Abstract
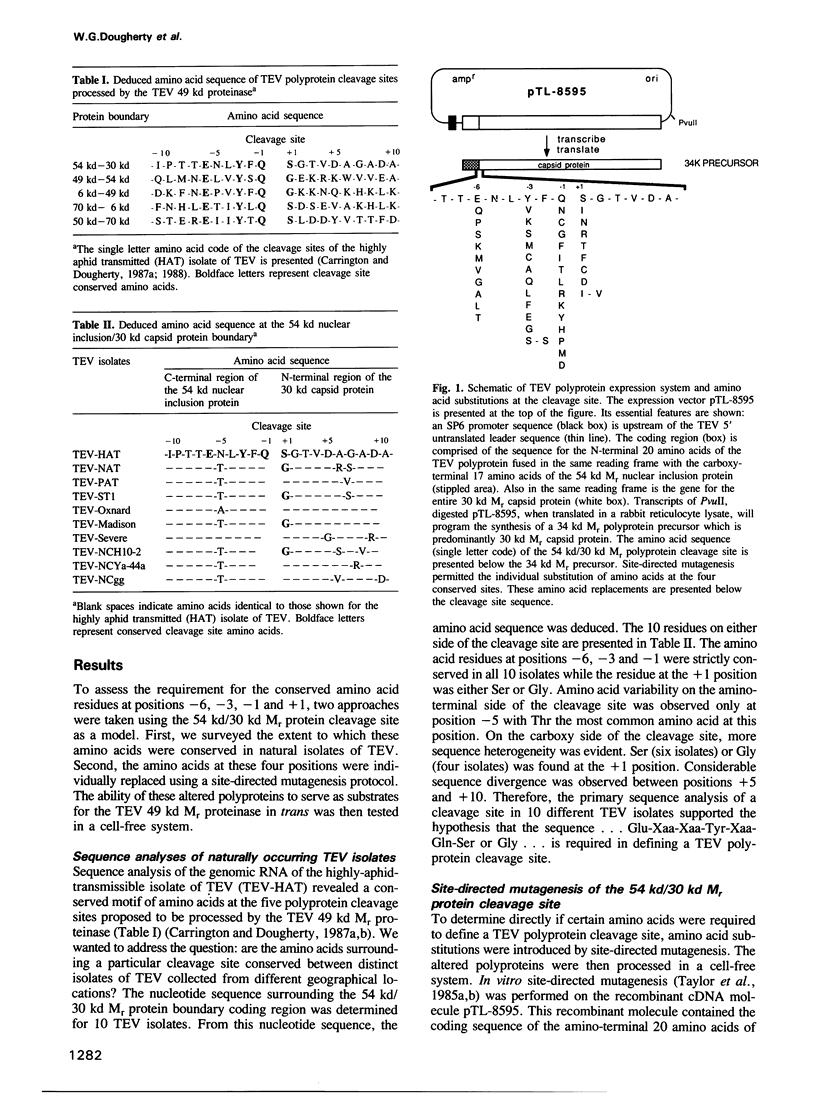
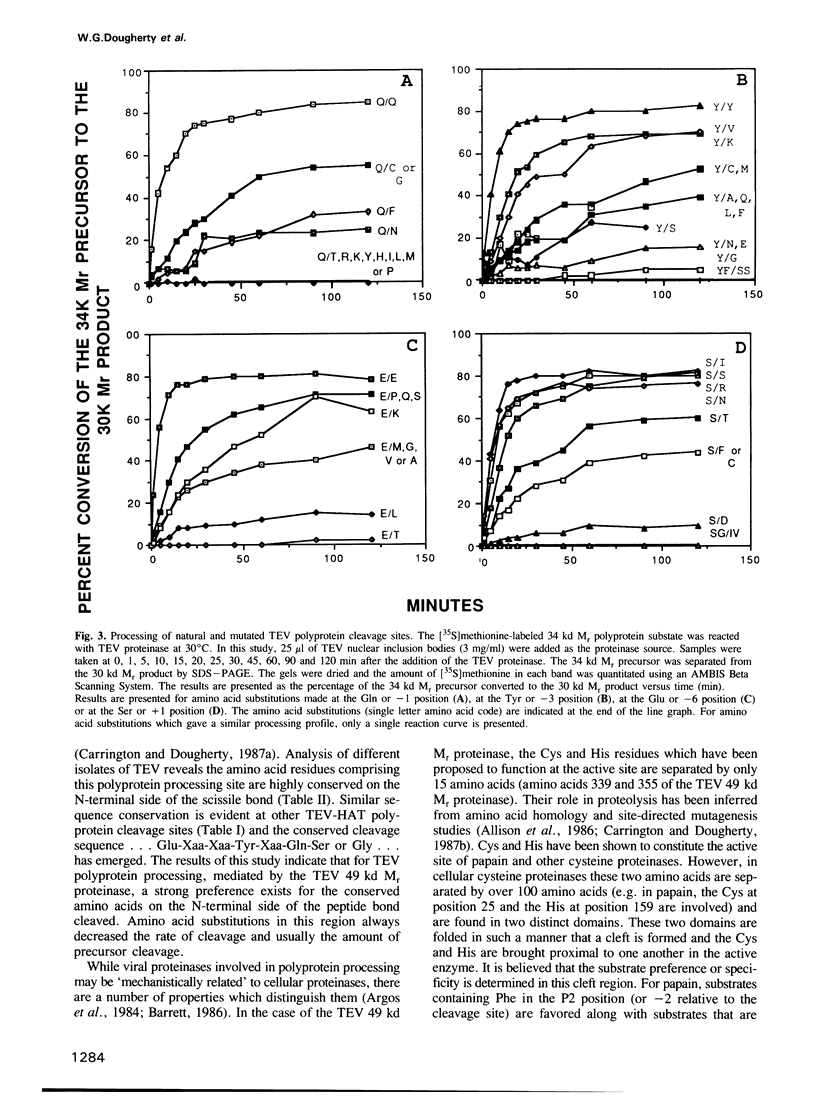
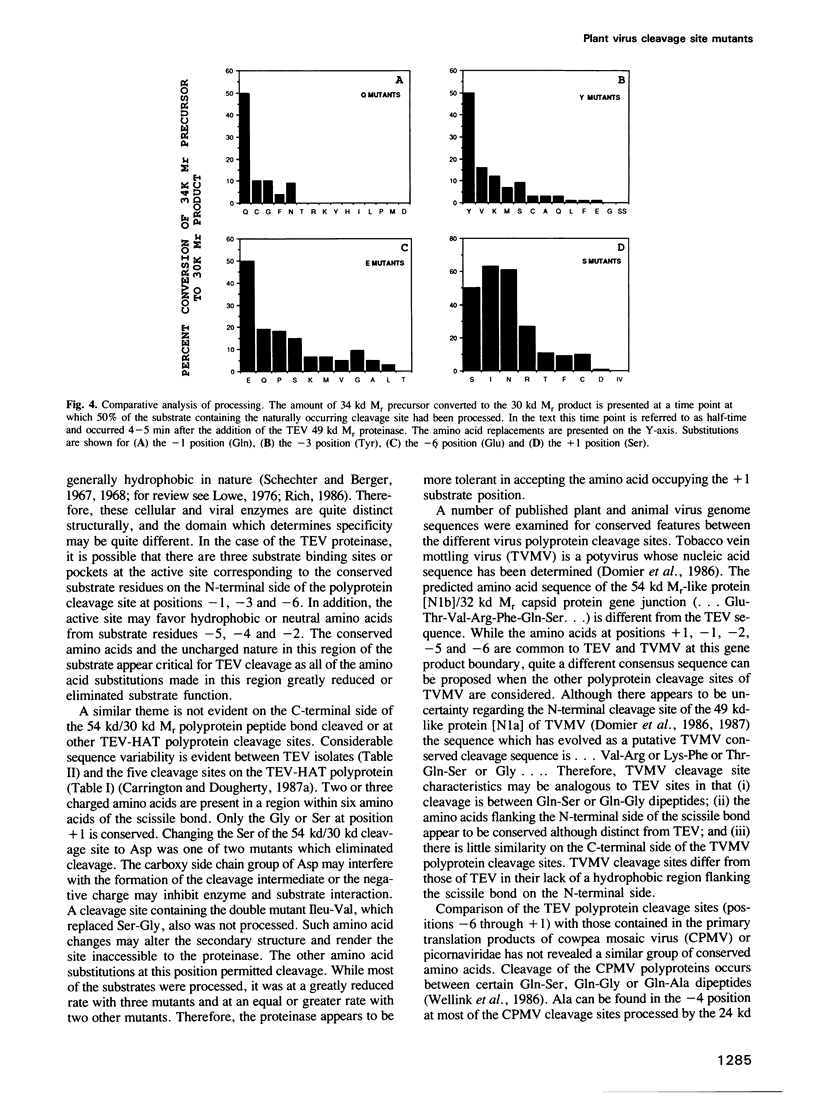
The RNA genome of tobacco etch virus (TEV) is organized as a single translational unit coding for a 346,000 (346 kd) mol. wt (Mr) polyprotein. The 346 kd Mr polyprotein is cleaved by a 49 kd Mr virus-encoded proteinase at five different sites between the dipeptides Gln-Ser or Gln-Gly. These cleavage sites or gene product boundaries are defined by the heptapeptide sequence...Glu-Xaa-Xaa-Tyr-Xaa-Gln-Ser or Gly.... We have used the 54 kd Mr nuclear inclusion protein/30 kd Mr capsid protein junction as a model to examine the role of these conserved amino acids in defining a cleavage site. The 54 kd/30 kd Mr protein cleavage site sequence of 10 TEV isolates from geographically distinct locations has been deduced. The conserved amino acids are present in all isolates. To determine if these four amino acids are an absolute requirement for polyprotein substrate activity, a site-directed mutational analysis has been performed. A recombinant cDNA molecule encoding the TEV 54 kd/30 kd Mr gene product cleavage site was mutated and polyprotein substrates were synthesized and processed