Abstract
Background
Beta-blocker therapy after acute myocardial infarction (MI) improves survival. Beta-blocker doses used in clinical practice are often substantially lower than those used in the randomized trials establishing their efficacy.
Objective
This study evaluated the association of beta-blocker dose with survival after acute MI, hypothesizing that higher dose beta-blocker therapy will be associated with increased survival.
Methods
A multicenter registry enrolled 7,057 consecutive patients with acute MI. Discharge beta-blocker dose was indexed to the target beta-blocker doses used in randomized clinical trials, grouped as >0% to 12.5%, >12.5% to 25%, >25% to 50%, and >50% of target dose. Follow-up vital status was assessed, with the primary endpoint of time-to-death right-censored at 2 years. Multivariable and propensity score analyses were used to account for group differences.
Results
Of 6,682 with follow-up (median 2.1 years), 91.5% were discharged on beta-blocker (mean dose 38.1%). Lower mortality was observed with all beta-blocker doses (p < 0.0002) versus no beta-blocker therapy. After multivariable adjustment, hazard ratios (HRs) for 2-year mortality compared with the >50% dose were 0.862 (95% confidence interval [CI]: 0.677 to 1.098), 0.799 (95% CI: 0.635 to 1.005), and 0.963 (95% CI: 0.765 to 1.213) for the >0% to 12.5%, >12.5% to 25%, and >25% to 50% of target dose, respectively. Multivariable analysis with an extended set of covariates and propensity score analysis also demonstrated that higher doses were not associated with better outcome.
Conclusions
These data do not demonstrate increased survival in patients treated with beta-blocker doses approximating those used in prior randomized clinical trials compared with lower doses. These findings provide the rationale to re-engage in research to establish appropriate beta-blocker dosing following MI to derive optimal benefit from this therapy.
(The PACE-MI Registry Study - Outcomes of Beta-blocker Therapy After Myocardial Infarction [OBTAIN]: NCT00430612)
Keywords: Adrenergic Beta-Antagonists, Follow-up Studies, Registries, Survival Analysis
Beta-blocker therapy following myocardial infarction (MI) improves survival. On the basis of randomized clinical trials (1,2) and large observational studies (3–5), guidelines for management of patients after ST-segment elevation MI (6) and non-ST-segment elevation MI (7) recommend beta-blocker therapy in essentially all post-MI patients without contraindications. The randomized clinical trials did not assess the effects of different doses of beta-blockers and there have been no large-scale studies that have addressed this. While the guidelines do not refer to specific beta-blockers or doses, basic evidence-based medicine principles support the use of beta-blockers that have been studied in trials at the doses used/targeted; trials that report dosing indicate that the majority of patients achieved target doses. However, clinically used beta-blocker doses are substantially lower (8,9). The impact of this large scale underdosing of beta-blockers on the beneficial effects of beta-blocker therapy is unknown. Analyses of post-MI beta-blocker trials have related mortality reduction to heart rate reduction (10,11); as heart rate reduction is dose-dependent, this supports the notion that there could be a dose-dependent reduction in mortality. The OBTAIN (Outcomes of Beta-Blocker Therapy After Myocardial INfarction) study is an observational multicenter registry in which beta-blocker dosing information was collected in all patients with acute MI at participating centers to assess the effect of dose on survival. The OBTAIN hypothesis was that higher dose beta-blocker therapy is associated with increased survival.
Methods
Study Design and Oversight
OBTAIN, initiated in 2007, was a companion registry to the PACE-MI (PACEmaker and β-blocker therapy post-Myocardial Infarction) trial (12). Detailed information on beta-blocker dosing was collected in the registry. There were 26 participating centers in the United States and 1 in Canada. When the trial was terminated in 2009, it was noted that beta-blocker utilization was nearly universal, but that most patients were treated with doses ≤25% of the target doses used in clinical trials. At that time, the decision was made to continue the registry and evaluate vital status for at least 2 years to test the hypothesis that there is a dose-response relationship in the beneficial effect of beta-blocker therapy on survival. After protocol modification to include vital status assessment and resubmission for Institutional Review Board approval, 21 of the original sites continued to participate (including 92% of the registry patients). An additional 5 U.S. sites were recruited.
The study was funded by the National Heart, Lung and Blood Institute (NHLBI). An Observational Study Monitoring Board, appointed by the NHLBI, monitored study conduct. The study was approved by each site’s Institutional Review Board with a waiver of consent for registry enrollment. Participating centers and study committees and personnel are listed in Online Appendix 1.
Patients
Consecutive patients admitted with acute MI at participating sites were entered into the registry. Acute MI was diagnosed by: 1) either creatine kinase elevation >2 times or troponin elevation >3 times the upper limit of normal; and 2) either chest pain (or equivalent symptoms suggestive of MI) or electrocardiographic changes consistent with MI.
Basic demographic, historical, and hospitalization information, as well as information regarding the index MI, was collected. Discharge beta-blocker type and dose were recorded. All data were collected at the site and deidentified patient information was entered in a web-based electronic data capture system.
Beta-Blocker Dosing
Beta-blocker type and dose was chosen by the managing physician. For the purposes of this study, target doses for the most commonly used beta-blockers were: metoprolol 200 mg/day (13,14); carvedilol 50 mg/day (15) (CoregCR equivalent dose 80 mg/day); propranolol 180 mg/day (16); timolol 20 mg/day (17); bisoprolol 10 mg/day (18); and atenolol 100 mg/day (19). On the basis of the dose administered, a proportion of the target dose was calculated (administered/target dose) only for patients taking 1 of these beta-blockers. Beta-blocker doses were divided into 5 pre-specified groups: no beta-blocker, >0% to 12.5%, >12.5% to 25%, >25% to 50%, and >50% of the target dose.
Study Endpoint
The pre-specified endpoint for this study was time to all-cause mortality with survival right-censored at 2 years. Vital status was assessed by either chart review, the Social Security Administration Death Master File, or direct communication with the patient/family. Per protocol, vital status was assessed 1 and 2 years after MI. Follow-up using the Social Security Administration Death Master File incorporated a 6-month delay to account for the lag time in recording deaths. Particularly for sites that participated in the original registry, longer-term follow-up (3+ years) was available.
Statistical Analysis
Patient characteristics were summarized as mean ± SD or count (%). Differences among groups were compared using chi-square tests for categorical variables and analysis of variance for continuous variables. Distribution-free rank sum tests were used for variables that deviated from normality. The median (interquartile range) was used to summarize these variables. The Kaplan-Meier method was used to calculate 1-, 2-, and 3-year survival in each study group.
Pre-specified analysis of the effect of the 5 pre-specified groups on 2-year survival was tested by comparing Kaplan-Meier survival curves with a log-rank test. Cox proportional hazards regression was used to test for the independent effects of beta-blocker dosing on survival. The following pre-specified patient characteristics were used in multivariable adjustment: age; sex; white race; Hispanic ethnicity; cardiac enzymes; left ventricular ejection fraction; diabetes; hypertension; hypercholesterolemia; ST-segment elevation MI; lytic therapy; primary percutaneous coronary intervention; length of stay; and other discharge medications (aspirin, angiotensin-converting enzyme [ACE] inhibitors/angiotensin receptor blockers (ARB), and statins). A pre-specified secondary analysis was performed comparing the outcomes for low-dose (≤25%) and high-dose (≥50%) beta-blocker therapy.
Further sensitivity analyses of the effect of the 4 beta-blocker doses on outcome included evaluation of 3-year outcomes. Multivariable analysis included an expanded set of all covariates listed in Table 1, including use of carvedilol versus metoprolol. Random effects (shared frailty model) were also included for each of the recruiting hospitals to better model differences in mortality among them. Quadratic and cubic polynomial terms for continuous predictors were included to account for potential non-linearity.
