We provide pharmacokinetic/pharmacodynamic data supporting that 4 weeks of daily rifapentine (approximately 10 mg/kg) and isoniazid (300 mg) can be coadministered with efavirenz without clinically meaningful reductions in efavirenz mid-dosing concentrations or virologic suppression.
Keywords: HIV/AIDS, tuberculosis, rifapentine, pharmacokinetics, pharmacodynamics
Abstract
Background. Concomitant use of rifamycins to treat or prevent tuberculosis can result in subtherapeutic concentrations of antiretroviral drugs. We studied the interaction of efavirenz with daily rifapentine and isoniazid in human immunodeficiency virus (HIV)–infected individuals receiving a 4-week regimen to prevent tuberculosis.
Methods. Participants receiving daily rifapentine and isoniazid with efavirenz had pharmacokinetic evaluations at baseline and weeks 2 and 4 of concomitant therapy. Efavirenz apparent oral clearance was estimated and the geometric mean ratio (GMR) of values before and during rifapentine and isoniazid was calculated. HIV type 1 (HIV-1) RNA was measured at baseline and week 8.
Results. Eighty-seven participants were evaluable: 54% were female, and the median age was 35 years (interquartile range [IQR], 29–44 years). Numbers of participants with efavirenz concentrations ≥1 mg/L were 85 (98%) at week 0; 81 (93%) at week 2; 78 (90%) at week 4; and 75 (86%) at weeks 2 and 4. Median efavirenz apparent oral clearance was 9.3 L/hour (IQR, 6.42–13.22 L/hour) at baseline and 9.8 L/hour (IQR, 7.04–15.59 L/hour) during rifapentine/isoniazid treatment (GMR, 1.04 [90% confidence interval, .97–1.13]). Seventy-nine of 85 (93%) participants had undetectable HIV-1 RNA (<40 copies/mL) at entry; 71 of 75 (95%) participants had undetectable HIV-1 RNA at week 8. Two participants with undetectable HIV-1 RNA at study entry were detectable (43 and 47 copies/mL) at week 8.
Conclusions. The proportion of participants with midinterval efavirenz concentrations ≥1 mg/L did not cross below the prespecified threshold of >80%, and virologic suppression was maintained. Four weeks of daily rifapentine plus isoniazid can be coadministered with efavirenz without clinically meaningful reductions in efavirenz mid-dosing concentrations or virologic suppression.
Clinical Trials Registration. NCT 01404312.
Human immunodeficiency virus (HIV) and tuberculosis are the leading causes of infection-related deaths globally [1]. With access to antiretroviral therapy (ART), HIV may become a manageable chronic disease. However, the presence of coinfection with tuberculosis leads to an increased level of morbidity and mortality if left untreated. In 2012, the World Health Organization (WHO) estimated there were 320 000 deaths in HIV-infected individuals related to tuberculosis [1]. HIV-infected individuals with latent tuberculosis (LTBI) are 21–34 times more likely to develop active tuberculosis than HIV-uninfected individuals [1]. Early detection and treatment of LTBI in HIV-infected individuals is imperative.
Isoniazid has long been the cornerstone of treatment to prevent active tuberculosis. However, current regimens are lengthy and may be associated with adverse effects. Difficulties with adherence to the current isoniazid recommendations lead to high rates of therapy discontinuation and poor rates of completion [2]. There is a compelling need to establish shorter and safer treatment regimens for LTBI in HIV-infected individuals.
Recent data have shown that a 3-month regimen of once-weekly rifapentine plus isoniazid is as effective as the current standard of care, 9 months of daily isoniazid [3, 4]. However, the US Centers for Disease Control and Prevention (CDC) guidelines currently do not recommend use of a rifapentine-containing regimen in HIV-infected individuals on ART because possible drug–drug interactions with rifapentine have not been fully evaluated [5]. Additionally, the International Standards for Tuberculosis Care, developed by TB CARE, which includes the WHO and the American Thoracic Society, recommend using isoniazid preventive therapy over rifapentine-containing regimens for treatment of LTBI in high-prevalence areas in HIV-infected individuals [6].
