SUMMARY
In the retina, rod and cone photoreceptors form distinct connections with different classes of downstream bipolar cells. However, the molecular mechanisms responsible for their selective connectivity are unknown. Here we identify a cell-adhesion protein, ELFN1, to be essential for the formation of synapses between rods and rod ON-bipolar cells in the primary rod pathway. ELFN1 is expressed selectively in rods where it is targeted to the axonal terminals by the synaptic release machinery. At the synapse, ELFN1 binds in trans to mGluR6, the postsynaptic receptor on rod ON-bipolar cells. Elimination of ELFN1 in mice prevents the formation of synaptic contacts involving rods, but not cones, allowing a dissection of the contributions of primary and secondary rod pathways to retinal circuit function and vision. We conclude that ELFN1 is necessary for the selective wiring of rods into the primary rod pathway and is required for high sensitivity of vision.
INTRODUCTION
In the nervous system, individual neurons assemble into elaborate but highly stereotypic networks, or circuits, that are thought to underpin information processing. It has been widely noted that neurons form these circuits by making highly specific connections that are evolutionary conserved (Sanes and Yamagata, 2009; Williams et al., 2010). Establishing specific synaptic connectivity is essential for normal neuronal function and many neuropsychiatric diseases are increasingly recognized to reflect the dysfunction of the circuit organization and operation (Akil et al., 2010; Arguello and Gogos, 2012; Luthi and Luscher, 2014). Studies over the last decade have uncovered several mechanisms that determine the specificity of synapse formation between individual neurons. These include the actions of guidance cues, which work over long distances, factors that specify laminar targeting by increasing the proximity of subsets of neurons, and various cell adhesion molecules that establish the physical contacts between neurons (Margeta and Shen, 2010; Robles and Baier, 2012; Sudhof, 2008; Zipursky and Sanes, 2010). The current hypothesis postulates that at the molecular level selective synaptogenesis is achieved through specific interactions between extracellular cell-adhesion proteins that bridge the synapse (Benson et al., 2001; de Wit et al., 2011; Williams et al., 2010). A number of such molecules with synaptogenic activity have been described, yet the examples of their contribution to the formation of specific circuits remain limited.
Meaningful information transfer at synapses ultimately requires the correct matching of postsynaptic receptors with the identity of the neurotransmitter (Lardi-Studler and Fritschy, 2007; Spitzer and Borodinsky, 2008). This process is perhaps the best understood at the neuromuscular junction where the expression of postsynaptic acetylcholine receptors is modulated by changes in electrical activity as the synapse develops (Borodinsky and Spitzer, 2007). The clustering of these receptors is then guided by the extracellular domain of the pre-synaptic molecule Agrin presented by the axonal terminals of the innervating neurons (Sanes and Lichtman, 2001). A similar role in positioning postsynaptic GABA and glutamate receptors has been described for the presynaptic cell adhesion molecule Neurexin at central synapses (Graf et al., 2004; Zhang et al., 2010). Thus, trans-synaptic interactions involving cell-adhesion molecules likely play an important role in coordinating synaptogenesis with receptor matching. However, with the large number of transmitters utilized by the nervous system and selective nature of the neuronal contacts during assembly of neuronal circuits, it is unclear how specificity in this process is achieved.
The retina is one of the best-understood mammalian circuits, were the neuronal connections in the primary image-forming pathway have been mapped and their functional relevance has been studied extensively (Hoon et al., 2014; Sanes and Zipursky, 2010). Vision in vertebrates is initiated by two types of photoreceptor neurons that respond to stimulation by light: rods and cones. Rods are highly sensitive and able to respond to single photons, thus setting absolute visual threshold. Cones are less sensitive but can respond to a broad range of light intensities of specific wavelengths and thus are essential for daytime and color vision (Kefalov, 2012; Korenbrot, 2012). The functional differences between rod and cone photoresponses are carried downstream through their selective connectivity with distinct classes of bipolar cells (BCs) forming established circuits with known properties that are conserved across vertebrate species (Ghosh et al., 2004; Lamb, 2013; Pahlberg and Sampath, 2011). In the mammalian retina rods establish synapses with a single class of BC, the rod ON-bipolar cell (ON-RBC), forming the highly-sensitive rod bipolar (primary) pathway (Dacheux and Raviola, 1986; DeVries and Baylor, 1993). In contrast, axonal terminals of cones make synapses with several classes of cone ON-bipolar cells (ON-CBCs) and OFF-bipolar cells (Ghosh et al., 2004) that express different types of postsynaptic glutamate receptors. The contacts for rods and cones are formed at stereotyped positions within close proximity of one another (Mumm et al., 2005; Sanes and Yamagata, 2009), and the connectivity of cones with many classes of cone bipolar cells (CBCs) is thought to play an essential role in contrast sensitivity and temporal tuning. Similarly, the exclusive connection of rods with ON-RBCs provides a dedicated channel for the high gain transmission of single-photon responses at low light intensities and is indispensable for scotopic vision (Okawa and Sampath, 2007). However, the molecular mechanisms that mediate wiring of photoreceptors with downstream ON-BC neurons and the molecular basis for this remarkable synaptic selectivity are entirely unknown.
In this study we identified the molecular mechanism responsible for the selective synaptic wiring of rod photoreceptors into the retinal circuitry. We found that rods express selectively the cell-adhesion molecule, ELFN1, which interacts trans-synaptically with the postsynaptic receptor mGluR6, expressed at ON-RBC dendrites. The loss of ELFN1 in mice ablates selectively the synapses of rods with ON-RBCs without affecting the connectivity of cone photoreceptors with CBCs. This results in night blindness via the specific elimination of the primary rod pathway for visual processing.
RESULTS
The ON-BC postsynaptic receptor, mGluR6, interacts with the cell adhesion molecule ELFN1
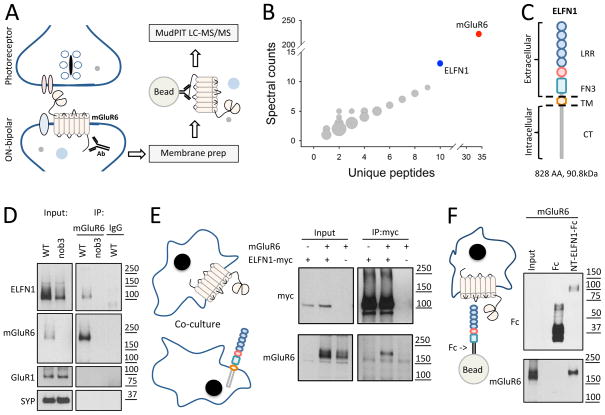
mGluR6 is the principal receptor on ON-BC dendrites that senses glutamate released from photoreceptors (Nakajima et al., 1993; Slaughter and Miller, 1981). It also plays an essential role in the assembly of the postsynaptic signaling complex in ON-BCs and formation of synapses between ON-BCs and photoreceptors (Cao et al., 2009; Dhingra and Vardi, 2012). Reasoning that mGluR6 may rely on yet unknown protein-protein interactions to coordinate its function in synapse formation, we performed a proteomic screen for its binding partners in mouse retinas (Figure 1A). Among proteins co-purified with mGluR6, and not found in the control experiment with retinas from nob3 mutant mice lacking mGluR6, we found ELFN1 as the highest scoring candidate by both the number of unique peptides identified and their spectral counts (Figure 1B). ELFN1 is a cell-adhesion protein of the leucine-rich repeat family that also contains a fibronectin type III domain (Figure 1C). We generated antibodies against ELFN1 and used them to validate co-immunoprecipitation of ELFN1 with mGluR6 in mouse retinas by Western blotting (Figure 1D). Binding between the two proteins was specific as no ELFN1 was detected in the immunoprecipitates from retinas lacking mGluR6 (nob3), or when non-immune IgG were used for the experiment (Figure 1D). Furthermore, we transfected ELFN1 and mGluR6 into cultured HEK293T cells and determined that they can also bind upon heterologous expression (Figure S1A). To determine the portion of ELFN1 that mediates this interaction, we evaluated two truncation mutants: one containing only the extracellular N-terminal portion (ELFN1-NT) and another containing the transmembrane segment and intracellular C-terminus (ELFN1-TM/CT). While the extracellular portion of ELFN1 was able to co-immunoprecipitate with mGluR6 as well as full-length protein, no co-immunoprecipitation was seen when the intracellular domain was used (Figure S1B). Next, we co-cultured HEK293T cells separately transfected with either mGluR6 or ELFN1 and followed their interaction by immunoprecipitation (Figure 1E). The results revealed robust pull-down of mGluR6 when ELFN1 was precipitated and vice versa, indicating that mGluR6 and ELFN1 can form a complex in trans.
