Abstract
The mitochondrial calcium uniporter (MCU) gene codifies for the inner mitochondrial membrane (IMM) channel responsible for mitochondrial Ca2 + uptake. Cytosolic Ca2 + transients are involved in sarcomere contraction through cycles of release and storage in the sarcoplasmic reticulum. In addition cytosolic Ca2 + regulates various signaling cascades that eventually lead to gene expression reprogramming. Mitochondria are strategically placed in close contact with the ER/SR, thus cytosolic Ca2 + transients elicit large increases in the [Ca2 +] of the mitochondrial matrix ([Ca2 +]mt). Mitochondrial Ca2 + uptake regulates energy production and cell survival. In addition, we recently showed that MCU-dependent mitochondrial Ca2 + uptake controls skeletal muscle trophism. In the same report, we dissected the effects of MCU-dependent mitochondrial Ca2 + uptake on gene expression through microarray gene expression analysis upon modulation of MCU expression by in vivo AAV infection. Analyses were performed on single skeletal muscle fibers at two time points (7 and 14 days post-AAV injection). Raw and normalized data are available on the GEO database (http://www.ncbi.nlm.nih.gov/geo/) (GSE60931).
Keywords: Mitochondrial calcium uniporter (MCU), Single skeletal muscle fibers, In-vivo analysis, Microarray
| Specifications | |
|---|---|
| Organism/cell line/tissue | Single skeletal muscle fibers from mouse EDL |
| Sex | Male |
| Sequencer or array type | Agilent-028005 SurePrint G3 Mouse GE 8 × 60K Microarray |
| Data format | Raw and normalized |
| Experimental factors | Two time points (7 days and 14 days) for each experimental factor: MCU up-regulation, MCU silencing, wild type mice, expression of control shRNA (shluc). |
| Experimental features | Two time points were considered for each condition (MCU up-regulation, MCU silencing, wild type, expression of control shRNA (shluc)). Each condition was analyzed with at least three biological replicates. AAV-MCU, AAV-shMCU and AAV-shluc were used for mice in-vivo infection. After mice sacrifice EDL skeletal muscles were excided and single myofibers were recovered and characterized. Only type 2b myofiber types were used in gene expression experiments. |
| Consent | In vivo experiments were performed in accordance with the Italian law D. L.vo n_26/2014. |
| Sample source location | CD1 mice from University of Padova, Italy |
1. Direct link to deposited data
Deposited data can be found here: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE60931.
2. Experimental design, materials and methods
2.1. Experimental design
To dissect the transcriptional effects of mitochondrial calcium uniporter (MCU) gene modulation in in-vivo single skeletal muscle fiber we performed a time series gene expression analysis after MCU up- or down-regulation [1]. The MCU gene encodes the channel of the inner mitochondrial membrane (IMM) responsible for mitochondrial Ca2 + uptake which controls the aerobic metabolism, cell death, and survival pathways [2], [3], [4]. Muscle activity leads to major swings in mitochondrial [Ca2 +], which, in turn controls muscle trophism [1].
2.2. Materials and methods
Adult 2 month old mice were used for the experiments. Untreated and adeno-associated virus (AAV) injected EDL muscles were collected. Muscles were digested in type I collagenase (10 mg/ml in DMEM). Single myofibers were dissociated. Intact, not contracted single myofibers were picked under a stereomicroscope and washed in PBS. For AAV-shluc and AAV-shMCU infected muscles, ZsGreen-expressing myofibers were selected using an inverted microscope (DMI4000, Leica). Each single isolated myofiber was lysed in 250 μl of TRIzol Reagent (Life Technologies) and RNA was extracted in the aqueous phase following the manufacturer's instructions. To purify RNA, spin-columns of the RNeasy Micro Kit (Qiagen) were used (Fig. 1). Before performing microarray experiments about 1/5 of the purified RNA was used for qRT-PCR measurements of MCU expression levels (Fig. 2) and for fiber characterization (Fig. 1). RNA was retrotranscribed using the SuperScript III Reverse Transcriptase (Life Technologies) according to the manufacturer's specifications. Gene-specific primers for exogenous MCU (AAV-MCU), and endogenous MCU were selected with Primer3Plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) and the specificity of each primer set was monitored by dissociation curve analysis. Thioredoxin 1 was chosen as the reference gene according to the results in [5]. The selected primers are described in Table 1.
Fig. 1.
