Abstract
So far, the digitization of health care is best exemplified by electronic medical records, which have been far from favorably or uniformly accepted. However, properly implemented digitization can enable better patient outcomes, improve convenience, potentially lower health care costs, and possibly lead to much greater physician satisfaction. Precision (also known as personalized or individualized) medicine is frequently discussed today, but, in reality, it is what physicians have attempted to do as best they could for millennia. But now we have new tools that can begin to give us a much more high-definition view of our patients; from affordable and rapid genetic testing to wearable sensors that track a wide range of important physiologic parameters continuously. Although seemingly counterintuitive, the digitization of health care can also markedly improve the physician-patient relationship, allowing more time for human interaction when care is bolstered by digital technologies that better individualize diagnostics and treatments.
Keywords: artificial intelligence, electronic health records, health care, patient care, physician-patient relations, technology
Digitalization: the administration of digitalis in a dosage schedule designed to produce and then maintain optimal therapeutic concentrations of its cardiotonic glycosides.
Digitization: the process of converting information into a digital format that can be processed by computers and many devices with computing capacity.
William Withering’s use of digitalis from foxglove plants for the treatment of dropsy in 1775 is often credited as launching the modern therapeutic era in medicine (1). Since that time 240 years ago, cardiovascular care has arguably led the medical field in evidence generation through clinical trials, integration of proven prevention strategies, and the incorporation of state-of-the-art technologies, such as cardiopulmonary bypass, percutaneous coronary interventions, implantable pacemakers, and much more. We are now at the cusp of the digital age of medicine—a time when pertinent data, previously locked away in a handwritten medical record or paper logbook, can be collected, aggregated, and analyzed at virtually the speed of light, and when novel, continuously gathered sensor data can be tracked, collated, summarized, and personalized. Digitization allows all of this to be transformed from scattered, unrelated bits of data into associated and potentially valuable information, and from this newly generated information can come greater knowledge of diseases and treatments.
The objective of this article is not to provide a comprehensive summary of the field of digital medicine and the many real challenges to transforming ideas and innovations into clinical reality. For this, we recommend several recent comprehensive reviews (2-5). Rather, our goal is to provide a review of the evidence supporting where digitization can potentially have the greatest impact, and a vision for what this could mean for patients and providers.
A hypothetical case study
Imagine that your clinic is about to begin. You sit down with your tablet computer and review your first patient’s record. He is a 64-year-old man who you first met over 3 years ago, when he was hospitalized for a non-ST-segment elevation myocardial infarction. He also has a long history of well-controlled hypertension, thanks, in part, to genome-guided therapy and routine 24/7 blood pressure (BP) tracking. Although you have only seen him once since that hospitalization, a quick look at the summary section of his health record shows that he has been well-cared for in all aspects of risk factor management, including behavior modification. A simple graph clearly summarizes the average daily fluctuations in his BP, along with its relationship to his measured stress levels, sleep, activity, and diet. You note that he is routinely maintaining excellent activity levels, is taking all of his prescribed medications 97% of days, and that his weight is stable and cholesterol levels excellent. In fact, everything looks so good that you begin to wonder why he has asked to see his cardiologist, rather than just continue with his primary health care team (centered at his local pharmacy, with mostly virtual visits).