Figure 1. ELFN1 is a novel binding partner of mGluR6.
A, Schematics of the immunoaffinity purification and identification strategy for mGluR6 interacting proteins. B, Rank order of proteins co-purifying with mGluR6 as identified by mass-spectrometry. ELFN1 is the most abundant, highest confidence candidate. C, Domain composition of ELFN1. The protein contains extracellular leucine-rich repeats (LRR) and fibronectin type 3 (FN3) domains, followed by the transmembrane segment (TM) and intracellular C-terminus. D, Co-immunoprecipitation of mGluR6 and ELFN1 from WT retinas as detected by Western blotting. Retinas lacking mGluR6 (nob3) and non-immune IgGs are used as specificity controls. About 1% of total ELFN1 was recovered in the precipitates under these experimental conditions and other unrelated synaptic proteins were not present in the eluates. E, ELFN1 and mGluR6 interact in-trans. Proteins co-immunoprecipitated following transient expression in separate HEK293 cells are detected by Western blotting. F, ELFN1 is sufficient for binding to mGluR6. Ectodomain of ELFN1 was purified as a fusion with Fc fragment and used to pull-down heterologously expressed mGluR6.
To obtain further evidence that the interaction with mGluR6 involves the extracellular domain of ELFN1, we fused the extracellular portion of ELFN1 (ELFN1-NT) with the Fc fragment for cellular secretion. This ELFN1-NT-Fc fusion protein, but not Fc alone, was able to pull-down specifically and effectively mGluR6 upon mixing the lysates of the cells expressing corresponding proteins (Figure S1C). Since the ELFN1-NT-Fc protein is secreted by cells, we could further demonstrate the binding when the mGluR6 cell lysate was incubated with the media expressing Fc proteins (Figure S1D). Finally, we purified extracellular ELFN1-NT-Fc as a recombinant protein and used it as bait in the pull-down assay. Unlike the recombinant Fc carrier, the ELFN1-NT-Fc protein retained effectively the heterologously-expressed mGluR6 on beads indicating that the interaction between these proteins is direct (Figure 1F). Together these results identify ELFN1 as a novel binding partner of mGluR6 in the retina and demonstrate direct protein-protein interactions involving their extracellular domains occur in trans.
ELFN1 is selectively expressed by rod photoreceptors
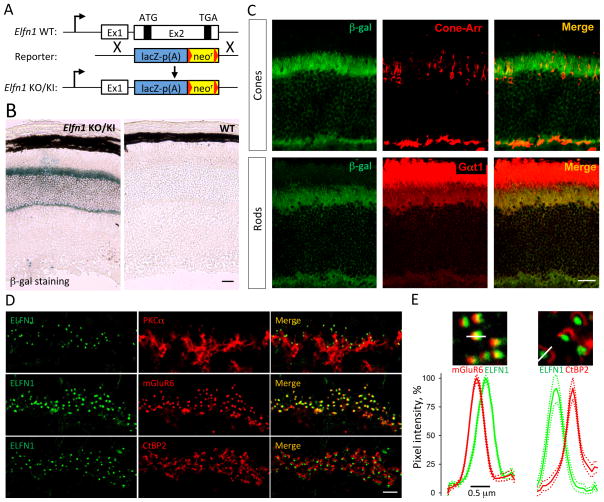
We studied the expression pattern of ELFN1 using a mouse line containing a β-gal reporter insertion into the native Elfn1 locus (Elfn1 KO/KI; Figure 2A). Immunohistochemistry on retinal cross-sections revealed that β-gal is present exclusively in the photoreceptor layer (Figure 2B). To confirm this observation, we performed fluorescent in situ hybridization detecting native Elfn1 mRNA in wild type (WT) retinas (Figure S2A). We also found that the signal was confined to the photoreceptor layer. Double staining with cell-specific markers further indicated that Elfn1 promoter driven β-gal expression was associated with rods but not cones (Figure 2C). High power magnification of the images confirmed these observations, revealing no readily detectable β-gal signal in cone-arrestin positive cell bodies of cone photoreceptors (Figure S2B).
Figure 2. ELFN1 is a synaptic protein exclusively expressed by rod photoreceptors.
A, Schematics of the Elfn1 allele used in the experiments. B, β-gal reporter knocked into Elfn1 gene is detected only in the photoreceptor layer of the retina. (Scale bar: 25 μm) C, Double labeling for β-gal and photoreceptor specific markers reveals that ELFN1 is co-expressed in Gαt1-containing rod but not cone arrestin (Cone-Arr) containing cone photoreceptors. (Scale bar: 25 μm.) D, ELFN1 is specifically localized at the synapses between rod photoreceptors and ON-RBC as revealed by co-labeling with ON-RBC marker (PKCα), presynaptic photoreceptor marker (CtBP2) and postsynaptic ON-BC marker (mGluR6). Scale bar: 5 μm. E, Punctate immunoreactivity of ELFN1 is apposed to CtBP2 and only partially overlaps with mGluR6 indicative of its extracellular position. Plots show quantification of relative fluorescence intensity distributions from 10 synaptic puncta. Dashed lines are plots of respective SEM values.
We analyzed the distribution of ELFN1 using specific antibodies against the protein. Using Elfn1 KO/KI retinas as a specificity control, we found that specific ELFN1 immunoreactivity was confined to the outer plexiform layer (OPL), where photoreceptors make synapses with bipolar cells (Figure S2C). Indeed, ELFN1 antibodies produced a characteristic punctate staining pattern, normally seen with other synaptic proteins (Figure 2D). Double labeling revealed that ELFN1-positive puncta were found close to the tips of PKCα-positive dendrites of ON-RBCs, co-clustered with the presynaptic ribbon, and partially overlapped with mGluR6 puncta. High-power confocal images of individual synaptic clusters revealed a layered organization of the synaptic proteins where ELFN1 is sandwiched between the presynaptic and postsynaptic markers, as would be expected for an extracellular molecule spanning the synaptic cleft (Figure 2E). We noticed that the ELFN1-positive puncta were distributed evenly in the upper sublamina of the OPL and did not appear to cluster at cone terminals (Figure S2D). Indeed, analysis of ELFN1 immunofluorescence distribution by triple staining with peanut agglutinin (PNA), a marker for cone synapses (Blanks and Johnson, 1983), and mGluR6 revealed that the ELFN1-specific signals appeared to be confined to rod synapses (Figure S2E). Together, these observations suggest that ELFN1 is a rod-specific protein targeted selectively to the synaptic terminals of these neurons.