Table 1.
Characteristics of the Study Population and Mortality by Beta-Blocker Dose
| Variable | Discharge Beta-Blocker | p Value |
Beta-Blocker Dose (% of the target dose) | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No vs. Yes |
>0%–12.5% | >12.5%– 25% |
>25%–50% | >50% | Among 5 Doses |
Among 4 Beta- Blocker Doses |
||
| n (%) | 567 (8.5) | 6,115 (91.5) | 1,448 (21.7) | 2,247 (33.6) | 1,541 (23.1) | 809 (12.1) | ||||
| PATIENT CHARACTERISTICS | ||||||||||
| Age (years) mean ± SD | 65.1 ± 14.7 | 63.7 ± 13.5 | 0.03 | 64.5 ± 13.6 | 62.6 ± 13.6 | 64.0 ± 13.3 | 64.2 ± 13.1 | <0.0001 | 0.0001 | |
| Male n (%) | 349 (61.6) | 4,195 (68.6) | 0.0006 | 971 (67.1) | 1,555 (69.2) | 1,056 (68.5) | 570 (70.5) | 0.004 | 0.35 | |
| Race n (%) | 0.16 | 0.002 | 0.002 | |||||||
| White | 438 (77.2) | 4,851 (79.3) | 0.24 | 1,164 (80.4) | 1,792 (79.8) | 1,220 (79.2) | 617 (76.3) | 0.13 | 0.12 | |
| Black | 69 (12.2) | 654 (10.7) | 0.28 | 116 (8.0) | 226 (10.1) | 185 (12.0) | 120 (14.8) | <0.0001 | <0.0001 | |
| Asian | 6 (1.1) | 150 (2.5) | 0.04 | 39 (2.7) | 51 (2.3) | 37 (2.4) | 22 (2.7) | 0.24 | 0.82 | |
| American Indian | 3 (0.5) | 26 (0.4) | 0.73 | 6 (0.4) | 11 (0.5) | 8 (0.5) | 1 (0.1) | 0.68 | 0.52 | |
| Pacific Islander | 2 (0.4) | 13 (0.2) | 0.37 | 4 (0.3) | 4 (0.2) | 3 (0.2) | 1 (0.1) | 0.87 | 0.87 | |
| Unknown | 50 (8.8) | 428 (7.0) | 0.11 | 120 (8.3) | 164 (7.3) | 91 (5.9) | 49 (6.1) | 0.04 | 0.05 | |
| Other | 1 (0.2) | 7 (0.1) | 0.51 | 1 (0.1) | 1 (0.0) | 3 (0.2) | 1 (0.1) | 0.66 | 0.52 | |
| Hispanic n (%) | 33 (6.4) | 441 (7.7) | 0.27 | 126 (9.5) | 176 (8.4) | 87 (6.0) | 49 (6.3) | 0.002 | 0.002 | |
| BMI (kg/m2) Mean ± SD | 28.0 ± 6.8 | 29.2 ± 6.5 | <0.000 1 |
28.1 ± 6.0 | 29.2 ± 6.3 | 29.6 ± 6.8 | 30.4 ± 6.9 | <0.0001 | <0.0001 | |
| MEDICAL HISTORY | ||||||||||
| Diabetes n (%) | 152 (26.9) | 1,985 (32.5) | 0.006 | 399 (27.6) | 688 (30.7) | 533 (34.6) | 336 (41.6) | <0.0001 | <0.0001 | |
| Hypertension n (%) | 357 (63.1) | 4,177 (68.4) | 0.01 | 857 (59.2) | 1,465 (65.3) | 1,131 (73.4) | 666 (82.4) | <0.0001 | <0.0001 | |
| Hyperlipidemia n (%) | 276 (48.8) | 3,336 (54.6) | 0.008 | 723 (49.9) | 1,154 (51.4) | 893 (58.0) | 523 (64.8) | <0.0001 | <0.0001 | |
| Previous MI n (%) | 115 (20.3) | 1,277 (20.9) | 0.74 | 250 (17.3) | 411 (18.3) | 378 (24.6) | 217 (26.9) | 0.0000 | <0.0001 | |
| CHF history n (%) | 70 (12.4) | 635 (10.4) | 0.14 | 135 (9.3) | 177 (7.9) | 174 (11.3) | 137 (17.0) | <0.0001 | <0.0001 | |
| CABG history n (%) | 69 (12.2) | 815 (13.3) | 0.44 | 143 (9.9) | 238 (10.6) | 240 (15.6) | 178 (22.0) | <0.0001 | <0.0001 | |
| ESRD n (%) | 21 (3.7) | 204 (3.3) | 0.64 | 37 (2.6) | 66 (2.9) | 51 (3.3) | 45 (5.6) | 0.002 | 0.0009 | |
| CVA/TIA n (%) | 58 (10.2) | 640 (10.5) | 0.86 | 133 (9.2) | 199 (8.9) | 183 (11.9) | 115 (14.2) | <0.0001 | <0.0001 | |
| COPD n (%) | 102 (18.0) | 618 (10.1) | <0.000 1 |
150 (10.4) | 222 (9.9) | 150 (9.7) | 83 (10.3) | <0.0001 | 0.94 | |
| Current smoker n (%) | 206 (36.9) | 1,997 (33.1) | 0.08 | 486 (33.9) | 804 (36.2) | 482 (32.0) | 211 (26.5) | <0.0001 | <0.0001 | |
| ICD* n (%) | 18 (3.2) | 208 (3.4) | 0.78 | 41 (2.8) | 66 (2.9) | 57 (3.7) | 44 (5.4) | 0.009 | 0.004 | |
| MI CHARACTERISTICS | ||||||||||
| STEMI n (%) | 201 (35.4) | 2,691 (44.0) | 0.0001 | 717 (49.6) | 1,002 (44.6) | 651 (42.3) | 297 (36.7) | <0.0001 | <0.0001 | |
| Anterior | 60 (29.9) | 904 (33.6) | 0.28 | 247 (34.4) | 311 (31.0) | 231 (35.5) | 108 (36.4) | 0.17 | 0.16 | |
| Inferior/Posterior | 114 (56.7) | 1,356 (50.4) | 0.08 | 362 (50.5) | 524 (52.3) | 329 (50.5) | 127 (42.8) | 0.02 | 0.04 | |
| Thrombolytic Therapy n (%) |
25 (12.4) | 365 (13.6) | 0.65 | 79 (11.0) | 146 (14.6) | 105 (16.1) | 32 (10.8) | 0.03 | 0.02 | |
| Primary PCI n (%) | 147 (73.1) | 2,241 (83.3) | 0.0002 | 630 (87.9) | 864 (86.3) | 526 (80.8) | 203 (68.4) | <0.0001 | <0.0001 | |
| In-hospital revascularization (nonprimary PCI and CABG) n (%) |
41 (20.4) | 444 (16.5) | 0.15 | 105 (14.6) | 149 (14.9) | 115 (17.7) | 71 (23.9) | 0.001 | 0.001 | |
| Diagnostic angiography n (%) |
18 (9.0) | 132 (4.9) | 0.02 | 25 (3.5) | 41 (4.1) | 37 (5.7) | 28 (9.4) | <0.0001 | 0.0004 | |
| NSTEMI N (%) | 366 (64.6) | 3,424 (56.0) | 0.0001 | 731 (50.5) | 1,245 (55.4) | 890 (57.8) | 512 (63.3) | <0.0001 | <0.0001 | |
| Thrombolytic Therapy n (%) |
14 (3.8) | 95 (7.5) | 0.25 | 22 (3.0) | 38 (3.1) | 20 (2.2) | 14 (2.7) | 0.62 | 0.70 | |
| Primary PCI n (%) | 126 (34.4) | 1,409 (41.2) | 0.01 | 334 (45.7) | 565 (45.4) | 333 (37.5) | 159 (31.1) | <0.0001 | <0.0001 | |
| In-hospital revascularization (nonprimary PCI and CABG) n (%) |
84 (23.0) | 1,106 (32.3) | 0.0002 | 233 (31.9) | 409 (32.9) | 292 (32.8) | 164 (32.0) | 0.006 | 0.96 | |
| Diagnostic angiography N (%) |
72 (19.7) | 508 (14.8) | 0.01 | 97 (13.3) | 168 (13.5) | 143 (16.1) | 91 (17.8) | 0.008 | 0.05 | |
|
Admission SBP (mm Hg) Mean ± SD |
