Abstract
Recently, we have showed that Tudor Staphylococcal Nuclease (TSN or Tudor-SN) proteins (TSN1 and TSN2) are localized in cytoplasmic messenger ribonucleoprotein (mRNP) complexes called stress granules (SG) and processing bodies (PB) under heat stress in Arabidopsis. One of the primary functions of these mRNP complexes is mRNA decay, which generates uncapped mRNAs by the action of endonucleases and decapping enzymes (Thomas et al., 2011) [1]. In order to figure out whether TSN proteins could be implicated in mRNA decay, we isolated uncapped and total mRNAs of Wild type (WT; Col and Ler) and TSN double knock-out (tsn1tsn2) seedlings grown under heat stress (39 °C for 40 min) and control (23 °C) conditions. Here, we provide the experimental procedure to reproduce the results (NCBI GEO accession number GSE63522) published by Gutierrez-Beltran et al. (2015) in The Plant Cell [2].
Keywords: Stress Granules, Stress, Arabidopsis, mRNA decay, Degradome
| Specifications | |
|---|---|
| Organism/cell line/tissue | Arabidopsis thaliana (Arabidopsis) Columbia (Col-O) and Landsberg erecta (Ler) Wild types and tsn1tsn2 double mutant/root tissue. |
| Sex | N/A |
| Sequencer or array type | Arabidopsis ArrayXS Thaliana chip |
| Data format | Data: GSM1551500-23, where raw data is provides as supplementary file (txt file) and processed data is included within Sample tables. |
| Experimental factors | Genomic DNA hybridizations of root tissues from 5-old-day Arabidopsis seedlings grown under control (23 °C) and heat stress (39 °C for 40 min) conditions |
| Experimental features | Isolation of uncapped and total mRNAs from WT (Col and Ler) and tsn1tsn2 plants |
| Consent | N/A |
| Sample source location | N/A |
1. Direct link to deposited data
1.1. Experimental design, materials and methods
1.2. Plant materials and experimental design
In the present study we have analyzed the degradome of WT (Col and Ler) and tsn1tsn2 Arabidopsis seedlings under specific (control or stress) conditions. To this end, plants were grown vertically on MS agar plates under a 16/8-h light/dark cycle and a light intensity of 150 μE m− 2 s− 1 for 5 days. For heat stress, plates with 5-day-old seedlings were incubated for 40 min on a thermoblock at 39 °C. For control condition, 5-day-old plants were directly harvested from the plate. Finally, root tissue was collected and used to purify uncapped and total mRNAs. Two independent sets of biological samples were used for the experiments.
1.3. Total and uncapped mRNA isolation
Total mRNA was isolated using RNeasy mini kit (Qiagen) following the manufacturer's instructions. Uncapped mRNA purification was performed as previously described, using a method based on the RNA ligase-mediated 5′ rapid amplification of cDNA ends (RLM 5´-RACE) [3], [4]. Briefly, T4 RNA ligase (Ambion) was used to add an RNA adaptor to poly(a)+ RNA having a free 5′ monophosphate (uncapped mRNAs). This adaptor was subsequently used for affinity purification and selective double-strand cDNA synthesis from uncapped mRNAs (dsDNA-uncapped). All RNA samples used in this study were subjected to a quality control analysis. To this end, an electrophoretic analysis via the 2100 Bioanalyzer (Agilent Technologies) and photometrical measurement with the Nanodrop 2000 spectrophotometer (Thermo Scientific) were performed for determination of the RNA integrity number (RIN) and detection of potential contaminants.
1.4. Microarray analysis
The dsDNA-uncapped along with total mRNA samples were sent to OakLabs (Germany), where total mRNA samples were reverse transcribed and the second strand cDNA (dsDNA-total) was synthesized using Agilent's LIQA kit. Both dsDNA-uncapped and dsDNA-total were used to synthetize fluorescent cRNA (complementary RNA) by in vitro transcription. Subsequently the cRNA samples were labeled using cyanine 3-CTP (Cy-3) dye and hybridized on Arabidopsis ArrayXS Thaliana chips (http://www.oak-labs.com/) following the manufacturer's protocol. Fluorescence signals on microarray were detected by using the SureScan Microarray Scanner (Agilent Technologies), which results in the raw data output (provided as txt files within GSM1551500-23). The samples were normalized using the ranked median quantiles as described previously [5]. Briefly, the mean signal of each target was ranked relative to all other targets. The ranked signal value was replaced with the median value of the sample rank. So the highest value in all samples became the mean of the highest, the second highest value became the mean of the second highest values, and so on. The normalized data are included within GSM1551500-23 table. The whole experiment is summarized in Fig. 1.