Ablation of ELFN1 in mice results in selective loss of rod photoreceptor contacts with ON-BC dendrites
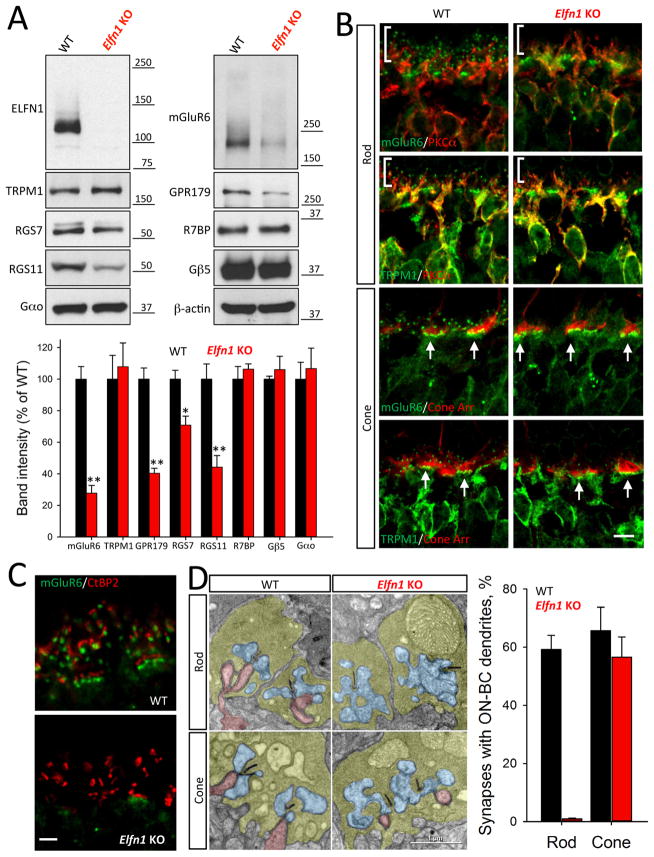
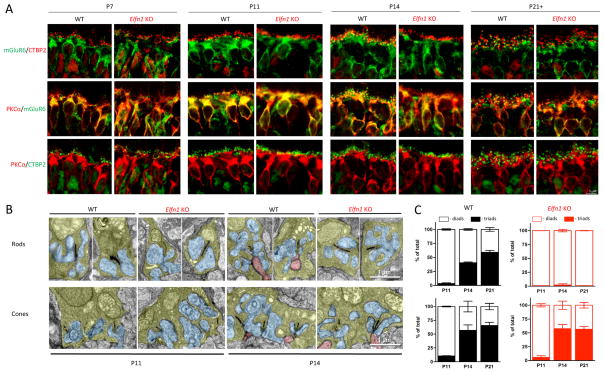
To determine the role of ELFN1 in the retina, we examined a line of Elfn1 knockout mice (Elfn1 KO), which eliminates ELFN1 protein expression (Figure 3A). Knockout of Elfn1 did not affect the viability of photoreceptors and mice displayed normal retinal morphology up to 5 months of age (Figure S3A, B). Interestingly, the levels of several ON-BC specific postsynaptic marker proteins were reduced. The largest change was seen in mGluR6 (~70% reduction), and reduced expression was observed for many components of the RGS complex (RGS7, RGS11, GPR179), which associate with mGluR6 and regulate its signaling (Figure 3A). In WT retinas, both mGluR6 and the effector channel TRPM1 accumulate at the dendritic tips of both ON-RBCs and ON-CBCs. In ON-RBCs these scattered postsynaptic puncta decorate the distal end of the PKCα-positive dendrites. In ON-CBCs, postsynaptic markers cluster in apposition to cone synaptic terminals (Figure 3B). Remarkably, in Elfn1 KO retinas the architecture for ON-RBCs was disrupted selectively. While the clustering of mGluR6 and TRPM1 in ON-CBCs contacting cone synaptic terminals was indistinguishable between genotypes, we could not detect their postsynaptic accumulation at the synapses made by ON-RBCs (Figure 3B; Figure S3C). These observations were further corroborated by examining the apposition of pre- and postsynaptic markers, which were intact in synapses with cones but undetectable in synapses with rods (Figure 3C). Electron microscopy further revealed a lack of ON-RBC dendrites invaginating rod terminals, but normal synaptic contacts of ON-CBCs with cone pedicles (Fig. 3D). We observed no deficits in the structure of the rod axonal terminals or their contacts with horizontal cell processes. Earlier studies found similar synapse formation deficits affecting ON-RBC synapses with rods in retinas with disrupted mGluR6 expression (Cao et al., 2009). We confirmed this observation and showed further that mGluR6 is also required for the formation of ON-CBC synapses with cones (Figure S3D).
Figure 3. Inactivation of ELFN1 specifically disrupts formation of rod to ON-RBC synapse.
A, Knockout of ELFN1 downregulates several key postsynaptic proteins of ON-BC. Protein expression is analyzed by Western blotting followed by quantification. *p<0.05; **p<0.01 (n=3, t-test). B, Lack of postsynaptic accumulation of mGluR6 and TRPM1 at the dendritic tips of ON-RBC but not ON-CBC in Elfn1 KO retinas. Retinal sections are counter-stained with cone arrestin (Cone Arr) to determine the location of cone synapses (arrows) and PKCα to identify the position of the rod synapses (vertical bracket). (Scale bar: 5 μm) C, High power confocal imaging reveals specific elimination of mGluR6-positive dendritic tips apposing rod but not cone terminals in Elfn1 KO retinas. (Scale bar: 2.5 μm) D, Electron microscopy shows no ON-BC dendrites (red) entering rod terminals of Elfn1 KO retinas. ON-BC dendrites are found contacting cone pedicles in both genotypes. Photoreceptor axonal terminals are colored in yellow, processes of horizontal cells in blue and ON-BC dendrites in pink. For the quantification, ~200–300 rod terminals and ~20–40 cone terminals from 2 separate mice were analyzed for each genotype.
Given the conservation of mGluR6’s role in synapse formation, we examined whether the ON-RBC dendrites in Elfn1 KO retinas would connect to cones instead of rods in the absence of their intended target (rod terminals). To facilitate detection of dendritic contacts with cone terminals we used an mGluR6-tdTomato reporter line (Kerschensteiner et al., 2009) that labels sparsely both ON-RBCs and ON-CBCs, distinguishing them by additional staining for an ON-RBC specific marker, PKCα. In both WT and Elfn1 KO retinas we found abundant evidence for the ON-CBC dendrites contacting PNA-positive cone pedicles (Figure S3E). All 22 ON-CBC cells in WT mice and 35 in Elfn1 KO examined made synaptic contacts with cones. In contrast, we found no evidence for ON-RBC contacting cone pedicles in either WT or Elfn1 KO retinas (45 and 65 ON-RBC neurons examined, in WT and Elfn1 KO retinas, respectively). We conclude that recruitment of mGluR6 by ELFN1 is required specifically for ON-RBCs to establish synaptic contacts with rod axonal terminals.