133.3 ± 31.2 | 141.0 ± 29.7 | <0.000 1 |
135.8 ± 27.5 | 140.1 ± 28.6 | 142.9 ± 30.6 | 148.7 ± 32.5 | <0.0001 | <0.0001 | |
|
Admission HR (beats/min) Mean ± SD |
82.9 ± 23.4 | 82.9 ± 21.2 | 0.94 | 81.3 ± 20.1 | 81.3 ± 20.2 | 84.2 ± 22.0 | 87.5 ± 23.6 | <0.0001 | <0.0001 | |
|
Heart Failure on Admission n (%) |
71 (12.5) | 616 (10.1) | 0.07 | 167 (11.5) | 179 (8.0) | 150 (9.7) | 112 (13.8) | <0.0001 | <0.0001 | |
| LVEF Mean ± SD | 48.8 ± 14.5 | 46.7 ± 12.8 | 0.001 | 45.3 ± 13.6 | 47.6 ± 12.4 | 46.4 ± 12.6 | 47.2 ± 12.8 | <0.0001 | <0.0001 | |
|
Troponin (ng/ml) Median (IQR) |
4.8 (1.3– 21.6) |
7.2 (2–28.1) | <0.000 1 |
12 (2.9– 42.5) |
7.2 (1.9– 28.8) |
6.2 (1.9– 22.4) |
4.3 (1.3– 17.7) |
<0.0001 | <0.0001 | |
| LOS Median (IQR) | 5 (3–9) | 5 (4–8) | 0.11 | 5 (4–8) | 4 (3–7) | 5 (4–8) | 6 (4–10) | <0.0001 | <0.0001 | |
| DISCHARGE MEDICATIONS | ||||||||||
|
BB Dose (% target) Mean ± SD |
12.0 ± 1.7 | 25.0 ± 0.4 | 49.1±3.2 | 100.3±29.0 | <0.0001 | |||||
| Mode (%) | 12.5 (92.4) | 25 (99.6) | 50 (93.1) | 100 (59.8) | ||||||
| Metoprolol n (%) | 923 (63.7) | 1,645 (73.2) | 1,052 (68.3) | 522 (64.5) | <0.0001 | |||||
| Carvedilol n (%) | 505 (34.9) | 468 (20.8) | 340 (22.1) | 174 (21.5) | <0.0001 | |||||
| ASA n (%) | 477 (84.1) | 5,708 (93.3) | <0.000 1 |
1,352 (93.4) | 2,099 (93.4) | 1,450 (94.1) | 745 (92.1) | <0.0001 | 0.33 | |
| ACE-I/ARB n (%) | 282 (49.7) | 4,150 (67.9) | <0.000 1 |
899 (62.1) | 1,541 (68.6) | 1,084 (70.3) | 581 (71.8) | <0.0001 | <0.0001 | |
| Statin n (%) | 404 (71.8) | 5,363 (87.8) | <0.000 1 |
1,246 (86.2) | 2,003 (89.2) | 1,330 (86.4) | 726 (89.7) | <0.0001 | 0.004 | |
| Clopidogrel n (%) | 333 (58.7) | 4,432 (72.5) | <0.000 1 |
1,034 (71.4) | 1,654 (73.6) | 1,118 (72.6) | 573 (70.8) | <0.0001 | 0.34 | |
| Dual antiplatelet n (%) | 314 (55.4) | 4,246 (69.4) | <0.000 1 |
997 (68.9) | 1,580 (70.3) | 1,074 (69.7) | 546 (67.5) | <0.0001 | 0.47 | |
| MORTALITY | ||||||||||
| 1 yr n (Kaplan-Meier %) | 97 (17.1) | 473 (7.7) | <0.000 1 |
117 (8.1) | 142 (6.4) | 136 (8.9) | 69 (8.6) | <0.0001 | 0.02 | |
| 2 yrs n (Kaplan-Meier %) | 123 (21.7) | 708 (11.7) | <0.000 1 |
165 (11.5) | 212 (9.5) | 197 (12.9) | 118 (14.7) | <0.0001 | 0.0002 | |
| 3 yrs n (Kaplan-Meier %) | 133 (25.4) | 795 (15.7) | <0.000 1 |
191 (16.2) | 237 (12.2) | 219 (17.6) | 131 (20.6) | <0.0001 | <0.0001 | |
includes patients with pre-admission ICD and those discharged with an ICD
ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ASA = aspirin; BB = beta-blocker; BMI = body mass index; CABG = coronary artery bypass graft surgery; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; CVA = cerebrovascular accident; ESRD = end stage renal disease; HR = heart rate; ICD = implantable cardioverter defibrillator; IQR = interquartile range; LOS = length of stay; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; SD = standard deviation; STEMI = ST-segment elevation myocardial infarction; TIA = transient ischemic attack.
Propensity score analysis was also performed as an alternative adjustment for patient differences in the 4 beta-blocker dose groups. To calculate the propensity score, we used mixed effects linear regression with random effects of the recruiting centers, continuous discharge beta-blocker dose (% of target dose) as a dependent variable, and the expanded control variable set reported in Table 1 (including quadratic and cubic polynomial terms for continuous predictors). In that way, the propensity scores represent the predicted discharge beta-blocker dose, given the extended set of patient characteristics. The propensity score was used as control variable in the proportional hazards frailty regression model. Further details are provided in Online Appendix 2.
All tests were 2-tailed and conventional 5% significance level was used. A gatekeeper hypothesis strategy for type I error control was utilized for pre-specified study endpoints—alpha levels were to be adjusted for subsequent tests if the gatekeeper null hypothesis were rejected. Analyses were performed using SAS software version 9.4.
Results
The registry included 7,057 patients. In-hospital mortality was 4.7%; 43 patients were lost to follow-up. Table 1 displays baseline characteristics of the 6,682 patients discharged alive, stratified by beta-blocker use. The mean age across groups was 63 to 65 years, with male predominance. Small to moderate group differences were noted for most characteristics.
Discharge therapy included beta-blockers (91.5%), aspirin (92.6%), ACE inhibitors/ARBs (66.3%), and statins (86.3%). There were 567 patients (8.5%) discharged without beta-blocker therapy. Reasons provided for not administering beta-blockers included low blood pressure (26%), conduction system disease (16%), pulmonary disease (17%), heart failure (9%), drug use (5%), debilitation (5%), and other (22%).