Rifapentine is a known cytochrome P450 (CYP) enzyme inducer [7, 8]. Efavirenz-based ART is a first-line treatment option for HIV in both US and international guidelines [9–13]. Efavirenz is a CYP substrate, leading to concern for decreased efavirenz concentrations and increased risk of virologic failure if dosed concurrently with rifapentine. The pharmacokinetic characteristics of this combination have not been evaluated. To this end, we designed a study to evaluate the effect of daily rifapentine plus isoniazid for 4 weeks on efavirenz pharmacokinetics.
METHODS
Study Population
The AIDS Clinical Trials Group (ACTG) study A5279 is an international, multicenter, randomized, open-label, phase 3 clinical trial comparing a 4-week daily rifapentine and isoniazid regimen with a standard 9-month daily isoniazid regimen for the prevention of tuberculosis in HIV-infected participants without evidence of active tuberculosis. An objective of A5279 was to investigate the effects of concomitant rifapentine and isoniazid administration on efavirenz pharmacokinetics and to determine the proportions of participants who had acceptable efavirenz concentrations during concomitant administration of rifapentine and isoniazid. Overall study inclusion criteria were as follows: HIV-infected men and women at least 13 years of age and 30 kg body weight, without evidence of active tuberculosis, who either have a positive tuberculin skin test or interferon-γ release assay or live in a high-burden area for tuberculosis (defined as prevalence of at least 60 cases per 100 000 population). The first 90 study participants taking an efavirenz-containing ART regimen for at least 4 weeks prior to study entry and randomized to the rifapentine plus isoniazid arm of A5279 were enrolled in the pharmacokinetic study. The institutional review boards or ethics committees of the participating institutions approved the study, and each participant gave written informed consent.
Randomization and Masking
Participants were randomly assigned to either 9 months of daily isoniazid plus pyridoxine (vitamin B6), or 4 weeks of daily weight-based rifapentine and isoniazid plus pyridoxine in an open-label fashion.
Outcomes
The primary outcome of the pharmacokinetic study was the proportion of participants with efavirenz plasma concentrations ≥1 mg/L while receiving daily rifapentine and isoniazid. The threshold of ≥1 mg/L was selected because mid-dosing interval concentrations <1 mg/L have been shown to increase the odds of virologic failure [14].
A priori rules of adequate proportions of patients with acceptable efavirenz plasma concentrations at both weeks 2 and 4 were included in the study protocol. Efavirenz pharmacokinetic data were judged to be acceptable if ≤20% of participants had efavirenz concentrations <1 mg/L at both weeks 2 and 4. Any participant with a baseline (week 0; pre–rifapentine and isoniazid) efavirenz plasma concentration <1 mg/L was taken as evidence for efavirenz nonadherence and deemed not evaluable for the purposes of the pharmacokinetic study.
Putative stopping rules were developed for the pharmacokinetic study should a large proportion of study participants have unacceptable plasma concentrations of efavirenz while taking the rifapentine and isoniazid. Pharmacokinetic data were evaluated after enrollment of 9, 21, 31, and 90 participants taking an efavirenz-containing antiretroviral regimen. At each of these enrollment milestones, the numbers of participants necessary to have acceptable efavirenz concentrations at both weeks 2 and 4 according to the stopping rules were ≥4 of 9 participants, ≥12 of 21 participants, and ≥20 of 31 participants, respectively. These rules were developed to have a high likelihood (≥95%) of continuing to accrue participants if the true underlying rate of having acceptable efavirenz concentrations is >80% [15].
The final evaluation occurred after 90 participants had been enrolled in the pharmacokinetic study. In the final evaluation, the exact lower 95% confidence bound was calculated for the proportion of participants with efavirenz concentrations >1 mg/L during the 4 weeks of LTBI therapy. The efavirenz pharmacokinetic data were deemed acceptable if the lower bound of the 95% confidence interval (CI) of the proportion of participants with acceptable efavirenz concentrations at both weeks 2 and 4 did not fall below the prespecified 80% level.
Procedures
Rifapentine dosing was based on body weight. Participants weighing 30–34.9 kg received 300 mg once daily, participants weighing 35–44.9 kg received 450 mg once daily, and participants weighing ≥45 kg received 600 mg once daily, administered as 150-mg tablets. Rifapentine was provided by sanofi-aventis (France) and isoniazid from multiple manufacturers. Efavirenz was obtained from local sources at each study site and was approved by national authorities. Patients were instructed to take all doses orally with food. The isoniazid dose was 300 mg, administered orally as a single tablet, daily with pyridoxine (vitamin B6) 25 mg; isoniazid was to be taken together with rifapentine and food.