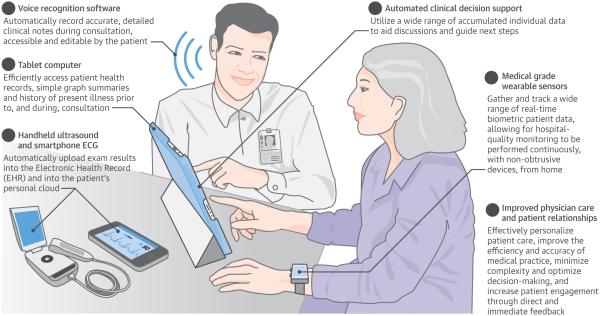
But, as you scan down to the computer-generated history of present illness (which your patient was guided through in a symptom-specific manner), you note that he has recently developed some infrequent chest discomfort, which concerns him, and he would like to discuss this with you. As you bring him back into the exam room to build upon what you just read of his history, you take out your handheld ultrasound and smartphone electrocardiogram (ECG) to assure that his echocardiogram and ECG will be automatically populated into the electronic health record (EHR) and, simultaneously, into the patient’s personal cloud. You discuss his symptoms, perform a physical exam, and share your assessment. All the while, voice recognition software has been expertly populating a detailed and accurate clinic note. About 30 min into the appointment, you begin to develop a plan in collaboration with your patient. Supported by automated clinical decision support (which concurs with your conclusion of a noncardiac etiology with 98% certainty, but also supplies you with the expected false positive rates of potential functional studies), you discuss the pros and cons of various next steps in depth, using a wide range of accumulated individual data, such as his imaging radiation exposure history and genome sequence, to together decide on a plan.
Is this a realistic possibility?
As unlikely as it is that this scenario might reflect anything even close to your own clinical experience, that is not due to a lack of the technology or ability to achieve it. The tools are available to enable routine care like this; the kind of care that can drive better patient outcomes and improved convenience, substantially lowering health care costs, and, in the process, provide much greater physician satisfaction.
As the case shows, digitization of care is much more far-reaching than just the institution of EHRs, which, more often than not, generate anger and frustration because they are designed primarily to improve billing, without a significant focus on improving either patient care or physician management (6). However, true patient-centric digitization of health care can take advantage of the remarkable strides in technology of the last several decades to completely reengineer how care is provided, with the ability to more effectively individualize patient care and markedly improve the efficiency of medical practice.
Individualizing patient care
Population-based data has been the foundation of medical practice for millennia. The population base of the earliest medical practitioners was restricted to their personal experience, and possibly that of their instructors. Over time, clinical trials were devised to guide therapies. This eventually led to the first randomized controlled trial (RCT) in 1946, ushering in a new age of evidence-based practice (7). Thanks to RCTs, we have been able to improve our treatments of a multitude of conditions, or, more precisely, populations of individuals with those conditions. But we know too well that every patient is different, and often what works for 1 patient with a specific phenotype (e.g., essential hypertension) doesn’t work in another. We currently do “trial-and-error” medicine to find the right drug to treat each patient’s hypertension.
Harnessing genetic data
The greatest barrier to better individualizing treatment has been the lack of an adequate understanding of phenotypic and genetic variability within various disease categories. New tools have rapidly emerged that give us a high-definition view of our patients (8). Whole-genome sequencing is now possible for <$2,000 compared with the original cost of $3 billion just over 10 years ago.
Although genetic testing has yet to achieve the revolutionary, across-the-board changes in the diagnosis and treatment of most human diseases that were initially anticipated (9), there have been some truly remarkable advances (10). For example, a recent analysis involving nearly 50,000 individuals with or at risk for coronary disease found a genetic risk score was not only predictive of the risk of incident or recurrent coronary disease, but was also able to identify those who derived the greatest benefit and those who received no benefit from statin therapy (11). If the data were more routinely available, pharmacogenomic information could potentially help better guide the individualization of therapeutic choices to improve both efficacy and safety (12).
However, taking advantage of genetic data will require more than just making the data available. Nearly three-fourths of physicians report they have “no to minimal knowledge” of genomics (13), and a recent study of medical school genetics course directors found that the majority felt that the genetics training the students received was inadequate preparation for clinical practice (14). So, for any genomics data to be actionable, it will likely need to be reinforced by substantial efforts in education and automated clinical decision support.