Beta-blockers administered at discharge included metoprolol (67.7%), carvedilol (24.3%), atenolol (3.8%), bisoprolol (2.8%), propranolol (0.2%), and others (1.1%). Of the patients discharged on a beta-blocker, 24.0%, 37.2%, 25.5%, and 13.4% received >0% to 12.5%, >12.5% to 25%, >25% to 50%, and >50% of the target dose, respectively. The mean administered dose was 38.1% of the target dose. Median follow-up was 2.1 years (IQR: 2.0 to 2.5). At last follow-up (n = 3,581), 52.4%, 20.2%. and 20.2% were taking the same, a higher, or a lower dose, respectively, with a 3.8% discontinuation rate and a 3.4% initiation rate in patients not discharged on beta-blockers. From discharge to 1 year, of the patients treated with >12.5% to 25% of the target dose, only 4% were subsequently in the >50% of target dose group. Of the patients treated with >50% of the target dose, only 12% were subsequently treated with ≤25% of target dose. In this cohort, beta-blocker therapy was associated with an unadjusted 51% (adjusted 45%; 95% confidence interval [CI]: 33% to 55%) lower mortality compared to no beta-blocker therapy.
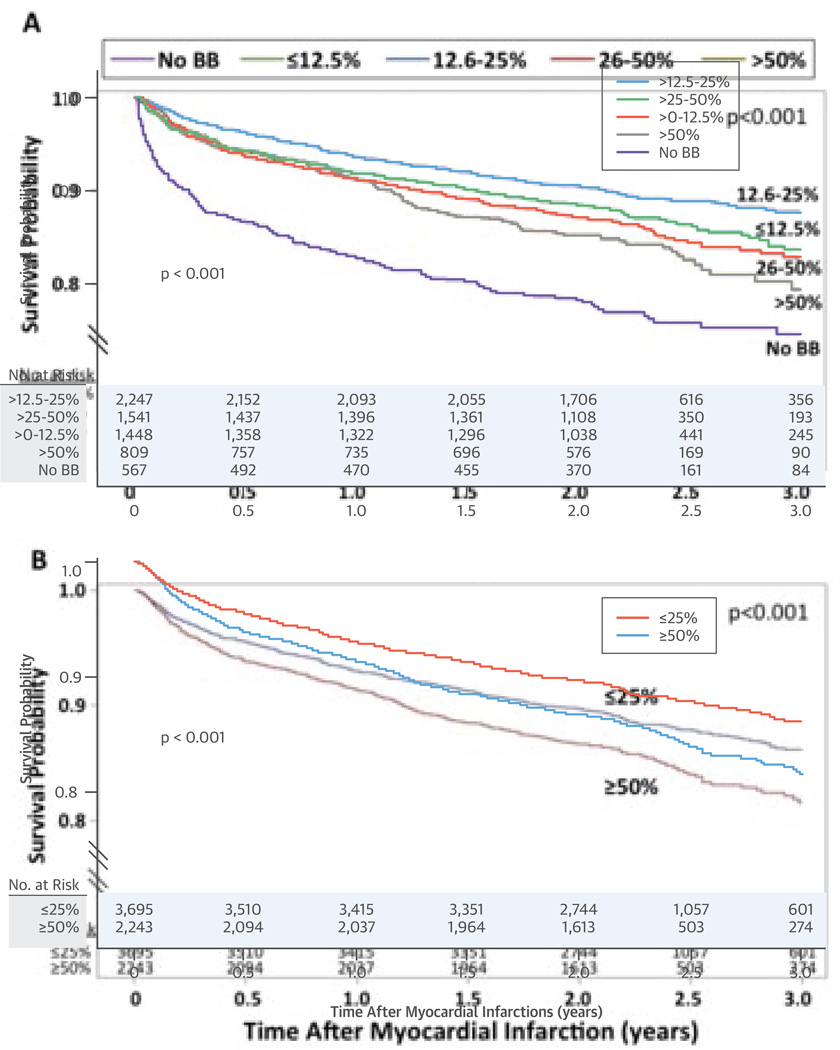
At 2 years, there were a total of 831 deaths (post-discharge mortality of 12.4%). The Central Illustration, Panel A shows the Kaplan-Meier curves for the primary analysis. Table 2 provides the hazard ratios relative to no beta-blocker and to the >50% target dose. Multivariable analysis identified that all tested parameters were independently related to survival (Table 3). After the pre-specified multivariable adjustment, relative to the >50% target dose, mortality did not differ for the >0% to 12.5% and >25% to 50% doses and was borderline statistically significant in those taking >12.5% to 25% of the target dose, but not after multivariable adjustment with the extended set of covariates (Table 2A).
Central Illustration. Beta-blockers After MI: Unadjusted Kaplan-Meier Survival Curves for the 5 Discharge Doses Analyzed and Low and High Dose Beta-Blocker Therapy.
Kaplan-Meier survival curves for (A) the primary (unadjusted) analysis comparing the 5 discharge doses (no beta-blocker [BB] and >0% to 12.5%, >12.5% to 25%, >25% to 50%, and >50% of the target dose) of beta-blockers and (B) the secondary (unadjusted) analysis comparing low-dose (≤25% of the target dose) to high-dose (≥50% of the target dose) beta-blocker therapy.
Table 2.
Hazard Ratios for the Pre-Specified Analyses and the Subsequent Analyses
| A. Univariable and multivariable adjusted hazard ratios for the primary 5 dose analysis and the secondary analysis comparing low (≤25% of target dose) to high (≥50% of target dose) on the basis of the pre-specified analyses and using the extended set of covariates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-specified Analyses | Extended Multivariable Analysis | |||||||||
| UNIVARIABLE | MULTIVARIABLE | |||||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | ||
| PRIMARY ANALYSIS | ||||||||||
| Vs. No Beta-blocker | ||||||||||
| >0%–12.5% (n = 1,448) | 0.486 | 0.385–0.614 | <0.0001 | 0.530 | 0.418–0.673 | <0.0001 | 0.576 | 0.379–0.877 | 0.01 | |
| >12.5%–25% (n = 2,247) | 0.395 | 0.316–0.493 | <0.0001 | 0.492 | 0.392–0.617 | <0.0001 | 0.562 | 0.374–0.843 | 0.005 | |
| >25%–50% (n = 1,541) | 0.547 | 0.437–0.685 | 0.0009 | 0.593 | 0.471–0.746 | <0.0001 | 0.649 | 0.435–0.970 | 0.04 | |
| >50% (n = 809) | 0.626 | 0.487–0.806 | 0.002 | 0.615 | 0.475–0.797 | 0.0002 | 0.666 | 0.440–1.007 | 0.05 | |
| Vs. >50% | ||||||||||
| >0%–12.5% | 0.776 | 0.612–0.983 | 0.05 | 0.862 | 0.677–1.098 | 0.23 | 0.865 | 0.667–1.123 | 0.28 | |
| >12.5%–25% | 0.630 | 0.503–0.789 | <0.0001 | 0.799 | 0.635–1.005 | 0.05 | 0.843 | 0.664–1.071 | 0.16 | |
| >25%–50% | 0.873 | 0.695–1.097 | 0.49 | 0.963 | 0.765–1.213 | 0.75 | 0.975 | 0.769–1.237 | 0.84 | |
| SECONDARY ANALYSIS | ||||||||||
| ≤25%vs. ≥50% | 0.758 | 0.651–0.883 | 0.0004 | 0.857 | 0.734–1.002 | 0.05 | 0.889 | 0.754–1.048 | 0.16 | |
| B. Sensitivity analyses showing adjusted hazard ratios for 3-year mortality compared to the >50% of target dose group using the pre-specified multivariable (MV) analysis, and the multivariable analysis with the extended set of covariates and propensity score analysis for the 4 beta-blocker dose groups. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta- blocker dose |
PRE-SPECIFIED MV ANALYSIS | EXTENDED MV ANALYSIS | PROPENSITY SCORE ANALYSIS | ||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| >0%–12.5% | 0.886 | 0.705–1.114 | 0.30 | 0.937 | 0.730– 1.202 |
0.61 | 1.163 | 0.910–1.486 | 0.23 |
| >12.5%–25% | 0.792 | 0.638–0.984 | 0.04 | 0.866 | 0.688– 1.091 |
0.22 | 0.834 | 0.664–1.047 | 0.12 |
| >25%–50% | 0.964 | 0.775–1.120 | 0.74 | 1.016 | 0.809– 1.275 |
0.89 | 1.041 | 0.832–1.302 | 0.73 |
Table 3.