Plasma samples were collected for pharmacokinetic evaluations at study entry (week 0, prior to initiation of rifapentine and isoniazid) and again at week 2 and week 4 (during rifapentine and isoniazid administration). A mid-dosing interval (12 hours postdose) plasma sample was collected for pharmacologic evaluation of efavirenz concentrations. Additional plasma samples were collected at baseline and at week 8 (post–study drug administration) for HIV type 1 (HIV-1) RNA quantitation. Efavirenz concentrations were quantified by validated ultra performance liquid chromatography assays as previously described [16].
Pharmacokinetic parameter estimation was accomplished using maximum a posteriori probability–Bayesian estimation implemented in ADAPT II (Biomedical Simulations Resource at the University of Southern California, Los Angeles) [17]. The primary pharmacokinetic parameter of interest for efavirenz was apparent oral clearance. Population mean and standard deviation values for efavirenz apparent oral clearance used in the Bayesian analysis were 8.0 ± 4 L/hour, and were taken from published literature [18]. Week 0 efavirenz concentrations were used to estimate the prerifapentine plus isoniazid apparent oral clearance of efavirenz. Plasma concentrations from weeks 2 and 4 were taken together to estimate the apparent oral clearance of efavirenz during concomitant treatment with rifapentine and isoniazid.
Statistical Analysis
Descriptive statistics were used to summarize continuous variables, including age, CD4 counts, and efavirenz treatment duration at study entry. Categorical variables, including race/ethnicity, country, whether HIV-1 RNA level was detectable, and ART at study entry, were summarized by frequency distribution. Participants with missing data were not included in the calculations. Participants with randomized treatment temporarily held prior to week 4 or incomplete pharmacokinetic evaluations were excluded from the analysis. Median and percentiles were used to describe the collection time of pharmacokinetic samples post–efavirenz dose and efavirenz concentration at weeks 0, 2, and 4. The geometric mean ratio (GMR) and 2-sided 90% CI of the pre- and on-rifapentine and isoniazid apparent oral clearance values were calculated. Analyses were done using SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
From May 2012 until May 2013, 97 HIV-infected individuals receiving efavirenz-containing ART were enrolled in the pharmacokinetic study from 5 countries: South Africa, Thailand, United States, Botswana, and Peru. Seven participants had incomplete (weeks 0, 2, and 4) PK sample collection. One participant was hospitalized and did not complete the pharmacokinetic evaluations. One participant had study medication (rifapentine and isoniazid) held and thus did not complete the pharmacokinetic study, and 1 participant was not included due to the clinic inadvertently not storing a collected pharmacokinetic sample. Eighty-seven participants had complete data sets (week 0, 2, and 4) available for pharmacokinetic analyses (Table 1). The median age of the 87 participants was 35 years (interquartile range [IQR], 29–44 years); 47 (54%) were female; 48 (55%) were black non-Hispanic; and 37 (43%) and 31 (36%) of the participants were from South Africa and Thailand, respectively.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Total (N = 87) |
|---|---|
| Age, y, median (IQR) | 35 (29–44) |
| Female sex | 47 (54) |
| Race/ethnicity | |
| White non-Hispanic | 1 (1) |
| Black non-Hispanic | 48 (55) |
| Hispanic (regardless of race) | 6 (7) |
| Asian/Pacific Islander | 32 (37) |
| Country of enrollment | |
| Botswana | 5 (6) |
| Peru | 2 (2) |
| South Africa | 37 (43) |
| Thailand | 31 (36) |
| United States | 12 (14) |
| HIV-1 RNA, log10 copies/mLa | |
| Mean (SD) | 1.6 (0.2) |
| Min, Max | 1.6, 3.0 |
| Undetectable (<40 copies/mL) | 79 (93) |
| Detectable | 6 (7) |
| CD4 count, cells/µL, median (IQR) | 508 (372–649) |
| EFV duration, wk, median (IQR) | 109 (61–233) |
Data are shown as No. (%) unless otherwise specified.
Abbreviations: EFV, efavirenz; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; SD, standard deviation.
a Entry HIV-1 RNA was available for 85 of 87 evaluable participants.