Integrating digital technologies
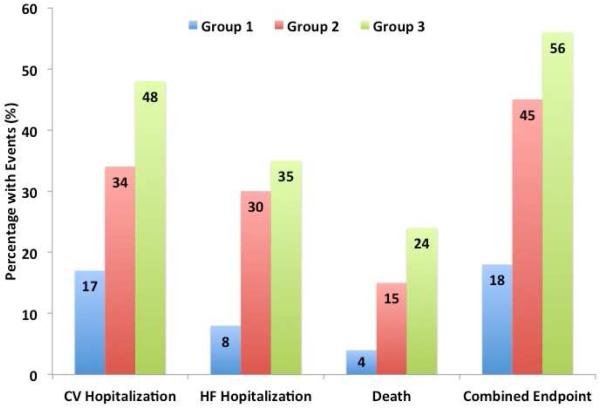
For many of the current phenotypic categories of diseases, a more complete understanding of interindividual variation in the character and extent of expression of signs and symptoms could substantially refine diagnoses, and likely treatments. Full integration of digital technologies can help accomplish this in several ways. Through longitudinal and searchable EHRs, an extensive and diverse library of unique characteristics of patients carrying the same diagnosis can be created to allow for clinically important subclassifications. For example, a recent study of nearly 400 individuals with a diagnosis of heart failure with preserved ejection fraction who underwent detailed clinical, laboratory, ECG, and echocardiographic phenotyping found that patients could be classified into 3 distinct groups with an over 4-fold difference in the risk of hospitalization or death (Central Illustration) (15).
Central Illustration. The Digitized Cardiovascular Physician Visit.
Summarized long-term sensor data presented via tablet computer, pocket ultrasound, handheld ECG acquisition, clinical decision support, and automated progress note development can all be components. ECG = electrocardiogram.
Possibly the most revolutionary, exciting, and challenging changes to come to medicine over the next few years will be the emergence of a wide selection of medical-grade wearable sensors that will allow real-time biometric data tracking of a wide range of physiological parameters. These technologies will allow hospital-quality monitoring to be performed continuously, with nonobtrusive devices, from the home. Cardiovascular-focused mobile health technologies will be particularly common, with devices that can measure BP without a cuff (even continuously), patches and shirts that provide continuous multilead ECG monitoring, noninvasive wearables tracking transthoracic impedance and cardiac output (also continuously), and smartphone-based ECGs for rhythm detection whenever needed (16).
The excessively expansive phenotype of essential hypertension is a great example of how continuous (beat-to-beat) monitoring can help identify clinically important subcategories of patients. A wide variability in nocturnal BP among patients has been well-described, with 52% of an untreated population being “dippers,” 8.8% “extreme dippers,” 35% “nondippers,” and 6% “risers” (17). In addition, there is compelling evidence that nocturnal BP is one of the stronger predictors of CV events, even when controlling for clinical BP (18). Of course, these data come from limited ambulatory BP monitoring, which is always challenging to acquire. But with a nonobtrusive, wearable device, like a watch, monitoring beat-to-beat BP continuously, day and night, could become simple and routine (19) (Figure 1). Not only would such technology allow a much greater understanding of nocturnal variation in BP in both known and unknown hypertensive patients, but the clinical importance of situational variability in BP could also be established (20).
Figure 1. An Example of Clinically-Important Subclassification of Phenotypes.
Three distinct subgroups developed through phenomapping of detailed clinical data in 397 patients with a diagnosis of heart failure with preserved ejection fraction. After adjustment for known risk factors, a patient’s subgroup was significantly associated with adverse outcomes. Data from (15)
These examples are early evidence that wearable sensors provide much more than just increased convenience for replicating what is currently done within clinics and hospitals. Rather, they offer the potential to not only refine, but also to completely redefine our current understanding of many pathophysiological states. With that understanding will come the ability to better individualize therapies if and when they are needed.