ELFN1 is required for the formation of the synaptic contacts between rods and ON-RBCs
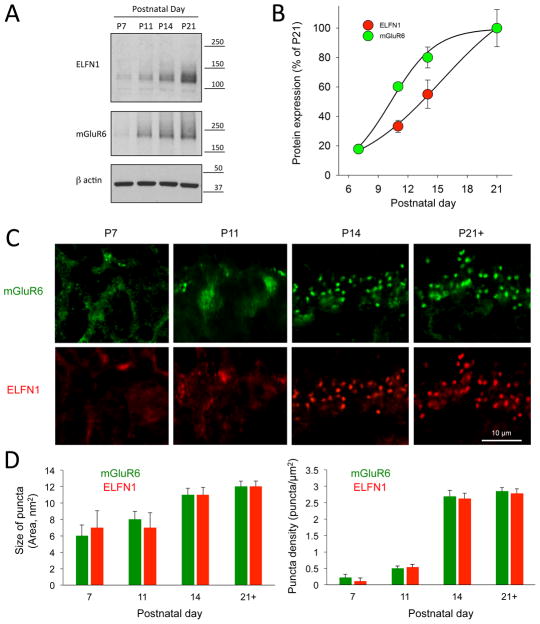
The lack of rod to ON-RBC synapses observed in adult Elfn1 KO retinas may reflect deficits in the synapse formation or stabilization/maintenance after initial assembly. To discriminate between these mechanisms we characterized the molecular and cellular events during the developmental window for rod photoreceptor synaptogenesis that begins at ~P7 and completes fully by P21 (Hoon et al., 2014). We found that the expression of ELFN1 protein was first detectable at P11 and coincided with the induction of mGluR6 (Figure 4A). The substantial rise in the levels of both of these proteins occurred at P14, when synaptogenesis of the photoreceptors was at the peak, and reached saturation by P21 (Figure 4B). Immunohistochemical analysis revealed that at early stages (P7–P11) ELFN1 was distributed diffusely throughout photoreceptor axons (Figure 4C), began forming detectable clusters at rod synaptic terminals at approximately P11, and was entirely in clusters within 3 days. The density of synaptic ELFN1 puncta increased approximately 5-fold (from 0.53±0.09 puncta/μm2 at P11 to 2.62±0.18 puncta/μm2 at P14) while nearly doubling their size (7±2 nm at P11 to 11±1 nm at P14). This organization remained stable until retinas reached maturity at P21 (Figure 4D). Distribution of mGluR6 mirrored this pattern as it progressed from diffuse in dendrites at P7–P11 to clustered at synapses at P14, greatly increasing both the density of the puncta and their size during this developmental stage.
Figure 4. Induction of ELFN1 during development correlates with the peak of rod photoreceptor synaptogenesis and postsynaptic recruitment of mGluR6.
A, Analysis of ELFN1 and mGluR6 protein expression in the retina lysates at different developmental stages by Western blotting. Equal amount of total protein lysates were loaded on the single gel and specific antibodies were used to probe indicated proteins. B, Quantification of ELFN1 and mGluR6 expression dynamics in WT retinas. Retinas from 3 mice were used, and values were averaged. C, Developmental dynamics of mGluR6 and ELFN1 recruitment to synaptic puncta. Immunostaining with indicated antibodies in the outer plexiform layer is shown. D, Quantification of the changes in the size (left) and number (right) of synaptic puncta positive for mGluR6 and ELFN1, respectively. Mean fluorescence intensity from 1 to ~ 30 puncta per retina section were measured from 3–6 sections per retina collected from 2 separate mice at each developmental time point. There were no differences between mGluR6 and ELFN1 across time points in either category (n>12 puncta per time point P>0.05, t-test).
We studied the effect of ELFN1 loss on synaptic development (Figure 5). Consistent with the earlier work (Nomura et al., 1994), we found at P7 that most PKCα-positive ON-RBC dendrites terminated in proximity of presynaptic rod ribbons. This organization was not changed in Elfn1 KO retinas, where ribbon/dendrite apposition was maintained throughout the development (Figure 5A). In WT retinas mGluR6 migrated from dendrites to synaptic clusters apposing ribbons at P14. However, this process failed to occur in ON-RBCs of Elfn1 KO mice, where mGluR6 maintained its diffuse dendritic distribution at P14 and P21.
Figure 5. Impact of ELFN1 on developmental dynamics of photoreceptor synapse formation.
A, Cytoarchitechture of retinal organization across indicated developmental stages (P7–P21) as revealed by immunostaining of retina cross-section. Regions corresponding to the position of ON-BC are shown. PKCα was used as a marker for rod ON-BC. B, Analysis of synaptic morphology by electron microscopy. Retinas at indicated developmental stages were dissected and studied by transmission electron microscopy. Photoreceptor axonal terminals are colored in yellow, processes of horizontal cells in blue and ON-BC dendrites in pink. C, Quantification of synaptic organization features. Rod and cone terminals containing ON-BC dendrites were designated as triads and devoid of ON-BC processes: diads. ~60–400 rod terminals and ~20–60 cone terminals from 2 separate mice were analyzed for each genotype and developmental stage.
We examined further the developmental dynamics of physical synapse formation in both Elfn1 KO and WT retinas by electron microscopy (Figure 5B). We were unable to identify characteristic hallmarks of the photoreceptor synapses in both mouse lines at P7, consistent with the lack of the photoreceptor synaptogenesis at this early stage. In WT retinas, we found many synapses containing only horizontal cell processes making contacts with both rod and cone terminals at P11. At this stage, ON-BC dendrites were detected inside very few synaptic terminals. This differed dramatically at P14 when ~40% of rod and a majority of cone terminals already contained ON-BC dendrites. Cones are known to complete their synaptogenesis earlier in time than rods (Blanks et al., 1974), and we found that the synaptic organization of cones remained unchanged after this point. However, the number of rod synapses with ON-RBCs increased further by P21 (Figure 5C). Importantly, in Elfn1 KO mice we did not detect ON-RBC dendrites within rod terminals at any developmental stage. Together, these data indicate that the lack of ELFN1 prevents the formation of rod synapses during synaptogenesis.
Axonal targeting of ELFN1 is dependent on intact neurotransmitter release and requires presynaptic calcium channels
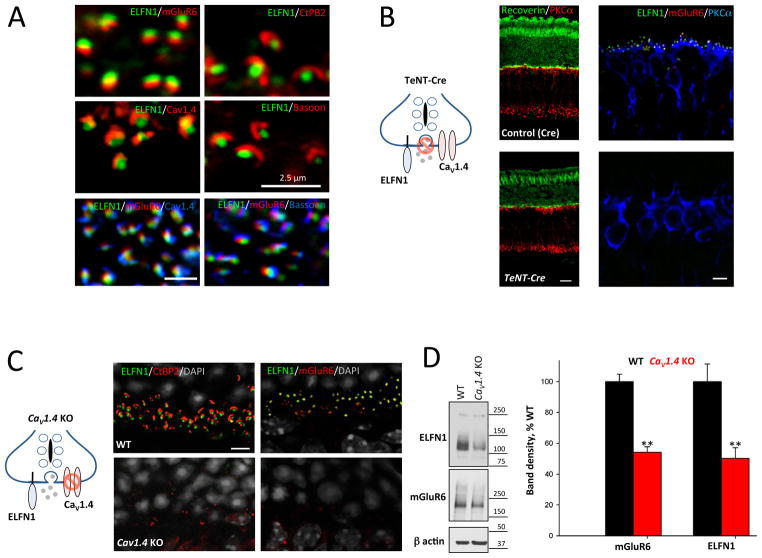
To initiate synapse formation with ON-RBCs at the site of the glutamate release, ELFN1 must be targeted close to the site of glutamate release. Indeed, ELFN1 in rods is present exclusively at synaptic terminals in close alignment with synaptic ribbons and Cav1.4 calcium channels (Figure 6A). Synaptic activity has been shown to play a role in establishing photoreceptor contacts with ON-BCs (Dunn et al., 2013) and the lack of photoreceptor calcium channels, Cav1.4, is known to disrupt physically rod and cone connections to ON-BCs (Liu et al., 2013; Mansergh et al., 2005; Zabouri and Haverkamp, 2013). We investigated whether synaptic transmission or Ca2+ channel localization impacted the ability of ELFN1 to form synapses.