in a cell-free system. Single amino acid substitutions made at the different positions reveal a strong preference for the naturally conserved amino acids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold E., Luo M., Vriend G., Rossmann M. G., Palmenberg A. C., Parks G. D., Nicklin M. J., Wimmer E. Implications of the picornavirus capsid structure for polyprotein processing. Proc Natl Acad Sci U S A. 1987 Jan;84(1):21–25. doi: 10.1073/pnas.84.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Brakke M. K., Van Pelt N. Properties of infectious ribonucleic acid from wheat streak mosaic virus. Virology. 1970 Nov;42(3):699–706. doi: 10.1016/0042-6822(70)90315-6. [DOI] [PubMed] [Google Scholar]
- Domier L. L., Franklin K. M., Shahabuddin M., Hellmann G. M., Overmeyer J. H., Hiremath S. T., Siaw M. F., Lomonossoff G. P., Shaw J. G., Rhoads R. E. The nucleotide sequence of tobacco vein mottling virus RNA. Nucleic Acids Res. 1986 Jul 11;14(13):5417–5430. doi: 10.1093/nar/14.13.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuhtsen H., Hiebert E., Purcifull D. E. Partial purification and some properties of tobacco etch virus induced intranuclear inclusions. Virology. 1974 Sep;61(1):200–209. doi: 10.1016/0042-6822(74)90254-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. Picornaviral processing: some new ideas. J Cell Biochem. 1987 Mar;33(3):191–198. doi: 10.1002/jcb.240330306. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem Biophys Res Commun. 1968 Sep 6;32(5):898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verver J., Goldbach R., Garcia J. A., Vos P. In vitro expression of a full-length DNA copy of cowpea mosaic virus B RNA: identification of the B RNA encoded 24-kd protein as a viral protease. EMBO J. 1987 Mar;6(3):549–554. doi: 10.1002/j.1460-2075.1987.tb04789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellink J., Rezelman G., Goldbach R., Beyreuther K. Determination of the proteolytic processing sites in the polyprotein encoded by the bottom-component RNA of cowpea mosaic virus. J Virol. 1986 Jul;59(1):50–58. doi: 10.1128/jvi.59.1.50-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D., Kaesberg P. 3'-terminal nucleotide sequence of encephalomyocarditis virus RNA determined by reverse transcriptase and chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4257–4261. doi: 10.1073/pnas.75.9.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]