Improving physician care
According to the Centers for Disease Control and Prevention, just over 611,000 patients died of heart disease and almost 585,000 died of cancer in 2013 (the latest available statistics) (21). A study published that same year estimated that more than 400,000 patients die annually due to preventable harm in the hospital, making it the 3rd leading cause of death in the United States (22). The investigator also estimated that the incidence of serious harm due to preventable errors in the hospital was 10- to 20-fold more common than lethal errors. Outpatient errors do not appear to be any less frequent than inpatient errors (23), with 1 study estimating that 1 in 20 U.S. adults are affected by an outpatient diagnostic error every year (24). Beyond these errors of commission are those of omission. A RAND study of over 7,400 adults evaluating 439 indicators of care quality for 30 conditions found that participants received only 54.9% of recommended care (25). These figures highlight both the complexity of patient care and how variable decision-making can be among health care providers. In addition, they emphasize the critical importance (truly life or death) of minimizing this complexity and optimizing decision-making through reducing variability with evidence-based guidance.
The challenge of complexity in intensive care
Complexity is greatest in the intensive care unit (ICU), where in an average-size ICU of 6 individuals, there are an average of 178 activities per patient every day, meaning over 1,000 activities per day, all with potential for error (26). Novel technologies, such as wireless, wearable sensors that continuously track all vital signs, as well as technologies that allow inpatient data and real-time monitored information to be viewed on smartphones and tablets, can allow better surveillance and greater access to the data, and therefore more timely decisions (16). But increasing the deluge of data that caregivers need to deal with is more likely to increase, rather than decrease errors through the sheer volume of noisy information. However, by incorporating predictive analytic engines that are always running in the background, the power of these multiple data streams and how they interact with each other can be harnessed to provide automated clinical decision support, improve care, and potentially save lives (27-29). These tools will not replace, but rather will empower physicians to perform their jobs more effectively.
Decision support in outpatient care
Outpatient care comes with its own unique challenges, often driven by a lack of adequate time brought on by excessive patient volumes necessitated by a fee-for-service-dominated reimbursement environment. Studies have found that clinicians have clinically-relevant questions, such as “What is the drug of choice for this condition?” or “What is the cause of this symptom?” a little more than once for every 2 patients they see (30). On average, the answers to those questions were pursued only half the time, and, in those instances, answers were found 78% of the time. This suggests that when a clinician has a question as to how to best treat a patient at the point of care, almost two-thirds of the time that question is never answered. In an age when virtually limitless information is available, literally, at our fingertips, the information-intense field of health care should have been one of the first to take advantage of cognitive computing capabilities, rather than a laggard.
Extensive incorporation of automated evidence-based clinical decision support as a standard component of EHRs could eliminate most unanswered clinical questions, as well as much of the variability in practice. But it is not yet that straightforward. For example, in a study of automated stroke and bleeding risk determination for patients with atrial fibrillation, although the decision support tool in this study recommended warfarin 49% of the time, it was only utilized in 10% of the patients (31). This suggests either that clinicians have a way to go to feel comfortable trusting automated support, or that algorithms are not yet able to capture many nuances of clinical decision-making. Automated clinical decision support is far from perfect, but early studies found that it improves practitioner performance two-thirds of the time (32). As more data are capable of being gathered and more data resources incorporated, automated support will be able to become more personalized, more accurate, and more effective at improving physician care.
The impossibility of staying current
As touched on earlier, physicians’ time is extremely limited and dominated by the demands of patient care and associated administrative duties. Yet we expect to remain state-of-the art throughout our careers, and our patients also expect this of us. With roughly 1 million new, peer-reviewed articles published and indexed annually on PubMed, staying on top of the literature is, in fact, impossible (33). Even if one were to assume that we need to read and understand a mere 0.1% of the new medical literature published every year, that would still require reading approximately 20 articles per week. The impossibility of staying current in practice is reflected in the nearly generational (17 years) delay between a definitive clinical trial and changing the majority of clinical practice (34). Even something as life-saving, safe, inexpensive, and simple as aspirin in the setting of an acute myocardial infarction was still not given to 1 of every 4 people admitted to the hospital with a ST-segment elevation myocardial infarction a full 9 years after the publication of ISIS-2 in 1987 (35). Accepting that we cannot possibly stay current is the first step toward fully embracing the potential of digital technology to improve the quality of care we can provide.