Figure 6. Inactivation of neurotransmitter release or knockout of Cav1.4 calcium channel prevents synaptic targeting of ELFN1.
A, Co-localization of ELFN1 with components of synaptic release machinery. Immunostaining for key components of the synaptic ribbon relative to ELFN1 and mGluR6 is shown. Scale bar: 2.5 μm B, Effect of inactivating vesicular fusion in photoreceptors on ELFN1 localization. Mice expressing TeNT in photoreceptors (TeNT-Cre) were compared to control littermates (Cre only) for retina morphology (left; scale bar 25 μm) and expression and localization of ELFN1, mGluR6 and synaptic marker proteins (right; scale bar: 5 μm). C, Effect of deleting presynaptic calcium channel CaV1.4 on ELFN1 localization. Localization of proteins in the outer plexiform layer was detected by immunohistochemistry. Scale bar: 5 μm. D, Destabilization of ELFN1 at the protein level in Cav1.4 knockout retinas as analyzed by Western blotting. **p<0.01 (n=4 mice, t-test)
First, we prevented photoreceptors from releasing the neurotransmitter glutamate by expressing tetanus toxin (TeNT) that abolishes vesicular fusion (Figure 6B). This was achieved by driving TeNT expression to photoreceptors using a Pcdh21-Cre driver line. Examination of the resulting mice (TeNT-Cre) indicated that blockade of the synaptic transmission did not affect the overall morphology of the photoreceptors or their survival, at least during the timeframe of examination (Figure 6B). Indeed, we found that in 6-week-old mice (n=3) used in the experiments, the thickness of the photoreceptor nuclear layer was similar between TeNT-Cre (10.4 ± 0.4 nuclei per row) and their control littermates (12.8 ± 0.4 nuclei per row). The majority of rod axonal terminals in TeNT-Cre mice reached the synaptic OPL layer, as demonstrated by PSD95 staining, and contained presynaptic specializations within the axonal terminals as demonstrated by CtBP2 CtBP2 staining (Figure S4A). However, synaptic ribbons became smaller and lost their “horseshoe”-like shape. We also noticed that a few axonal terminals were located in the photoreceptor nuclear layer, as observed previously in several models of synaptic dysfunction (Liu et al., 2013; Samuel et al., 2014; tom Dieck et al., 2012). Remarkably, we observed no ELFN1 staining in the photoreceptor axonal terminals of TeNT-Cre retinas (Figure 6B). Consistent with this observation, we found that postsynaptic clustering of mGluR6 was also lost from the tips of ON-BC dendrites.
Because similar synaptic disorganization was described previously in the retinas with mutant or absent Cav1.4, the Ca2+ channel that triggers vesicular release from photoreceptors, we examined the expression and localization of ELFN1 in CaV1.4 KO (Figure 6C). In accordance with earlier reports (Liu et al., 2013; Mansergh et al., 2005; Zabouri and Haverkamp, 2013), we found a disorganization of synapses between photoreceptors and ON-BCs in Cav1.4 KO. Synaptic ribbons displayed irregular shapes and mGluR6 was no longer detectable at ON-BC dendritic tips, consistent with our demonstrated deficits in the formation of photoreceptor to ON-BC synapses (Figure 6C). Interestingly, we found that in the Cav1.4 KO the accumulation of ELFN1 at the synaptic terminal was prevented (Figure 6C). Immunohistochemistry revealed further that ELFN1 protein expression was decreased substantially, suggesting that Cav1.4 is required for ELFN1’s stability (Figure 6D). In contrast, expression and localization of Cav1.4 and the synaptic ribbon were normal in Elfn1 knockout retinas (Figure S4B).
We sought to delineate whether targeting of ELFN1 to synapses may be sensitive to general alterations in the synaptic transmission, or more specifically to its physical interaction with the presynaptic glutamate release machinery. For this we examined the consequences of eliminating TRPM1, the effector channel in the dendritic tips of ON-BCs responsible for generating the depolarizing response. We found that in Trpm1 knockout retinas that ELFN1 was localized normally to synapses in apposition to mGluR6, in a manner indistinguishable from WT retinas (Figure S4C). Thus, we conclude that vesicular release machinery and Cav1.4 likely regulate photoreceptor synapse formation through the recruitment of ELFN1 to axonal terminals.
Mice lacking ELFN1 have selective deficits in synaptic transmission between rod photoreceptors and rod ON-BC neurons
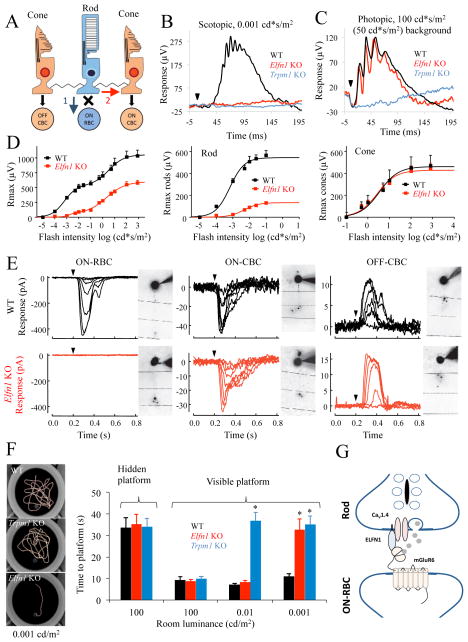
We analyzed the functional consequences of the selective loss of rod to ON-RBC synapses by electroretinography (ERG). Rods transmit light-evoked responses through either the most sensitive primary (rod bipolar) pathway that involves direct synaptic input onto ON-RBCs (Dacheux and Raviola, 1986) or the less sensitive secondary (rod-cone) pathway that involves passive transmission of signals through gap junctions to cone terminals (Raviola and Gilula, 1973) that in turn relays them onto ON-CBCs (Figure 7A). Activation of these pathways produces depolarizing activity that is reflected by the ERG b-wave. In Elfn1 KO mice, dim scotopic flashes that activate only the primary rod pathway produced no b-wave response (Figure 7B). This was indistinguishable from the phenotype observed in mouse mutants that lack responsiveness of ON-bipolar neurons to light, such as Trpm1 KO mice, suggesting complete lack of synaptic transmission to ON-RBC. In contrast, when a background light was applied to suppress the activity of rods, bright photopic flashes mediated by cones led to ERGs in Elfn1 KO mice that were indistinguishable from WT (Figure 7C). Stimulation of retinas with higher scotopic flash strengths revealed the presence of partial b-wave responses (Figure 7D), consistent with transduction of rod-driven signals via the less sensitive secondary pathway that is observed in retinas that completely lack ON-RBCs (Abd-El-Barr et al., 2009). The separation of the ERG b-wave components confirmed further that the rod-driven responses were reduced severely while the cone-driven response was unaffected by ELFN1 elimination (Figure 7D). The a-wave, reflecting the hyperpolarizing response of photoreceptors, was indistinguishable between WT and Elfn1 KO across all light intensities, indicating intact rod and cone photoreceptor phototransduction (Figure S5).