Samples for HIV-1 RNA were collected from 85 of 87 participants at baseline (week 0) and from 75 of 87 participants at week 8 HIV. Seventy-nine of the 85 (93%) participants had undetectable (<40 copies/mL) plasma HIV-1 RNA at study entry. Seventy-one of 75 (95%) participants with available week 8 HIV-1 RNA had a undetectable level (<40 copies/mL). Of 75 participants with HIV-1 RNA results at week 8, 71 had undetectable HIV-1 RNA at entry. Two of these 71 (2.8%) had detectable HIV-1 RNA (43 and 47 copies/mL) at week 8. The median duration of efavirenz containing ART before study entry was 109 weeks (IQR, 61–233 weeks).
Interim safety evaluations were conducted by assessing the proportion of participants with acceptable efavirenz concentrations when study enrollment reached 9, 21, and 31 evaluable participants. As all passed their respective thresholds, the study was allowed to enroll to full accrual.
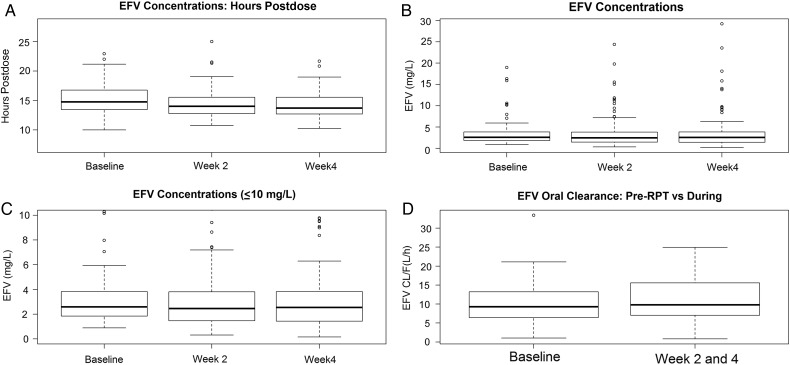
Two hundred sixty-one pharmacokinetic samples (87 samples at each week 0, 2, and 4) were collected at a median post–efavirenz dose sampling time of 14.8 hours (IQR, 13.5–16.8 hours) at week 0, 14.0 hours (IQR, 12.8–15.6 hours) at week 2, and 13.7 hours (IQR, 12.7–15.5 hours) at week 4 (Figure 1A). Median week 0 (efavirenz only, no rifapentine/isoniazid) efavirenz concentrations were 2.59 mg/L (IQR, 1.85–3.83 mg/L). Median week 2 (efavirenz + rifapentine + isoniazid) efavirenz concentrations were 2.46 mg/L (IQR, 1.47–3.81 mg/L), and median week 4 (efavirenz + rifapentine + isoniazid) efavirenz concentrations were 2.54 mg/L (IQR, 1.44–3.83 mg/L). Eighty-five (98%) participants had week 0 efavirenz plasma concentrations ≥1 mg/L, 81 (93%) participants had week 2 efavirenz plasma concentrations ≥1 mg/L, and 78 (90%) study participants had week 4 plasma efavirenz concentrations ≥1 mg/L. Seventy-five (86%) participants had week 2 and week 4 plasma efavirenz concentrations ≥1 mg/L with a lower 95% CI bound of 78.6%. Excluding (as per protocol) the 2 participants with efavirenz <1 mg/L at entry, 75 of 85 (88.2%) had efavirenz ≥1 mg/L at both weeks 2 and 4 with a lower 95% CI bound of 80.8% (Figure 1B and 1C). The median apparent oral clearance of efavirenz was 9.3 L/hour (range, 1.0–33.4) for week 0, prior to initiation of rifapentine and isoniazid, and 9.8 L/hour (range, .8–24.9) for combined weeks 2 and 4, during rifapentine and isoniazid treatment (Figure 1D). The GMR of efavirenz apparent oral clearance was 1.04 (on rifapentine and isoniazid to off rifapentine and isoniazid; 90% CI, .97–1.13).
Figure 1.
Efavirenz (EFV) sampling times, hours postdose by week (A), EFV concentrations by week (B), EFV concentrations by week (truncated concentration axis) (C), and EFV apparent oral clearance (CL/F) before and after rifapentine (RPT) and isoniazid administration (D).