Experimental flow. Adult CD1 mice [1] were infected with adeno-associated viruses (AAVs) [2] carrying a short hairpin to silence MCU (AAV-shMCU) (group 1), a control short hairpin (AAV-shluc) (group 2), and MCU cDNA to up-regulate its expression (AAV-MCU) (group 3). Group 4 comprises not-infected control mice (wild type). EDL skeletal muscles were collected 7 days and 14 days after injection, respectively [3] and fibers were dissociated [4]. From each isolated myofiber [5] mRNA was extracted [6] and used to quantify MCU expression and classify myofibers [7] according the positive expression of myosin, heavy polypeptide 4 (Myh4) and negative expression of myosin, light polypeptide 3 (Myl3). RNA from slow type 2b myofibers was used for Whole Transcriptome Amplification (WTA) [8] and cDNA produced was labeled and used for microarray hybridization [9]. After microarray scanning data were normalized and analyzed [10].
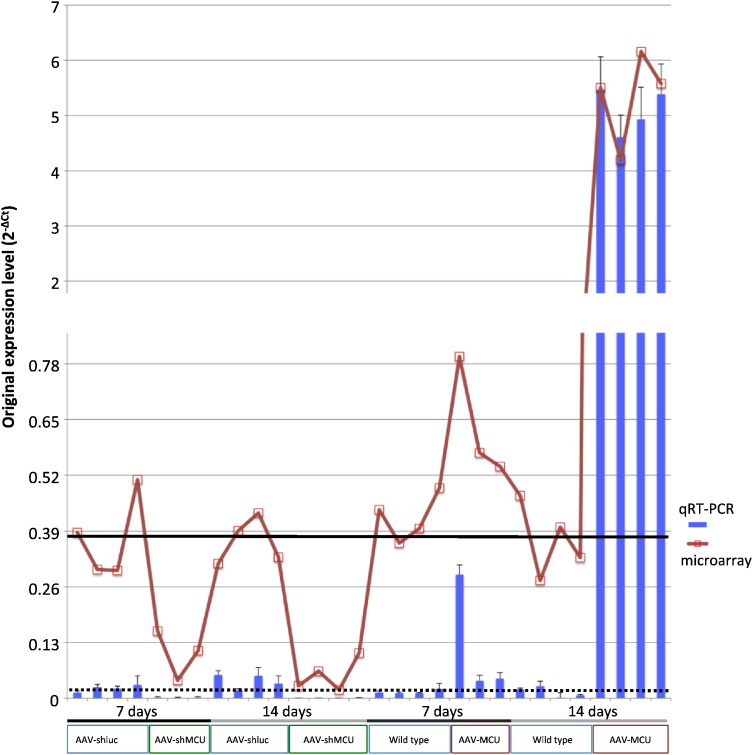
Fig. 2.
qRT-PCR and microarray results for MCU gene. The red line represents MCU expression derived from microarray experiments while histograms represent MCU expression derived from qRT-PCR experiments. Correlation between them is 98.8%. The black line represents MCU average expression in wild type mice from microarray experiments and dashed one from qRT-PCR. See caption of Fig. 1 for the description of X axis names.
Table 1.
Primers used for qRT-PCR.
| Primer name | Sequence | Description |
|---|---|---|
| MCU_endo_FOR | AAAGGAGCCAAAAAGTCACG | Endogenous MCU |
| MCU_endo_REV | AACGGCGTGAGTTACAAACA | |
| MCU_eso_FOR | AATTGCTCAGGCAGAAATGGA | Exogenous MCU from AAV-MCU |
| MCU_eso_REV | CTTATCGTCGTCATCCTTGTAATC | |
| Txn1_FOR | TCCAATGTGGTGTTCCTTGA | Reference gene |
| Txn1_REV | GGCTTCAAGCTTTTCCTTGTT |
The qRT-PCR experiments were performed in a 7500 Real-time PCR System (Life Technologies) using the SYBR Green technology of GoTaq qPCR Master Mix (Promega). qRT-PCR confirmed the induction and silencing of MCU in selected fibers for microarray analysis (Fig. 2). Moreover, MCU induction is time dependent evidencing a smaller MCU up-regulation 7 days after AAV injection (~ 8 fold) than that 14 days after AAV injection (~ 360 fold).
The remaining purified RNA of the selected fibers was exponentially amplified using the TransPlex Whole Transcriptome Amplification 2 Kit (Sigma-Aldrich) to obtain a sufficient amount of cDNA for the microarray experiments. Briefly, RNA was reverse transcribed in a cDNA library, and then the library was exponentially amplified for 18 cycles, a few cycles below the amplification “plateau” observed in a PCR test reaction. To remove the residual primers and nucleotides, the amplification product was purified with the GenElute PCR Clean-up columns (Sigma-Aldrich). The resulting cDNA was quantified with a Nanodrop ND-1000 spectrophotometer (Thermo Scientific). 2 μg of amplified-purified cDNA was directly labeled using the Genomic DNA Enzymatic Labeling Kit (Agilent Technologies). The kit uses random primers and the exo-Klenow fragment to directly label cDNA samples with Cy3-dUTP nucleotides. Labeled cDNA was purified using Amicon 30 kDa filters (Millipore) and quantified using the NanoDrop ND-1000 spectrophotometer (Thermo Scientific). On average, cDNA yield was about 4 μg and the specific activity of 30 pmol Cy3 per μg of cDNA (Fig. 1).