Figure 7. ELFN1 mediates functional wiring of a primary rod pathway.
A, Cartoon representation of synapse connectivity between rods, cones, and bipolar cells. Blue arrow denotes primary rod pathway; red arrow denotes secondary. B, Representative ERG traces elicited by a scotopic flash of 0.001 cd*s/m2 (~0.6 R*/rod) to activate the primary rod pathway only. C, Representative ERG traces to a photopic flash of 100 cd*s/m2 (~58,000 R*/rod) under a 50 cd*s/m2 (~29,000 R*/rod/s) light background to activate cone pathway only. D, (left), Dose response plot of maximal b-wave amplitudes from WT and Elfn1 KO mice plotted against their eliciting flash-intensities. (Middle) Rod-only ON-BC dose-response component. Half-maximal flash intensity for rods are 0.0007 ± 0.0001 cd*s/m2 (0.4 ± 0.1 R*/rod/s) for WT and 0.0050 ± 0.0001 cd*s/m2 (2.9 ± 0.1 R*/rod/s) for Elfn1 KO. (Right) Cone-only ON-BC dose-response component. Half-maximal flash intensity for cones were 0.53 ± 0.10 cd*s/m2 (310 ± 58 R*/rod) for WT and 0.44 ± 0.10 cd*s/m2 (260 ± 58 R*/rod) for Elfn1 KO (right). The data are averaged from 3 mice of each genotype. E, Light-evoked responses of ON-RBCs, ON-CBCs, and OFF-CBCs in dark-adapted slices from WT and Elfn1 KO mice. Flash strengths range from 0.34 to 4.0 R*/rod for RBCs and CBCs, spanning a range of strengths that account for activity generated by the primary and secondary rod pathways. Cells are filled during recordings with the fluorophore Alexa-750 and imaged to confirm the bipolar cell identity. Responses of RBCs and CBCs are from the same slices, typically within 100 μm of one another. In total we recorded from 3 ON-RBCs and 6 ON-CBCs across 4 WT mice, and 8 ON-RBCs and 6 ON-CBCs across 7 Elfn1 KO mice. ON-CBCs subtype was not characterized further. It should be noted that light-evoked responses of ON-RBCs in Elfn1 KO mice were never observed, even during the presentation of very bright background light. F, Evaluation of behavioral sensitivity of mice to light in a water-maze task. Mice were trained to find a randomly placed visible escape platform in a water maze, and their tracks and time to escape are recorded. Under a bright photopic light environment of 100 cd*/m2 (58,000 R*/rod/s), all genotypes readily found an escape platform. Elfn1 KO mice fail to locate the platform at the dimmest illumination range of 0.001 cd*s/m2 (~0.6 R*/rod) but are capable of performing the task at the higher level of 0.01 cd/m2 (5.8 R*/rod/s) (* P<0.05, t-test, n=5 for all groups). Error bars are SEM. G, Schematic diagram depicting possible arrangements of elements in the rod synapses. ELFN1 plays critical role in bridging presynaptic components with postsynaptic cascade of the ON-RBC.
To determine the cellular basis for the lack of dim scotopic b-waves in Elfn1 KO mice, we performed single-cell patch clamp recordings from dark-adapted retinal slices. Light-evoked responses were totally absent from ON-RBCs of Elfn1 KO mice (Figure 7E), consistent with the lack of a high sensitivity b-wave (Figure 7B) and the selective loss of rod to ON-RBC synapses (Figures 2, 3), thereby confirming the elimination of the rod bipolar pathway. However, both ON- and OFF-CBCs displayed robust responses at higher scotopic flash strengths in Elfn1 KO mice, reflecting that the secondary rod pathway remained intact in Elfn1 KO mice. Thus, ELFN1 is necessary for synaptic transmission between rods and ON-RBCs, but not cones and ON-CBCs or rods and ON-CBCs through cones.
Behavioral contribution of the primary rod pathway to vision
To ascertain the behavioral consequences of the selective disruption of rod to ON-RBC synapses along the primary rod pathway, we evaluated the visual performance of mice in a water-maze task that relies on their ability to navigate to an escape platform at different levels of illumination. Trained WT and Elfn1 KO mice did not differ in their ability to locate quickly the platform under photopic, or cone-driven, conditions. When illumination was reduced to the low scotopic range approaching visual threshold (0.001 cd/m2, or ~0.6 R*/rod/s), WT mice remained able to find the escape platform, but neither Elfn1 KO nor Trpm1 KO mice could do so. Thus, at this low light intensity synaptic transmission from rods to ON-RBCs is required for vision. To discriminate the behavioral contributions of primary and secondary rod pathways, we increased the light intensity to 0.01 cd/m2 (~5.8 R*/rod/s), at which only rods are active and transmit their photoresponses to both ON-RBCs and ON-CBCs. At this level of stimulation Trpm1 KO mice remained unable to detect the platform, while the behavioral performance of Elfn1 KO mice became indistinguishable from WT (Figure 7F). These results are consistent with the selective loss of the primary rod pathway in Elfn1 KO mice (Figure 7A) and suggest that Elfn1 KO mice locate the escape platform based on the flow of rod-driven signals through the secondary rod pathway. ELFN1 is thus a key molecular determinant enabling the high sensitivity primary rod pathway, making mice lacking this protein a valuable tool for dissecting the functional integration of rod circuits and their role in vision.
DISCUSSION
Our findings provide the molecular identity of the factor used by rod photoreceptors for recognition and selective wiring with their postsynaptic partners, the ON-RBCs. This is the first demonstration of a molecule that mediates specific connectivity between defined subclasses of neurons in the retinal circuitry. Rod photoreceptors display an exquisite light sensitivity that is mediated by a G-protein coupled cascade (phototransduction) that signals reliably the absorption of single photons (Burns and Arshavsky, 2005; Yau and Hardie, 2009). This high detection sensitivity requires the selective synaptic integration of rods into a specialized retinal circuit, referred to as the primary rod (or rod bipolar) pathway. We found that deletion of ELFN1 prevents rods from forming synapses with ON-RBCs, while not effecting their survival, anatomical organization, or phototransduction. Furthermore, light-driven signals from rods continue to be carried through the secondary rod pathway, which is formed by electrical coupling by gap junctions from rods to cones and the consequent propagation of signals via ON-CBCs. We showed that the selective loss of rod to ON-RBC synaptic contacts had a profound effect on mouse vision and prevented the detection of low light signals while preserving behavioral responses mediated by the less sensitive secondary pathway. These results allowed a dissection of the relative contributions of primary versus secondary pathways to visual detection. This genetic model will provide a framework for investigating how parallel channels in the retina establish the full range of visual function.