DISCUSSION
In this study of coadministration of rifapentine and isoniazid for 4 weeks in HIV-infected persons receiving an efavirenz-containing antiretroviral regimen, we found that 88% of participants maintained efavirenz concentrations above the minimum target concentration of 1 mg/L. A nonsignificant increase in the apparent oral clearance of efavirenz was observed, consistent with the well-known CYP induction properties of rifapentine [7, 8]. Of those participants who had HIV-1 RNA <40 copies/mL at baseline and an available week 8 HIV-1 RNA sample, 97% remained at <40 copies/mL after completion of rifapentine and isoniazid therapy. One limitation of the study was that week 8 HIV-1 RNA data were not available for 12 of the patients included in the pharmacokinetic study. However, week 8 HIV-1 RNA data were available on 86% of the patients with complete pharmacokinetic data available. The findings provide a pharmacokinetic and pharmacodynamic basis to support 4 weeks of rifapentine and isoniazid coadministration for prevention of tuberculosis in HIV-infected persons also taking efavirenz.
The CDC currently recommends once-weekly directly observed treatment with rifapentine and isoniazid for 12 weeks as an alternative to 9 months of daily isoniazid for the treatment of LTBI [5]. This recommendation is based on 3 randomized clinical trials that have shown the shorter-course regimens to be as effective as 9 months of daily isoniazid therapy [3, 4, 19]. Utilization rates of efavirenz-containing ART are high in countries of high tuberculosis prevalence. The rifapentine-isoniazid regimen, however, is not recommended for use in HIV-infected persons receiving ART because potential drug–drug interactions have not been evaluated. Efavirenz is metabolized by CYP2B6 and CYP2A6 [20], and rifapentine, like rifampin, is a known inducer of CYP-mediated drug metabolism, with the potential to lower efavirenz concentrations [7, 8]. In contrast, isoniazid has been shown to inhibit CYP2A6, which may counteract an induction effect of rifapentine in a select genotypic subset of individuals [21]. The role of isoniazid in this 3-way drug–drug interaction among rifapentine and efavirenz appears to better predict and explain high efavirenz plasma concentrations, which may cause patients to experience adverse drug effects from efavirenz, as opposed to lower efavirenz concentrations, which may lead to virologic failure [22–26]. With regard to the interaction between efavirenz and rifampin, the US Department of Health and Human Services guidelines for treatment of HIV infection suggest an increased efavirenz dose of 800 mg daily as an option in patients receiving rifampin who weigh >60 kg [10]. Similarly, the US Food and Drug Administration–approved package insert for efavirenz recommends the higher dose of 800 mg daily in patients weighing ≥50 kg receiving rifampin [27]. The basis for these recommendations is pharmacokinetic studies that have shown an increase in efavirenz apparent oral clearance and a reduction in efavirenz concentrations by approximately 30%, and an attempt to minimize the potential for subtherapeutic concentrations by increasing the dose of efavirenz [28]. A number of clinical trials, however, have shown sustained virologic efficacy during concomitant standard-dose efavirenz and rifampin therapy [29–32]. Nonetheless, the lack of any pharmacokinetic and pharmacodynamic data when daily rifapentine is given with efavirenz has precluded a recommendation for coadministration in HIV-infected individuals on ART.
The challenges of concomitant therapy for HIV infection and LTBI are not trivial. Current treatment options for LTBI in patients receiving ART are limited to ≥6 months of daily isoniazid preventive therapy. Despite the demonstrated efficacy of isoniazid preventive therapy, poor rates of uptake and the high proportion of individuals who fail to complete even 6 months of isoniazid preventive therapy highlight the need for alternative, more effective short-course approaches for treatment of LTBI [33]. ACTG A5279 represents an effort to extend the benefits of a novel, short-course rifapentine-containing regimen for LTBI treatment to HIV-infected individuals on ART. We chose to study the rifapentine–efavirenz interaction in this intended patient population, rather than the more common evaluation of drug–drug interactions in healthy volunteers. To accomplish this, we used a convenient and minimally invasive pharmacokinetic sampling strategy. Published data support a single mid-dosing interval efavirenz concentration as informative of the pharmacokinetics and pharmacodynamics of efavirenz [14]. Efavirenz is typically dosed prior to bedtime; therefore, a 12-hour postdose sampling time also was convenient for the participants and the clinical sites. Bayesian analysis allowed us to evaluate the sparse individual participant pharmacokinetic data as it accrued, instead of after complete enrollment. This enabled early protocol stopping rules to limit enrollment had a clinically significant decrease in efavirenz concentrations been detected. The measurement of plasma HIV-1 RNA before and after rifapentine provided a pharmacodynamic endpoint. These characteristics of this clinical pharmacologic evaluation, designed as a companion to the larger A5279 clinical trial, illustrate how necessary drug–drug interaction information can be efficiently and safely obtained. Our finding that a 4-week regimen of daily rifapentine and isoniazid can be administered to HIV-infected persons who are receiving an efavirenz-containing antiretroviral drug regimen, without clinically meaningful reductions in efavirenz concentrations or virologic suppression, is one important step that advances the field of concomitant therapy for HIV infection and latent tuberculosis.