The performance of IBM’s Watson on the television show Jeopardy gives a hint of what modern-day cognitive computing power can bring to health care. Watson and similar systems are capable of storing and instantaneously retrieving enormous amounts of information. Watson has “read” all of PubMed, can understand natural language, and can determine the strength of the hypotheses it generates (36). It will not be long before physicians will be able to routinely consult the artificial intelligence of a Watson for immediate access to the most up-to-date medical information and guidance. The ability to take advantage of resources like this will allow for more evidence-based, error-free, and intellectually satisfying interactions with our patients.
What will digitization mean?
A major concern of clinicians is that increased use of technology will damage the doctor-patient relationship, and with it professional satisfaction. A recent survey of over 20,000 US physicians tells an already discouraging story of the current state of professional satisfaction.(37) At least half of the respondents described their morale as negative, considered themselves pessimistic about the future of medicine, and would not recommend health care as a profession to their children. Reinforcing the importance of our interactions with patients, the vast majority of respondents (78.6%) felt that their relationships with their patients were the most satisfying aspect of their practice. Rather than harm this, we believe that the digitization of health care can eventually help build a markedly improved physician-patient relationships, allowing greater time for interaction when a patient requires the care of a physician. This is because when care is bolstered by digital technologies that better individualize diagnostics and treatments, simplify real-world monitoring, and provide evidence-based guidance at the point of need, then much of what physicians currently spend their time doing can be handled through automated systems or by others in the care team, maintaining the physician’s time to serve primarily as a diagnostician and educator. Furthermore, the increased reliance on patient-generated data, with direct feedback to the patient, will lead to marked increases in engagement.
Synergizing human and artificial intelligence
Although ever-increasing computational power, ubiquitous connectivity, and continually shrinking high-fidelity sensors are a powerful set of tools, there will always be a need for the expertise and, especially, the compassion of a clinician: someone with the skills of a diagnostician and educator, and with the ability (and time) to synthesize multiple complicated inputs, act upon, and explain them. The artificial intelligence that prodigious computational power brings does not replace human intellect. Rather, it complements and reinforces it.
An example comes from the world of chess, which was the first real test of human intellect versus machine. In 1997 IBM’s Deep Blue beat the reigning chess grand master Garry Kasparov, a tremendous feat of computational capability for the time. Today, however, in freestyle chess matches where anything is allowed—man alone, machine alone, or man plus machine— the combination of human plus artificial intelligence is the most frequent winner (38).
Another example of how the synergy of human intellect plus computer support is better than either alone is in weather forecasting. Predicting the weather requires analyzing numerous streams of disparate, but potentially related data from a plethora of sensors. Weather prediction is aided by the National Center for Atmospheric Research supercomputer, which is capable of 77 trillion calculations per second. Thanks to the ability of the supercomputer to model weather patterns, the accuracy of hurricane forecasting has improved 350% over the last 25 years (39). Yet, according to the National Weather Service, when humans interact with the computer models, they improve precipitation forecasts by 25% and temperature forecasts by 10% compared with the computer model alone (39).
As the era of big data per individual comes into play, with terabytes of biological, anatomic, physiological, and environmental data becoming fully integrated, humans will no longer be capable of processing the information. This requires synergistic interaction between man and machine, which ultimately will transform medicine into a digitized data science and, in the process, markedly improve health care.
Figure 2. Example of a Wrist-Wearable Multiparametric Sensor.
An early prototype of the Quanttus device measures multiple physiological parameters, including continuous beat-to-beat blood pressure. It is currently being evaluated in clinical studies. Photo courtesy of Dr. Maulik Majmudar, Chief Clinical Officer, Quanttus.