This work allows us to propose a model for the molecular mechanism mediating synaptic integration of rods into the retinal circuitry (Figure 7G). ELFN1 plays a key role in this model by virtue of its selective expression at rod synaptic terminals. There, its extracellular domain is engaged in a trans-synaptic interaction with mGluR6 expressed by ON-RBCs, recruiting mGluR6 to the dendritic tips. This binding of ELFN1 with mGluR6 plays an essential role in the formation of this synaptic contact, as elimination of either component results in a similar loss of synapses. Although ELFN1 is required for synapse formation during early developmental stages of photoreceptor synaptogenesis, it remains to be determined whether it also acts in mature retinas to support stabilization of synaptic contacts. We found further that the expression and positioning of ELFN1 at the axonal terminal is dependent on synaptic release machinery. There are two significant implications of this observation. First, it may provide a possible explanation for the spatial specificity of postsynaptic receptor recruitment: integration of ELFN1 with Cav1.4 calcium channels presynaptically ensures the proper positioning of postsynaptic mGluR6 in the immediate vicinity of glutamate release. Second, this dependence provides a possible mechanism for the effects of neuronal activity on synaptogenesis: by modulating ELFN1 expression and localization, changes in synaptic release would affect physical assembly of the synapse.
While ELFN1 is indispensable for rod-to-ON-RBC synaptogenesis, it may not be sufficient for ensuring specificity in synapse formation. For example, ON-CBCs express mGluR6 at their dendritic tips but do not connect to ELFN1-expressing rod terminals. Rather, they synapse selectively with cone terminals that appear to be devoid of ELFN1. Thus, other molecules likely play a role similar to ELFN1 in cones, and the two pathways may compete with one another to achieve specificity. Indeed, when all photoreceptors develop into cone-like cells in retinas lacking the transcription factor Nrl, ON-RBCs form connections with them (Strettoi et al., 2004). Alternatively, it is possible that the wiring selectivity is determined by setting separate developmental time windows. Cones have been shown to form synaptic contacts ~4 days before rods (Blanks et al., 1974; Miller et al., 1999). Therefore if ELFN1 is not expressed by rods at that time, when cones are expressing their ‘ELFN1-like’ synaptogenic molecule, ON-CBC would only have one potential target (cones). Then with time as ON-RBCs become competent to establish contacts, they would also have only one synaptic partner (rods). Additionally, cell adhesion-like proteins expressed by different classes of the ON-BC neurons might regulate specificity of the synaptogenesis. These could include ELFN1-like molecules, such as LRIT3 (Neuille et al., 2014; Zeitz et al., 2013). Although LRIT3 is expressed by both ON-RBC and ON-CBC and plays an essential role in the synaptic communication with both rods and cones, it appears to selectively impact the organization of presynaptic elements in cones (Neuille et al., 2015).
Our study for the first time describes ELFN1 as a key presynaptic component with an essential role in the physical assembly of synapses between specific subtypes of neurons in the retina. Different function has been previously ascribed to ELFN1 in the brain. It was reported that in the hippocampus ELFN1 is expressed in postsynaptic somatostatin positive interneurons, where it is recruited to their synapses with the CA1 pyramidal cells (Sylwestrak and Ghosh, 2012). At those synapses ELFN1 was shown to act across the synapse to inhibit synaptic vesicle release probability at presynaptic terminals of CA1 neurons. A subsequent study further determined that the effect on release probability is likely mediated by presynaptic mGluR7, with which ELFN1 was found to form trans-synaptic contacts (Tomioka et al., 2014). However, the mechanistic role of ELFN1 in the hippocampus appears to differ from the retina. In hippocampal neurons it is expressed postsynaptically and acts to regulate presynaptic release mechanisms (Sylwestrak and Ghosh, 2012; Tomioka et al., 2014). Furthermore, ELFN1 at these synapses has no impact on the physical connectivity of hippocampal neurons, and its loss does not abolish synaptic transmission. Instead, it adjusts synaptic properties of select hippocampal circuits. Thus it appears that ELFN1 plays a distinct, previously unappreciated role in the retina as a pre-synaptic guidance receptor that matches a specific photoreceptor type with their postsynaptic targets. While in the retina ELFN1 is expressed by a single type of the neuron, in the brain it is present in several distinct populations across many brain regions (Dolan and Mitchell, 2013). This broader expression is reflected by marked behavioral alterations associated with the loss of ELFN1 that include hyperactivity, seizures, and an abnormal response to psychostimulants (Dolan and Mitchell, 2013). Accordingly, mutations in ELFN1 were also found to be associated with epilepsy in humans (Tomioka et al., 2014). These observations suggest that ELFN1 may affect several circuits in the brain outside of hippocampus. Demonstration of the presynaptic function of ELFN1 and its involvement in physical assembly of synaptic connections in this study suggests that it may play similar role in guiding selective axonal wiring in other circuits.
Interestingly, mGluR7 identified in a previous study as a binding partner for ELFN1 (Tomioka et al., 2014) is homologous to mGluR6 and belongs to the same subfamily. In both retina and hippocampus interactions involving ELFN1 occur trans-synaptically and are involved in positioning glutamate GPCRs at discrete subcellular locations. Together, these findings raise a provocative possibility that ELFN1 may serve as an adapter molecule that recruits mGluRs to the synaptic sites in the nervous system thus endowing circuits with specific properties. Given the large number of neurotransmitter receptors and their precise localization patterns, targeting GPCRs via extracellular interactions with ELFN1-like cell adhesion molecules to the site of the neurotransmitter release may constitute a general principle in the development of metabotropic synapses and cellular signaling.
EXPERIMENTAL PROCEDURES
All experiments involving mice were approved by the IACUC committees at the Scripps Research Institute and University of California, Los Angeles. Experiments were conducted in accordance with the ARVO statement for the use of animals in vision research and guidelines set forth by NIH.
Detailed protocols for the procedures are provided in Extended Experimental Procedures. Protein purification, cell culture, transfection, immunoprecipitaiton, electron microscopy, electroretinography, single cell electrophysiology, animal behavior, and in situ hybridizations were performed using conventional, previously described methods.
Supplementary Material
Acknowledgments
We wish to thank Dr. Lee Ann Higgins (University of Minnesota) for performing mass-spectrometry experiments, Ms. Natalia Martemyanova for producing and maintaining mice examined in this study, and Ms. Montina Van Meter in Histology Core, Scripps Florida for toluidine blue staining. This work was supported by NIH grants: EY018139 (KAM), EY017606 (APS), NS084190 (AL), DC009433 (AL), a Carver Research Program of Excellence award (AL) and the Jules Stein Eye Institute Vision Core Grant EY000331 (APS). This work is also supported by an Unrestricted Grant from Research to Prevent Blindness, Inc. to the Department of Ophthalmology at UCLA. The R26floxstop-TeNT mouse strain was provided generously by Martin Goulding and mGluR6-tdTomato line was a gift from Rachel Wong.