Predictors From Multivariable Analyses
| A. Hazard ratios and 95% confidence intervals from multivariable analysis of 2-year mortality in the 2 pre-specified analyzed cohorts by predictor: 1) the 5 pre-specified dose groups (none, >0%–12.5%, >12.5%–25%, >25%-50%, >50% of the target dose) and 2) the low (≤25% of target dose) and high (≥50% of the target dose) dose groups. | ||||
|---|---|---|---|---|
| 5 Discharge Doses | ≤25% vs. ≥50% of Target Dose | |||
| Predictor | HR [95% CI] | p Value | HR [95% CI] | p Value |
| Beta-blocker dose | See Table 2 | <0.001 | 0.857 (0.734–1.002) | 0.05 |
| Age | 1.054 (1.048–1.060) | <0.0001 | 1.057 (1.050–1.064) | <0.0001 |
| ln (Troponin) | 1.070 (1.023–1.119) | 0.003 | 1.068 (1.018–1.121) | 0.008 |
| LVEF | 0.980 (0.975–0.986) | <0.0001 | 0.981 (0.975–0.987) | <0.0001 |
| ln(LOS) (days) | 1.320 (1.188–1.467) | <0.0001 | 1.312 (1.167–1.476) | <0.0001 |
| Male Sex | 1.240 (1.070–1.436) | 0.004 | 1.213 (1.031–1.427) | 0.02 |
| White Race | 0.822 (0.698–0.969) | 0.02 | 0.824 (0.688–0.988) | 0.04 |
| Hispanic Ethnicity | 0.678 (0.496–0.926) | 0.02 | 0.665 (0.479–0.924) | 0.02 |
| Diabetes | 1.453 (1.256–1.680) | <0.0001 | 1.550 (1.320–1.820) | <.0001 |
| Hypertension | 1.313 (1.090–1.581) | 0.004 | 1.275 (1.037–1.568) | 0.02 |
| Hyperlipidemia | 0.815 (0.706–0.940) | 0.005 | 0.811 (0.693–0.949) | 0.009 |
| STEMI | 0.694 (0.575–0.837) | 0.0001 | 0.690 (0.561–0.848) | 0.0004 |
| Thrombolytic therapy | 0.646 (0.445–0.937) | 0.02 | 0.587 (0.381–0.905) | 0.02 |
| Primary PCI | 0.614 (0.516–0.731) | <0.0001 | 0.596 (0.492–0.721) | <0.0001 |
| ASA | 0.599 (0.494–0.727) | <0.0001 | 0.677 (0.539–0.849) | 0.001 |
| ACE-I/ARB | 0.763 (0.662–0.878) | 0.0002 | 0.810 (0.693–0.947) | 0.008 |
| Statin | 0.752 (0.633–0.892) | 0.001 | 0.818 (0.671–0.998) | 0.05 |
| B. Hazard ratios and 95% confidence intervals from the multivariable analysis with the extended set of covariates of 3-year mortality in the 4 treated beta-blocker dose groups (>0%–12.5%, >12.5%–25%, >25%–50%, >50% of the target dose). | ||||
|---|---|---|---|---|
| Parameter Estimates | ||||
| HR (95% CI) | p Value | |||
| Beta-blocker dose | See Table 2 | |||
| Age | 1.529 (1.044–2.240) | 0.03 | ||
| Age (quadratic) | 0.994 (0.989–1.000) | 0.05 | ||
| Age (cubic) | 1.000 (1.000–1.000) | 0.04 | ||
| ln(Troponin) (cubic) | 1.006 (0.999–1.013) | 0.09 | ||
| ln(LOS) | 2.838 (0.578–13.937) | 0.20 | ||
| Male Sex | 1.374 (1.170–1.615) | 0.0001 | ||
| Diabetes | 1.370 (1.163–1.614) | 0.0002 | ||
| Hypertension | 1.219 (0.999–1.487) | 0.05 | ||
| STEMI | 0.842 (0.666–1.065) | 0.15 | ||
| Thrombolytic therapy | 0.739 (0.498–1.098) | 0.13 | ||
| Primary PCI | 0.580 (0.474–0.710) | <0.0001 | ||
| ASA | 0.784 (0.625–0.984) | 0.04 | ||
| ACE-I/ARB | 0.878 (0.754–1.023) | 0.10 | ||
| Statin | 0.778 (0.632–0.958) | 0.02 | ||
| History of MI | 1.200 (1.015–1.419) | 0.03 | ||
| History of CABG | 1.285 (1.073–1.538) | 0.006 | ||
| In-hospital revascularization | 0.526 (0.431–0.641) | <0.0001 | ||
| History of COPD | 1.708 (1.411–2.066) | <0.0001 | ||
| History of ESRD | 2.366 (1.836–3.049) | <0.0001 | ||
| History of CHF | 1.327 (1.104–1.595) | 0.003 | ||
| History of CVA/TIA | 1.256 (1.048–1.504) | 0.01 | ||
| ICD | 1.360 (1.006–1.840) | 0.05 | ||
| BMI | 0.745 (0.617–0.899) | 0.002 | ||
| BMI (quadratic) | 1.007 (1.002–1.013) | 0.01 | ||
| BMI (cubic) | 1.000 (1.000–1.000) | 0.03 | ||
Hazard ratios for continuous variables are associated with 1 unit increase in the measure.
Abbreviations as in Table 1.
The Kaplan-Meier curves for low-dose (≤25%) versus high-dose (≥50%) beta-blocker therapy (Central Illustration, Panel B) show a significantly higher mortality with high-dose therapy as compared with low-dose therapy (hazard ratio [HR]: 1.319; 95% CI: 1.133 to 1.536; p = 0.0004). After multivariable adjustment (Table 3), there was higher mortality (HR: 1.167; 95% CI: 0.998 to 1.363; p = 0.05) with high- versus low-dose therapy, but not after multivariable adjustment with the extended set of covariates (Table 2A).