The microarray experiments were performed using SurePrint G3 Mouse Gene Expression 8 × 60K microarrays (Agilent Technologies) (GEO platform: GPL13912). 800 ng of labeled cDNA target was mixed with 5 μl of 10 × Blocking Agent (Agilent Technologies) and water to a final volume of 25 μl. Samples were denaturated at 95 °C for 2 min and added to 25 μl of 2 × GEx Hybridization Buffer HI-RPM (Agilent Technologies). A 40 μl mix was dispensed onto the array. Slides were loaded into the Agilent SureHyb chambers and hybridization was performed in a hybridization oven at 65 °C for 17 h with a 10 rpm rotation. After hybridization, slides were washed using a Wash Buffer Kit (Agilent Technologies) and dried at RT.
Microarray slides were scanned using a G2505C scanner (Agilent Technologies) at a 3 μm resolution. Probe features were extracted using the Feature Extraction Software v. 10.7.3.1 with GE_1_Sep09 protocol (Agilent Technologies). The Feature Extraction Software directly performed intra-array normalizations. Raw data are available in the GEO database (accession number GSE60931). The genomic results described in [1] were produced as following described. Inter-array normalization of expression levels was performed with a quantile method [6] and the values for within-array replicate spots were then averaged. Feature Extraction Software, which provided spot quality measures, was used to evaluate the quality and reliability of the hybridization. In particular, the flag “glsFound” (set to 1 if the spot had an intensity value significantly different from that of the local background and to 0 when otherwise) was used to filter out unreliable probes: the flag equals to 0 was to be noted as “not available” (NA). Probes with a high proportion of NA values were removed from the dataset in order to carry out more solid and unbiased statistical analyses. 45% of NA was used as the threshold in the filtering process, and a total of 30,073 of 39,570 probes were obtained. The comparison of MCU expression obtained with qRT-PCR and microarray evidenced a correlation of 98.8% (Fig. 2). To identify the differentially expressed probes in at least one condition, one way ANOVA analysis was performed using a threshold p-value ≤ 0.01. Significant differentially expressed probes were used to search specific expression clusters according to the Self Organizing Tree Algorithm (SOTA) [7] as implemented in MultiExperiment Viewer version 4.8.1 (tMev) of the TM4 Microarray Software Suite [8]. Gene ontology analysis was performed using DAVID web tool [9], while pathway analysis was performed applying Gene Set Enrichment Analysis (GSEA) as implemented in Graphite web tool [10]. The KEGG pathway database was used and only pathways that presented at least 10 mapped genes in common with our gene expression matrix were considered in the analysis. Interestingly, the analysis evidenced that, after MCU up-regulation, genes involved in Ca2 + homeostasis are activated, while, but when MCU was down-regulated, the same genes were lesser activacted or down-regulated (Fig. 3).
Fig. 3.
Heat map of differentially expressed genes between AAV-MCU and AAV-shMCU after 14 days. Selected genes are those enriched for the cytoskeleton, myofibrils, sarcomere organization and calcium ion homeostasis functions. Gene expression values are relative to the average expression in the control condition: the blue color means low expression, the red color high expression.
References
- 1.Mammucari C., Gherardi G., Zamparo I., Raffaello A., Boncompagni S., Chemello F., Cagnin S., Braga A., Zanin S., Pallafacchina G., Zentilin L., Sandri M., De Stefani D., Protasi F., Lanfranchi G., Rizzuto R. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep. 2015;10:1269–1279. doi: 10.1016/j.celrep.2015.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kiloDalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., Koteliansky V., Mootha V.K. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 5.Chemello F., Bean C., Cancellara P., Laveder P., Reggiani C., Lanfranchi G. Microgenomic analysis in skeletal muscle: expression signatures of individual fast and slow myofibers. PLoS One. 2011;6:e16807. doi: 10.1371/journal.pone.0016807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolstad B.M., Irizarry R.A., Astrand M., Speed T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 7.Herrero J., Valencia A., Dopazo J. A hierarchical unsupervised growing neural network for clustering gene expression patterns. Bioinformatics. 2001;17:126–136. doi: 10.1093/bioinformatics/17.2.126. [DOI] [PubMed] [Google Scholar]
- 8.Saeed A.I., Bhagabati N.K., Braisted J.C., Liang W., Sharov V., Howe E.A., Li J., Thiagarajan M., White J.A., Quackenbush J. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 9.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 10.Sales G., Calura E., Martini P., Romualdi C. Graphite Web: web tool for gene set analysis exploiting pathway topology. Nucleic Acids Res. 2013;41:W89–W97. doi: 10.1093/nar/gkt386. [DOI] [PMC free article] [PubMed] [Google Scholar]