Acknowledgments
Disclosures: Dr. Steinhubl is supported by the National Institutes of Health (NIH) /National Center for Advancing Translational Sciences grant UL1TR001114 and a grant from the Qualcomm Foundation. He serves as Medical Advisor for Agile Edge Technologies, Airstrip Technologies, BridgeCrest Medical, Dynosense, Electrozyme, FocusMotion, and PhysIQ and serves on the board of directors of Vantage mHealthcare, Inc. Dr. Topol received research funding from the Qualcomm Foundation and the NIH/National Center for Advancing Translational Sciences grant UL1TR001114. He is currently serving on the board of directors of Dexcom and has previously served on the board of directors of Sotera Wireless and Volcano. Dr. Topol is the Editor-in-Chief of Medscape (WebMD).
Abbreviations
- BP
blood pressure
- ECG
electrocardiogram
- EHR
electronic health record
- ICU
intensive care unit
- RCT
randomized clinical trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Krikler DM. Withering and the foxglove: the making of a myth. Br Heart J. 1985;54:256–7. doi: 10.1136/hrt.54.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci Transl Med. 2015;7:283rv3. doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood) 2014;33:194–9. doi: 10.1377/hlthaff.2013.0992. [DOI] [PubMed] [Google Scholar]
- 4.Free C, Phillips G, Watson L, et al. The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med. 2013;10:e1001363. doi: 10.1371/journal.pmed.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neubeck L, Lowres N, Benjamin EJ, et al. The mobile revolution--using smartphone apps to prevent cardiovascular disease. Nat Rev Cardiol. 2015;12:350–60. doi: 10.1038/nrcardio.2015.34. [DOI] [PubMed] [Google Scholar]
- 6.Verdon DR. EHRs: the real story. Why a national outcry from physicians will shake the health information technology sector. Med Econ. 2014;91:18–20. 27. [PubMed] [Google Scholar]
- 7.Bhatt A. Evolution of clinical research: a history before and beyond James Lind. Perspect Clin Res. 2010;1:6–10. [PMC free article] [PubMed] [Google Scholar]
- 8.Topol EJ, Steinhubl SR, Torkamani A. Digital medical tools and sensors. JAMA. 2015;313:353–4. doi: 10.1001/jama.2014.17125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wade N. A decade later, genetic map yields few new cures. New York Times. 2010 Jun 12;:A1. [Google Scholar]
- 10.Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187–97. doi: 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- 11.Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264–71. doi: 10.1016/S0140-6736(14)61730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden DM, Johnson JA, Kimmel SE, et al. Cardiovascular pharmacogenomics. Circ Res. 2011;109:807–20. doi: 10.1161/CIRCRESAHA.110.230995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selkirk CG, Weissman SM, Anderson A, et al. Physicians' preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomarkers. 2013;17:219–25. doi: 10.1089/gtmb.2012.0165. [DOI] [PubMed] [Google Scholar]
- 14.Plunkett-Rondeau J, Hyland K, Dasgupta S. Training future physicians in the era of genomic medicine: trends in undergraduate medical genetics education. Genet Med. 2015 Feb 12; doi: 10.1038/gim.2014.208. [E-pub ahead of print], http://dx.doi.org/doi:10.1038/gim.2014.208. [DOI] [PubMed]
- 15.Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–79. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh JA, III, Topol EJ, Steinhubl SR. Novel wireless devices for cardiac monitoring. Circulation. 2014;130:573–81. doi: 10.1161/CIRCULATIONAHA.114.009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Sierra A, Redon J, Banegas JR, et al. Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–72. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- 18.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin Outcome Study. Hypertension. 2005;46:156–61. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 19.Comstock J. Quanttus: Why continuous blood pressure monitoring is a game-changer. mobihealthnews. 2015 Apr 7; [Google Scholar]