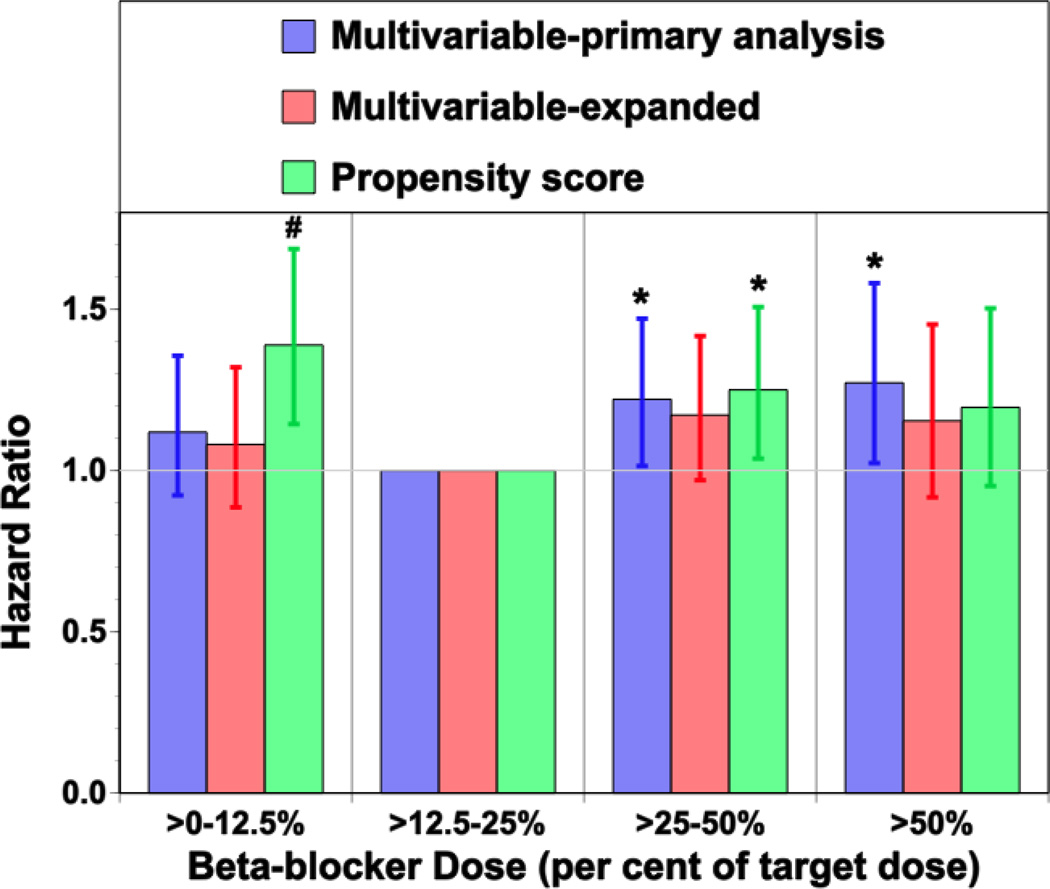
Table 2B demonstrates the multivariable hazard ratios with extended follow-up to 3 years, using the pre-specified multivariable analysis, and the analyses focusing on the 4 beta-blocker dose groups using multivariable analysis with the expanded set of covariates and the propensity score analysis. Relative to the >50% dose group, there were no significant differences between the >0% to 12.5% and >25% to 50% dose groups. Although there were lower hazard ratios in the >12.5% to 25% dose group, these were not consistently significant across all analyses. As the >12.5% to 25% group was the largest group and experienced the lowest mortality, Figure 1 shows the hazard ratios relative to the >12.5% to 25% dose group. Increased hazard ratios were noted in the >0% to 12.5% (expanded multivariable HR: 1.092, 95% CI: 0.896 to 1.331, p = 0.38; propensity score HR: 1.394, 95% CI: 1.148 to 1.692, p = 0.0008) and >25% to 50% (expanded multivariable HR: 1.176, 95% CI: 0.973 to 1.420, p = 0.09; propensity score HR: 1.248, 95% CI: 1.035 to 1.505, p = 0.02) dose groups.
Figure 1. Adjusted Hazard Ratios for 3-Year Survival With Multivariable Analyses and Propensity Score Analysis Relative to the >12.5–25% of Target Dose Group.
Adjusted hazard ratios for 3-year survival with multivariable analysis incorporating the pre-specified variable set, multivariable analysis incorporating the expanded variable set, and propensity score analysis comparing mortality with each beta-blocker dose to the mortality observed in the >12.5% to 25% of the target dose group.* p < 0.03, †p < 0.001.
Subgroup analyses were performed for patients taking metoprolol versus carvedilol, ST-segment elevation MI versus non-ST-segment elevation MI, patients with LVEF above or below 40%, and patients who were or were not revascularized during their admission (primary PCI, later PCI, or surgery). There was a significant interaction with the effect of beta-blocker dose only for revascularization (p = 0.037). In revascularized patients, the HRs compared with the >50% dose were 0.649 (95% CI 0.472 to 0.891), 0.546 (95% CI: 0.403 to 0.740), and 0.768 (95% CI: 0.563 to 1.048) for the >0% to 12.5%, >12.5% to 25%, and >25% to 50% doses, respectively. In nonrevascularized patients, these effects were less pronounced (HR: 1.294; 95% CI: 0.940 to 1.782 vs. HR: 0.963; 95% CI: 0.709 to 1.308; vs. HR: 1.223; 95% CI: 0.901 to 1.660) for the >0% to 12.5%, >12.5% to 25%, and >25% to 50% doses, respectively.
Discussion
This study was designed to evaluate whether higher-dose beta-blocker therapy is associated with increased survival compared to lower doses in patients discharged alive from the hospital after MI. Contrary to our hypothesis, improved outcome with higher dose beta-blocker therapy, specifically the target beta-blocker doses used in prior randomized clinical trials, was not observed. While baseline differences in the treatment groups preclude a definitive determination of the dose-response relationship between beta-blocker dose and mortality post-MI, the lowest observed mortality was at 25% of the target dose (i.e., metoprolol 50 mg/day). However, there was not a consistent statistically significant reduction in mortality with this dose with the various analyses used to adjust for baseline differences among the groups. In relation to these findings, the existing evidence base from randomized clinical trials incorporated primarily target doses and provided no information regarding the dose-response of post-MI beta-blocker therapy on subsequent survival. Thus, the present registry data remain consistent with prior clinical trials that show a benefit of full dose beta-blocker therapy. Yet, they raise the question of whether lower doses may result in equivalent outcomes compared to the target dose. These data support the need for further testing to determine optimal dosing of beta-blockers after MI.
The intriguing findings from this registry require careful explication, as there are several potential explanations for these results. First, it remains possible, though unlikely, that target dose beta-blocker therapy is still associated with better survival than lower doses; this would be possible in this registry if some unmeasured confounder(s) were unequally represented in the target and lower-dose groups making the former a substantially higher risk group than the low-dose group in which accounting for this parameter would substantially alter (reverse) the estimates of the adjusted survival. It is more feasible that further adjusting for other unmeasured confounders would show that there is not a strong dose-dependence of beta-blocker effect. In other words, once one achieves a threshold dose, further increments in the dose do not provide further benefit. In addition, the registry data are consistent with a greater benefit at lower doses than the target doses used in the clinical trials, but this would need to be tested prospectively. Finally, it is conceivable that there is not a single optimal dose for all post-MI patients with some patients benefiting from lower doses and some patients requiring higher doses. As the trial hypothesis was that higher doses would be associated with improved outcomes, an a priori noninferiority analysis was not proposed to show noninferiority of the >12.5% to 25% target dose. While it would not be appropriate to conduct noninferiority testing with a margin determined in a post-hoc manner, our post-hoc calculations showed that the noninferiority margin that would change the conclusion about noninferiority of the >12.5% to 25% target dose would have to be relatively small. Further studies will need to determine whether fixed target dosing for all post-MI patients or individualized dosing on the basis of patient or MI characteristics will optimize outcomes.
A variety of data support the biologic plausibility for the lack of a uniform improved survival with target dose versus low-dose beta blocker therapy post-MI. As most of the randomized clinical trial data for the beneficial effects of beta-blocker therapy were derived before thrombolysis, primary angioplasty, and routine use of aspirin, statins, and ACE-inhibitors, the benefit of beta-blockers in the modern era has often been questioned. Meta-analyses including >50,000 patients from the early randomized trials of post-MI beta-blocker therapy (1,2) demonstrated 19% to 23% reductions in mortality. The more contemporary CAPRICORN (15) randomized trial of carvedilol in post-MI patients with left ventricular ejection fraction ≤40% also demonstrated a 23% reduction in all-cause mortality. Notably, in CAPRICORN, 74% of patients achieved the target dose and an additional 11% achieved 50% of the target dose. Large-scale observational studies (3–5) from Medicare databases documented the benefits of beta-blocker therapy in an era of rampant underuse. The largest contemporary trial, COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) (20), randomized 45,852 patients with suspected acute MI to metoprolol (initially intravenous followed by 200 mg orally daily) versus placebo and noted no reduction in mortality at 28 days. A 2014 meta-analysis (21) comparing the effect of beta-blockers on mortality after MI in the pre- versus post-reperfusion eras noted no benefit in the post-reperfusion era. Finally, a contemporary observational report identifed a 15% reduction in mortality with beta-blocker therapy after MI (22). The changes in the therapeutic landscape of MI care and the variable reported outcomes provide further rationale to re-explore the effect of beta-blocker treatment and dosing on outcomes after MI.