Notes
Acknowledgments. We thank the study participants and the staff at each of the clinical trials sites as well as the Antiviral Pharmacology Laboratory at the University of Nebraska Medical Center. Additionally, we thank Laura Moran, clinical trials specialist for A5279; Ann Walawander and Jimi Tutko, data managers for A5279; and Marilyn Maroni and Sanofi Aventis.
Author contributions. A. T. P., S. S., R. E. C., J. W. A., A. G., C. A. B., and C. V. F. were involved in study design, data collection, data analysis, data interpretation, and manuscript writing. T. M., K. S., and L. M. were site investigators and were involved in data collection and data analysis. Y. B. was a study statistician and was involved in data analysis, data interpretation, and manuscript writing. P. K. was a member of study oversight as well as a clinical affairs safety associate. The National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) also approved the design of the study. Conduct of the study was entirely the responsibility of the investigators, with regulatory oversight by the NIAID. Data collection, management, and interpretation were entirely the responsibility of the investigators. All authors had full access to all data. The corresponding author had the final responsibility to submit for publication.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH.
Financial support. This work was supported by the NIAID of the NIH (UM1 AI068634, UM1 AI068636, and UM1 AI106701). Clinical site support was provided in part by the NIAID (UM1 AI069463, UM1 AI069438, UM1 AI068632, UM1 AI069432, UM1 AI069477, UM1 AI069481 and UM AI069423).
Potential conflicts of interest. C. A. B. has received grants from NIH/NIAID, Gilead, and MBio Diagnostics; has received compensation from ViiV and GlaxoSmithKline (GSK) for participation in Data Safety and Monitoring Board, her institution receives contract funds from Gilead to support conduct of a clinical trial sponsored by Gilead for which C. A. B. is the site investigator, has received research grant and contract support from MBio Diagnostics as well as being a member of the Scientific Advisory Board of MBio Diagnostics; and her spouse is on the scientific advisory board for Gilead, CytoDyne, Monogram Biosciences, and MBio Diagnostics and has received research contract/grant support for unrelated clinical trials from Merck and Boehringer Ingelheim. L. M. has received grants from NIH/NIAID, Bristol-Myers Squibb, Johnson & Johnson, Pfizer, Merck Sharp & Dohme, and GSK and has received nonfinancial support from Sanofi-Aventis. R. E. C. has received personal fees and consultant fees from Merck. S. S. and C. V. F. have received grants from NIH/NIAID. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global tuberculosis report 2013.
- 2.Horsburgh CR, Goldberg S, Bethel J et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest 2010; 137:401–9. [DOI] [PubMed] [Google Scholar]
- 3.Sterling TR, Villarino ME, Borisov AS et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365:2155–66. [DOI] [PubMed] [Google Scholar]
- 4.Martinson NA, Barnes GL, Moulton LH et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med 2011; 365:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep 2011; 60:1650. [PubMed] [Google Scholar]
- 6.TB CARE I. International standards for tuberculosis care, 3rd ed The Hague: TB CARE I, 2014. [Google Scholar]
- 7.Sanofi-Aventis: Priftin (rifapentine) [package insert] August 2007 Available at: http://products.sanofi.us/priftin/priftin.pdf. Accessed 15 January 2015.
- 8.Keung A, Reith K, Eller M, McKenzie K, Cheng L, Weir S. Enzyme induction observed in healthy volunteers after repeated administration of rifapentine and its lack of effect on steady-state rifapentine pharmacokinetics: part I. Int J Tuberc Lung Dis 1999; 3:426–36. [PubMed] [Google Scholar]
- 9.US Department of Health Human Services. Panel on antiretroviral therapy and medical management of HIV infected children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2012. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/PediatricGuidelines.pdf. Accessed 15 January 2013.