Footnotes
AUTHOR CONTRIBUTIONS
Y.C. performed all biochemical and cell biological experiments described in the paper, including affinity purification of mGluR6 complexes, immunoprecipitations, immunostaining of retina cross-sections, Western blotting, and ectodomain binding assays; I.S. performed ERG, behavioral analysis of mouse strains and immunostaining; K.E.F. performed single cell patch clamp recordings and analysis of electrophysiological data; N.K. conducted EM experiments; C.O. performed in situ hybridization experiments, K.N.J., J.L.H and K.B. generated and characterized R26floxstop-TeNT/Pcdh21-Cre mouse model and supplied tissues for analysis, M.R.G. and M.F. helped express and purify ELFN1 proteins, A.L. generated critical reagent (antibodies), S.B. helped producing and characterizing Cav1.4 KO mice. A.P.S. designed patch clamp experiments, analyzed electrophysiological data, and wrote the paper; K.A.M. designed the study, analyzed data, and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd-El-Barr MM, Pennesi ME, Saszik SM, Barrow AJ, Lem J, Bramblett DE, Paul DL, Frishman LJ, Wu SM. Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. J Neurophysiol. 2009;102:1945–1955. doi: 10.1152/jn.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Brenner S, Kandel E, Kendler KS, King MC, Scolnick E, Watson JD, Zoghbi HY. Medicine. The future of psychiatric research: genomes and neural circuits. Science. 2010;327:1580–1581. doi: 10.1126/science.1188654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello PA, Gogos JA. Genetic and cognitive windows into circuit mechanisms of psychiatric disease. Trends Neurosci. 2012;35:3–13. doi: 10.1016/j.tins.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Benson DL, Colman DR, Huntley GW. Molecules, maps and synapse specificity. Nat Rev Neurosci. 2001;2:899–909. doi: 10.1038/35104078. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Adinolfi AM, Lolley RN. Synaptogenesis in the photoreceptor terminal of the mouse retina. J Comp Neurol. 1974;156:81–93. doi: 10.1002/cne.901560107. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Johnson LV. Selective lectin binding of the developing mouse retina. J Comp Neurol. 1983;221:31–41. doi: 10.1002/cne.902210103. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc Natl Acad Sci U S A. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. doi: 10.1016/j.neuron.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP, et al. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Hong W, Luo L, Ghosh A. Role of leucine-rich repeat proteins in the development and function of neural circuits. Annual review of cell and developmental biology. 2011;27:697–729. doi: 10.1146/annurev-cellbio-092910-154111. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Baylor DA. Synaptic circuitry of the retina and olfactory bulb. 1993;72(Suppl):139–149. doi: 10.1016/s0092-8674(05)80033-9. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Vardi N. “mGlu Receptors in the Retina” - WIREs Membrane Transport and Signaling. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:641–653. doi: 10.1002/wmts.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan J, Mitchell KJ. Mutation of Elfn1 in mice causes seizures and hyperactivity. PLoS One. 2013;8:e80491. doi: 10.1371/journal.pone.0080491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Della Santina L, Parker ED, Wong RO. Sensory experience shapes the development of the visual system’s first synapse. Neuron. 2013;80:1159–1166. doi: 10.1016/j.neuron.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Okawa H, Della Santina L, Wong RO. Functional architecture of the retina: development and disease. Progress in retinal and eye research. 2014;42:44–84. doi: 10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov VJ. Rod and cone visual pigments and phototransduction through pharmacological, genetic, and physiological approaches. J Biol Chem. 2012;287:1635–1641. doi: 10.1074/jbc.R111.303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot JI. Speed, sensitivity, and stability of the light response in rod and cone photoreceptors: facts and models. Progress in retinal and eye research. 2012;31:442–466. doi: 10.1016/j.preteyeres.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD. Evolution of phototransduction, vertebrate photoreceptors and retina. Progress in retinal and eye research. 2013;36:52–119. doi: 10.1016/j.preteyeres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Lardi-Studler B, Fritschy JM. Matching of pre- and postsynaptic specializations during synaptogenesis. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2007;13:115–126. doi: 10.1177/1073858406296803. [DOI] [PubMed] [Google Scholar]
- Liu X, Kerov V, Haeseleer F, Majumder A, Artemyev N, Baker SA, Lee A. Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels. 2013;7:514–523. doi: 10.4161/chan.26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Luscher C. Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci. 2014;17:1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Margeta MA, Shen K. Molecular mechanisms of synaptic specificity. Molecular and cellular neurosciences. 2010;43:261–267. doi: 10.1016/j.mcn.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ED, Tran MN, Wong GK, Oakley DM, Wong RO. Morphological differentiation of bipolar cells in the ferret retina. Vis Neurosci. 1999;16:1133–1144. doi: 10.1017/s0952523899166136. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Godinho L, Morgan JL, Oakley DM, Schroeter EH, Wong RO. Laminar circuit formation in the vertebrate retina. Progress in brain research. 2005;147:155–169. doi: 10.1016/S0079-6123(04)47012-5. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Neuille M, El Shamieh S, Orhan E, Michiels C, Antonio A, Lancelot ME, Condroyer C, Bujakowska K, Poch O, Sahel JA, et al. Lrit3 deficient mouse (nob6): a novel model of complete congenital stationary night blindness (cCSNB) PLoS One. 2014;9:e90342. doi: 10.1371/journal.pone.0090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuille M, Morgans CW, Cao Y, Orhan E, Michiels C, Sahel JA, Audo I, Duvoisin RM, Martemyanov KA, Zeitz C. LRIT3 is essential to localize TRPM1 to the dendritic tips of depolarizing bipolar cells and may play a role in cone synapse formation. Eur J Neurosci. 2015;42:1966–1975. doi: 10.1111/ejn.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Okawa H, Sampath AP. Optimization of single-photon response transmission at the rod-to-rod bipolar synapse. Physiology. 2007;22:279–286. doi: 10.1152/physiol.00007.2007. [DOI] [PubMed] [Google Scholar]
- Pahlberg J, Sampath AP. Visual threshold is set by linear and nonlinear mechanisms in the retina that mitigate noise: how neural circuits in the retina improve the signal-to-noise ratio of the single-photon response. Bioessays. 2011;33:438–447. doi: 10.1002/bies.201100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Baier H. Assembly of synaptic laminae by axon guidance molecules. Curr Opin Neurobiol. 2012;22:799–804. doi: 10.1016/j.conb.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Voinescu PE, Lilley BN, de Cabo R, Foretz M, Viollet B, Pawlyk B, Sandberg MA, Vavvas DG, Sanes JR. LKB1 and AMPK regulate synaptic remodeling in old age. Nat Neurosci. 2014;17:1190–1197. doi: 10.1038/nn.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Many paths to synaptic specificity. Annual review of cell and developmental biology. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Borodinsky LN. Implications of activity-dependent neurotransmitter-receptor matching. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:1393–1399. doi: 10.1098/rstb.2007.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Mears AJ, Swaroop A. Recruitment of the rod pathway by cones in the absence of rods. J Neurosci. 2004;24:7576–7582. doi: 10.1523/JNEUROSCI.2245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwestrak EL, Ghosh A. Elfn1 regulates target-specific release probability at CA1-interneuron synapses. Science. 2012;338:536–540. doi: 10.1126/science.1222482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Specht D, Strenzke N, Hida Y, Krishnamoorthy V, Schmidt KF, Inoue E, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, et al. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci. 2012;32:12192–12203. doi: 10.1523/JNEUROSCI.0752-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka NH, Yasuda H, Miyamoto H, Hatayama M, Morimura N, Matsumoto Y, Suzuki T, Odagawa M, Odaka YS, Iwayama Y, et al. Elfn1 recruits presynaptic mGluR7 in trans and its loss results in seizures. Nat Commun. 2014;5:4501. doi: 10.1038/ncomms5501. [DOI] [PubMed] [Google Scholar]
- Williams ME, de Wit J, Ghosh A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron. 2010;68:9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabouri N, Haverkamp S. Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One. 2013;8:e63853. doi: 10.1371/journal.pone.0063853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Jacobson SG, Hamel CP, Bujakowska K, Neuille M, Orhan E, Zanlonghi X, Lancelot ME, Michiels C, Schwartz SB, et al. Whole-exome sequencing identifies LRIT3 mutations as a cause of autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2013;92:67–75. doi: 10.1016/j.ajhg.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Atasoy D, Arac D, Yang X, Fucillo MV, Robison AJ, Ko J, Brunger AT, Sudhof TC. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010;66:403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.