Several studies (8,9) have noted beta-blocker underdosing relative to clinical trial doses. There are scant data and no randomized clinical trial data addressing whether this represents an acceptable or “poor” clinical practice. A 1998 retrospective cohort study (23) of 1,165 post-MI patients, of whom 365 were treated with beta-blockers, is the only prior study evaluating the effect of dose on outcome. Unadjusted mortality at a mean follow-up of approximately 2 years in those treated with ≥50% and <50% of the target dose was 6.9% and 3.4%, respectively. Multivariable analysis demonstrated a 67% reduction in cardiovascular mortality associated with low-dose beta-blockers. Interestingly, a study of 208 post-MI patients (24), of whom 154 were treated with a mean beta-blocker dose of 34% of the target dose, demonstrated a 60% reduction in all-cause mortality at a mean follow-up of 58.5 months. As no prior randomized clinical trials evaluated whether low-dose or target-dose beta-blocker therapy results in improved outcomes after MI, the OBTAIN registry establishes clinical equipoise for this issue and justifies further evaluation.
Dose-dependent effects of beta-blockers in the setting of heart failure have been examined with somewhat inconsistent results (25–27). Whereas some trials (26,27) have shown a direct relationship of dose to survival, a meta-analysis (28) demonstrated no significant difference in mortality reduction between the trials in which patients received ≥50% of the target dose versus low doses (RR: 0.74 and 0.78, respectively), though a relationship to heart rate reduction was noted. Although there may be some commonality of purpose in the use of beta-blockers post-MI and in heart failure, it is also possible that the dose-response relationships are different, reflecting important differences in underlying global and regional autonomic abnormalities, particularly in the degree of sympathoexcitation, between the 2 conditions. Furthermore, it is possible that the dose-response relationships for the beneficial effects of beta-blockers, even among subgroups of patients with MI, may be flatter than the dose-response relationships for adverse effects, including those that may affect the conduction system or cause metabolic side effects, such as hyperlipidemia or insulin resistance (29).
The predominant mechanisms of benefit for beta-blocker therapy after MI are reductions in ischemia, reinfarction, and sudden death. In the era of revascularization, aspirin, and statin use, it is plausible that the contribution of beta-blocker therapy to reductions in ischemia and reinfarction are not as prominent as when the initial beta-blocker clinical trials were performed. In fact, a 41% reduction in sudden death was reported in a pooled analyis of 5 studies evaluating trials of metoprolol post-MI, accounting for virtually all the difference in total mortality between the metoprolol and placebo-treated patients (30). While it is possible that this benefit plays an even more prominent role in the modern era of post-MI treatment, it is also interesting to note that the presenting rhythms for out-of-hospital cardiac arrest have undergone transformation over the last decades, with a decline in ventricular fibrillation and an increase in pulseless electrical activity/asystole (31). The natural history of this change is uncertain, but may reflect, at least in part, the use of beta-blockers. Of particular interest is a report that noted an adjusted odds ratio of 5 for beta-blocker use among out-of-hospital cardiac arrest survivors presenting with pulseless electrical activity versus ventricular fibrillation (32). The potential importance of bradyarrhythmias was further highlighted in the CARISMA (Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction) study (33), in which post-MI patients with LVEF <40% received an implantable cardiac monitor. At 2-year follow-up, 17% of patients had either high-degree atrioventricular block, significant sinus bradycardia, or sinus arrest.
Personal factors that might influence the optimal beta-blocker dose include individual risk on the basis of patient and MI characteristics, genetic polymorphisms, and observed beta-blocker effect. For example, the OACIS (Osaka Acute Coronary Insufficiency Study) registry (34) found improved survival with beta-blocker therapy after ST-segment elevation MI only in the higher-risk subgroup. There are data suggesting that beta-adrenergic receptor polymorphisms influence outcomes in acute coronary syndromes and heart failure (35–38), but the dose-response effect is unknown. Furthermore, genetic polymorphisms may affect beta-blocker metabolism and concentration (39,40). A number of analyses have suggested that mortality reduction post-MI is related more to the degree of heart rate reduction than to beta-blocker type (10,11). Whether these factors can allow for optimal titration of beta-blocker dose for an individual post-MI patient requires further study.
There are several reasons for the current high rate of low-dose beta-blocker therapy post-MI. This may represent either physician or patient inertia. Some patients may not be able to tolerate higher doses for hemodynamic reasons or due to noncardiac side effects or a more severe medical condition. Finally, advanced conduction system or myocardial disease may also preclude dose up-titration. There is no a priori reason for these factors to bias toward greater benefit with lower doses.
An important caveat for the current findings is that they do not represent randomized clinical trial results. As such, multiple beta-blockers were used and the doses were indexed to doses used in clinical trials. While this does not assure equivalent effects, it should be noted that 93% of the treated patients in this registry received either metoprolol or carvedilol, which was accounted for in the sensitivity analyses. In addition, the survival analysis was indexed to the discharge beta-blocker dose. Although dose changes do occur over time, only a minority of patients had their doses up-titrated. Being a registry, there was also nonuniform distribution of risk factors among groups. In addition, the specific rationale for the individual dosing regimens is unknown. Thus, the multivariable/propensity score analyses may have incompletely adjusted for these differences and there may be unmeasured covariates, such as the extent of coronary artery disease or follow-up heart rate and blood pressure, which may affect the findings. Yet multivariable adjustment and propensity score analyses consistently showed no greater benefit with full-dose beta-blocker therapy, contrary to the orginal hypothesis. Thus, despite these limitations, it is apparent that there is need to stimulate further randomized trials of post-MI beta-blocker therapy from their currently dormant state.
Current practice is characterized by the use of low-dose beta-blocker therapy post-MI. To date, no data support this practice, as all the randomized clinical trials used higher target doses. As these trials did not perform dose titration studies, the present findings are not in conflict with the randomized clinical trial data. Importantly, further research is needed to establish optimal (personalized) beta-blocker dosing following MI.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE: Therapy with beta-adrenergic antagonist drugs is recommended for patients after MI, but the most commonly prescribed doses are one-quarter of the dose evaluated in the randomized clinical trials that demonstrated efficacy and optimum doses have not been validated.
TRANSLATIONAL OUTLOOK: Additional research is needed to compare various doses of beta-blockers in survivors of MI and identify factors that influence optimum dose selection.
Acknowledgment
Funding Source: This research was supported by grant #5U01HL080416 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
The authors thank Daya Alexander for her special contributions to the conduct and management of this study.