- 10.US Department of Health Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 12 February 2013. 2013. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 12 February 2013.
- 11.World Health Organization. Consolidated guidelines on general HIV care and the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed 1 August 2013. [PubMed]
- 12.Thompson MA, Aberg JA, Hoy JF et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA panel. JAMA 2012; 308:387–402. [DOI] [PubMed] [Google Scholar]
- 13.Williams I, Churchill D, Anderson J et al. British HIV Association guidelines for the treatment of HIV‐1‐positive adults with antiretroviral therapy 2012 (updated November 2013). HIV Med 2014; 15(suppl 1):1–6. [DOI] [PubMed] [Google Scholar]
- 14.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 2001; 15:71–5. [DOI] [PubMed] [Google Scholar]
- 15.Chen TT. Optimal three‐stage designs for phase II cancer clinical trials. Stat Med 1997; 16:2701–11. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher C, Brundage R, Fenton T et al. Pharmacokinetics and pharmacodynamics of efavirenz and nelfinavir in HIV-infected children participating in an area-under-the-curve controlled trial. Clin Pharmacol Ther 2007; 83:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource, 2009. [Google Scholar]
- 18.Fattinger K, Fellay J, Telenti A, Biollaz J, Buclin T. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther 2003; 73:20–30. [DOI] [PubMed] [Google Scholar]
- 19.Schechter M, Zajdenverg R, Falco G et al. Weekly rifapentine/isoniazid or daily rifampin/pyrazinamide for latent tuberculosis in household contacts. Am J Respir Crit Care Med 2006; 173:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Iulio J, Fayet A, Arab-Alameddine M et al. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet Genomics 2009; 19:300–9. [DOI] [PubMed] [Google Scholar]
- 21.Wen X, Wang J-S, Neuvonen PJ, Backman JT. Isoniazid is a mechanism-based inhibitor of cytochrome P 450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol 2002; 57:799–804. [DOI] [PubMed] [Google Scholar]
- 22.Almutairi FE, Greenblatt DJ, Hazarika S et al. Isoniazid mediates the CYP2B6* 6 genotype-dependent interaction between efavirenz and antituberculosis drug therapy through mechanism-based inactivation of CYP2A6. Antimicrob Agents Chemother 2014; 58:4145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas DW, Kwara A, Richardson DM et al. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. J Antimicrob Chemother 2014; 69:2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee L, Soon G, Chew N, Else L, Amara A, Knoo S. Differential induction of efavirenz metabolism by rifampin without and with isoniazid in healthy volunteers with CYP2B6 516GG and TT genotypes [abstract 516]. In: 20th Conference on Retroviruses and Opportunistic Infections, 2013:3–6. [Google Scholar]
- 25.Luetkemeyer AF, Rosenkranz SL, Lu D et al. Combined effect of CYP2B6 and NAT2 genotype on plasma efavirenz exposure during rifampin-based antituberculosis therapy in the STRIDE study. Clin Infect Dis 2015; 60:1860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIlleron HM, Schomaker M, Ren Y et al. Effects of rifampin-based antituberculosis therapy on plasma efavirenz concentrations in children vary by CYP2B6 genotype. AIDS 2013; 27:1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanofi-Aventis: Rifadin (rifampin) [package insert] June 2014.
- 28.Liu J, Chan-Tack K, Jadhav P et al. Why did the FDA approve efavirenz 800 mg when co-administered with rifampin? Int J Clin Pharmacol Ther 2014; 52:446–53. [DOI] [PubMed] [Google Scholar]
- 29.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother 2006; 58:1299–302. [DOI] [PubMed] [Google Scholar]
- 30.Manosuthi W, Kiertiburanakul S, Sungkanuparph S et al. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS 2006; 20:131–2. [DOI] [PubMed] [Google Scholar]
- 31.Luetkemeyer AF, Rosenkranz SL, Lu D et al. Relationship between weight, efavirenz exposure, and virologic suppression in HIV-infected patients on rifampin-based tuberculosis treatment in the AIDS Clinical Trials Group A5221 STRIDE Study. Clin Infect Dis 2013; 57:586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dooley KE, Denti P, Martinson N et al. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis co-infection. J Infect Dis 2015; 211:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 1:CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]