Fig. 1.
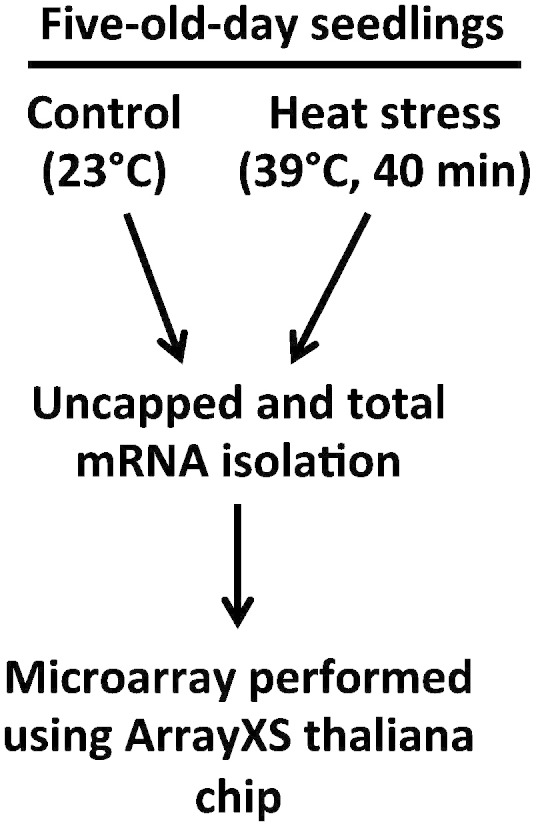
Schematic description of the steps involved in this study. Root tissue from 5-day-old WT (Col and Ler) and tsn1tsn2 Arabidopsis seedlings grown under control (23 °C) or heat stress conditions (39 °C for 40 min) were harvested and used to isolate uncapped and total mRNAs. Two independent sets of biological samples were used for the experiment. Therefore, a total of 24 samples were hybridized with ArrayXS thaliana chip (OakLabs) and which analysis is provided in the Gutierrez-Beltran article [2].
2. Discussion
This study provides a complete view of uncapping-mediated mRNA degradation under control and heat stress condition in WT (Col and Ler) and tsn1tsn2 plants. Notably, our results analyze the mRNA degradome after PB and SG appearance in order to figure out whether mRNAs are selectively decapped in these cytoplasmic mRNP complexes. These dataset has been used in a study that shows the importance of TSN presence in mRNA catabolism. In this work, the abundance of uncapped mRNAs was compared with the levels of the corresponding total (uncapped and capped) mRNAs. This analysis furnished lists of transcripts that were either enriched or depleted in uncapped from in the individual genetic backgrounds [2].
Conflict of interest
The author declares no conflict of interest.
Acknowledgments
This work was supported by grants from Knut and Alice Wallenberg Foundation and Pehrssons Fund.
References
- 1.Thomas M.G. RNA granules: the good, the bad and the ugly. Cell. Signal. 2011;23(2):324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutierrez-Beltran E. Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell. 2015 doi: 10.1105/tpc.114.134494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao Y., Riechmann J.L., Meyerowitz E.M. Transcriptome-wide analysis of uncapped mRNAs in Arabidopsis reveals regulation of mRNA degradation. Plant Cell. 2008;20(10):2571–2585. doi: 10.1105/tpc.108.062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao Y., Riechmann J.L. Genome-wide profiling of uncapped mRNA. Methods Mol. Biol. 2012;876:207–216. doi: 10.1007/978-1-61779-809-2_17. [DOI] [PubMed] [Google Scholar]
- 5.Bolstad B.M. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]


