic accuracy of computer-aided detection of pulmonary tuberculosis in chest radiographs: a validation study from sub-Saharan Africa. PLoS One 2014; 9:e106381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belard S, Andronikou S, Pillay T, Grobusch MP, Zar HJ. New imaging approaches for improving diagnosis of childhood tuberculosis. South Afr Med J 2014; 104:181–2. [DOI] [PubMed] [Google Scholar]
- 32.Moore DP, Klugman KP, Madhi SA. Role of Streptococcus pneumoniae in hospitalization for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study. Pediatr Infect Dis J 2010; 29:1099–4. [DOI] [PubMed] [Google Scholar]
- 33.Mpofu N, Moyo S, Mulenga H, et al. Time to symptom resolution in young children treated for pulmonary tuberculosis. Pediatr Infect Dis J 2014; 33:1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumacher SG, Pai M. Xpert® MTB/RIF for extra-pulmonary tuberculosis: time to look beyond accuracy. Int J Tuberc Lung Dis 2015; 19:2. [DOI] [PubMed] [Google Scholar]
- 35.Holm LL, Rose MV, Ravn P. Perspectives in implementing standardized case definitions for tuberculosis research involving children in a low-income, high-burden setting. J Infect Dis 2013; 207:870–1. [DOI] [PubMed] [Google Scholar]