ABBREVIATIONS
- ACE
angiotensin-converting enzyme
- ARB
angiotensin receptor blocker
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- NHLBI
National Heart, Lung and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Disclaimer: The views expressed in this manuscript are the authors’ and do not necessarily reflect those of the National Institutes of Health or the Department of Health and Human Services. Dr. Goldberger and Mr. Subačius had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Teo KK, Yusuf S, Furberg CD. Effects of prophylactic antiarrhythmic drug therapy in acute myocardial infarction. An overview of results from randomized controlled trials. JAMA. 1993;270:1589–1595. [PubMed] [Google Scholar]
- 2.Freemantle N, Cleland J, Young P, et al. β blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998;339:489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM, Radford MJ, Wang Y, et al. National use and effectiveness of β-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998;280:623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 5.Soumerai SB, McLaughlin TJ, Spiegelman D, et al. Adverse outcomes of underuse of β-blockers in elderly survivors of acute myocardial infarction. JAMA. 1997;277:115–121. [PubMed] [Google Scholar]
- 6.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction— Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:652–726. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Arnold SV, Spertus JA, Masoudi FA, et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62:1791–1801. doi: 10.1016/j.jacc.2013.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberger JJ, Bonow RO, Cuffe M, et al. PACE-MI Investigators. β-Blocker use following myocardial infarction: Low prevalence of evidence-based dosing. Am Heart J. 2010;160:435–442. doi: 10.1016/j.ahj.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucherat M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: a meta-regression of randomized clinical trials. Eur Heart J. 2007;28:3012–3019. doi: 10.1093/eurheartj/ehm489. [DOI] [PubMed] [Google Scholar]
- 11.Kjekshus JK. Importance of heart rate in determining beta-blocker efficacy in acute and long-term acute myocardial infarction intervention trials. Am J Cardiol. 1986;57:43F–49F. doi: 10.1016/0002-9149(86)90888-x. [DOI] [PubMed] [Google Scholar]
- 12.Goldberger JJ, Bonow RO, Cuffe M, et al. Post-myocardial infarction β-blocker therapy: the bradycardia conundrum. Rationale and design for the Pacemaker & β-blocker therapy post-MI (PACE-MI) trial. Am Heart J. 2008;155:455–464. doi: 10.1016/j.ahj.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 13.The MIAMI Trial Research Group. Metroprolol in acute myocardial infarction (MIAMI): A randomized placebo-controlled international trial. Eur Heart J. 1985;6:199–226. [PubMed] [Google Scholar]
- 14.Hjalmarson A, Elmfeldt D, Herlitz J, et al. Effect on mortality of metoprolol in acute myocardial infarction: A double-blind randomised trial. Lancet. 1981;2:823–827. doi: 10.1016/s0140-6736(81)91101-6. [DOI] [PubMed] [Google Scholar]
- 15.The CAPRICORN Investigators. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 16.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–1714. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 17.Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 18.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 19.Viskin S, Kitzis I, Lev E, et al. Treatment with beta-adrenergic blocking agents after myocardial infarction: From randomized trials to clinical practice. J Am Coll Cardiol. 1995;25:1327–1332. doi: 10.1016/0735-1097(94)00552-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZM, Pan HC, Chen YP, et al. COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622–1632. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 21.Bangalore S, Makani H, Radford M, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. 2014;127:939–953. doi: 10.1016/j.amjmed.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 22.Andersson C, Shilane D, Go AS, et al. β-blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64:247–252. doi: 10.1016/j.jacc.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Barron HV, Viskin S, Lundstrom RJ, et al. β-blocker dosages and mortality after myocardial infarction: data from a large health maintenance organization. Arch Intern Med. 1998;158:449–453. doi: 10.1001/archinte.158.5.449. [DOI] [PubMed] [Google Scholar]
- 24.Siu CW, Pong V, Jim MH, et al. β-blocker in post-myocardial infarct survivors with preserved left ventricular systolic function. Pacing Clin Electrophysiol. 2010;33:675–680. doi: 10.1111/j.1540-8159.2010.02694.x. [DOI] [PubMed] [Google Scholar]
- 25.Wikstrand J, Hjalmarson A, Waagstein F, et al. MERIT-HF Study Group. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT-HF) J Am Coll Cardiol. 2002;40:491–498. doi: 10.1016/s0735-1097(02)01970-8. [DOI] [PubMed] [Google Scholar]
- 26.Metra M, Torp-Pedersen C, Swedberg K, et al. Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J. 2005;26:2259–2268. doi: 10.1093/eurheartj/ehi386. [DOI] [PubMed] [Google Scholar]
- 27.Dobre D, van Veldhuisen DJ, Mordenti G, et al. SENIORS Investigators. Tolerability and dose-related effects of nebivolol in elderly patients with heart failure: data from the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS) trial. Am Heart J. 2007;154:109–115. doi: 10.1016/j.ahj.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 28.McAlister FA, Wiebe N, Ezekowitz JA, et al. Meta-analysis: β-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca VA. Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin. 2010;26:615–629. doi: 10.1185/03007990903533681. [DOI] [PubMed] [Google Scholar]
- 30.Olsson G, Wikstrand J, Warnold I, et al. Metoprolol-induced reduction in postinfarction mortality: Pooled results from five double-blind randomized trials. Eur Heart J. 1992;13:28–32. doi: 10.1093/oxfordjournals.eurheartj.a060043. [DOI] [PubMed] [Google Scholar]
- 31.Hulleman M, Berdowski J, de Groot JR, et al. Implantable cardioverter-defibrillators have reduced the incidence of resuscitation for out-of-hospital cardiac arrest caused by lethal arrhythmias. Circulation. 2012;126:815–821. doi: 10.1161/CIRCULATIONAHA.111.089425. [DOI] [PubMed] [Google Scholar]
- 32.Youngquist ST, Kaji AH, Niemann JT. Beta-blocker use and the changing epidemiology of out-of-hospital cardiac arrest rhythms. Resuscitation. 2008;76:376–380. doi: 10.1016/j.resuscitation.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloch Thomsen PE, Jons C, Raatikainen MJ, et al. Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) Study Group. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation. 2010;122:1258–1264. doi: 10.1161/CIRCULATIONAHA.109.902148. [DOI] [PubMed] [Google Scholar]
- 34.Nakatani D, Sakata Y, Suna S, et al. Osaka Acute Coronary Insufficiency Study (OACIS) Investigators. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cardiol. 2013;111:457–464. doi: 10.1016/j.amjcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Liu WN, Fu KL, Gao HY, et al. β1 adrenergic receptor polymorphisms and heart failure: a meta-analysis on susceptibility, response to β-blocker therapy and prognosis. PLoS One. 2012;7:e37659. doi: 10.1371/journal.pone.0037659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanfear DE, Jones PG, Marsh S, et al. β2-adrenergic receptor genotype and survival among patients receiving β-blocker therapy after an acute coronary syndrome. JAMA. 2005;294:1526–1533. doi: 10.1001/jama.294.12.1526. [DOI] [PubMed] [Google Scholar]
- 37.Cresci S, Dorn GWII, Jones PG, et al. Adrenergic-pathway gene variants influence beta-blocker-related outcomes after acute coronary syndrome in a race-specific manner. J Am Coll Cardiol. 2012;60:898–907. doi: 10.1016/j.jacc.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aleong RG, Sauer WH, Robertson AD, et al. Adrenergic receptor polymorphisms and prevention of ventricular arrhythmias with bucindolol in patients with chronic heart failure. Circ Arrhythm Electrophysiol. 2013;6:137–143. doi: 10.1161/CIRCEP.111.969618. [DOI] [PubMed] [Google Scholar]
- 39.Koytchev R, Alken RG, Vlahov V, et al. Influence of the cytochrome P4502D6*4 allele on the pharmacokinetics of controlled-release metoprolol. Eur J Clin Pharmacol. 1998;54:469–474. doi: 10.1007/s002280050495. [DOI] [PubMed] [Google Scholar]
- 40.Ismail R, Teh LK. The relevance of CYP2D6 genetic polymorphism on chronic metoprolol therapy in cardiovascular patients. J Clin Pharm Ther. 2006;31:99–109. doi: 10.1111/j.1365-2710.2006.00699.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.