- 20.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–48. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 21.CDC/National Center for Health Statistics . Deaths and Mortality. Centers for Disease Control and Prevention; 2015. Available at: http://www.cdc.gov/nchs/fastats/deaths.htm. Accessed August 3, 2015. [Google Scholar]
- 22.James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Safety. 2013;9:122–8. doi: 10.1097/PTS.0b013e3182948a69. [DOI] [PubMed] [Google Scholar]
- 23.Bishop TF, Ryan AM, Casalino LP. Paid malpractice claims for adverse events in inpatient and outpatient settings. JAMA. 2011;305:2427–31. doi: 10.1001/jama.2011.813. [DOI] [PubMed] [Google Scholar]
- 24.Singh H, Meyer AN, Thomas EJ. The frequency of diagnostic errors in outpatient care: estimations from three large observational studies involving US adult populations. BMJ Qual Saf. 2014;23:727–31. doi: 10.1136/bmjqs-2013-002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. New Engl J Med. 2003;348:2635–45. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 26.Donchin Y, Gopher D, Olin M, et al. A look into the nature and causes of human errors in the intensive care unit. Crit Care Med. 1995;23:294–300. doi: 10.1097/00003246-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt PE, Meredith P, Prytherch DR, et al. Impact of introducing an electronic physiological surveillance system on hospital mortality. BMJ Qual Saf. 2015;24:10–20. doi: 10.1136/bmjqs-2014-003073. [DOI] [PubMed] [Google Scholar]
- 28.Winters B, Custer J, Galvagno SM, Jr., et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. 2012;21:894–902. doi: 10.1136/bmjqs-2012-000803. [DOI] [PubMed] [Google Scholar]
- 29.Summers RL, Pipke M, Wegerich S, et al. Functionality of empirical model-based predictive analytics for the early detection of hemodynamic instability. Biomed Sci Instrum. 2014;50:219–24. [PubMed] [Google Scholar]
- 30.Del Fiol G, Workman TE, Gorman PN. Clinical questions raised by clinicians at the point of care: a systematic review. JAMA Intern Med. 2014;174:710–8. doi: 10.1001/jamainternmed.2014.368. [DOI] [PubMed] [Google Scholar]
- 31.Wess ML, Schauer DP, Johnston JA, et al. Application of a decision support tool for anticoagulation in patients with non-valvular atrial fibrillation. J Gen Intern Med. 2008;23:411–7. doi: 10.1007/s11606-007-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–38. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 33.Neuroskeptic Science: Growing Too Fast? Neuroskeptic blog. Discover Magazine. 2012 Sep 30; Available at: http://blogs.discovermagazine.com/neuroskeptic/2012/09/30/science-growing-too-fast/-.VcAjnHiZ43w. Accessed August 3, 2015.
- 34.Balas EA, Boren SA. Managing clinical knowledge for health care improvement. In: Bemmel J, McCray AT, editors. Yearbook of Medical Informatics 2000: Patient-Centered Systems. Schattauer Verlagsgesellschaft mbH; Stuttgart, Germany: 2000. pp. 65–70. [PubMed] [Google Scholar]
- 35.Becker RC, Burns M, Gore JM, et al. Early and pre-discharge aspirin administration among patients with acute myocardial infarction: current clinical practice and trends in the United States. J Thromb Thrombolysis. 2000;9:207–15. doi: 10.1023/a:1018706425864. [DOI] [PubMed] [Google Scholar]
- 36.Friedman LF. IBM's Watson Supercomputer May Soon Be The Best Doctor In The World. Business Insider. 2014 Apr 22; Available at: http://www.businessinsider.com/ibms-watson-may-soon-be-the-best-doctor-in-the-world-2014-4. Accessed August 3, 2015.
- 37.Merritt Hawkins . 2014 Survey of America's Physicians: Practice Patterns and Perspectives. The Physicians Foundation; 2014. Available at: http://www.physiciansfoundation.org/uploads/default/2014_Physicians_Foundation_Biennial_Physician_Survey_Report.pdf. Accessed August 3, 2015. [Google Scholar]
- 38.Kelly K. The Three Breakthroughs that have Finally Unleashed AI on the World. Wired. 2014:22–11. [Google Scholar]
- 39.Silver N. The Signal and the Noise: Why So Many Predictions Fail--But Some Don't. Penguin Press; New York, NY: 2012. [Google Scholar]