Abstract
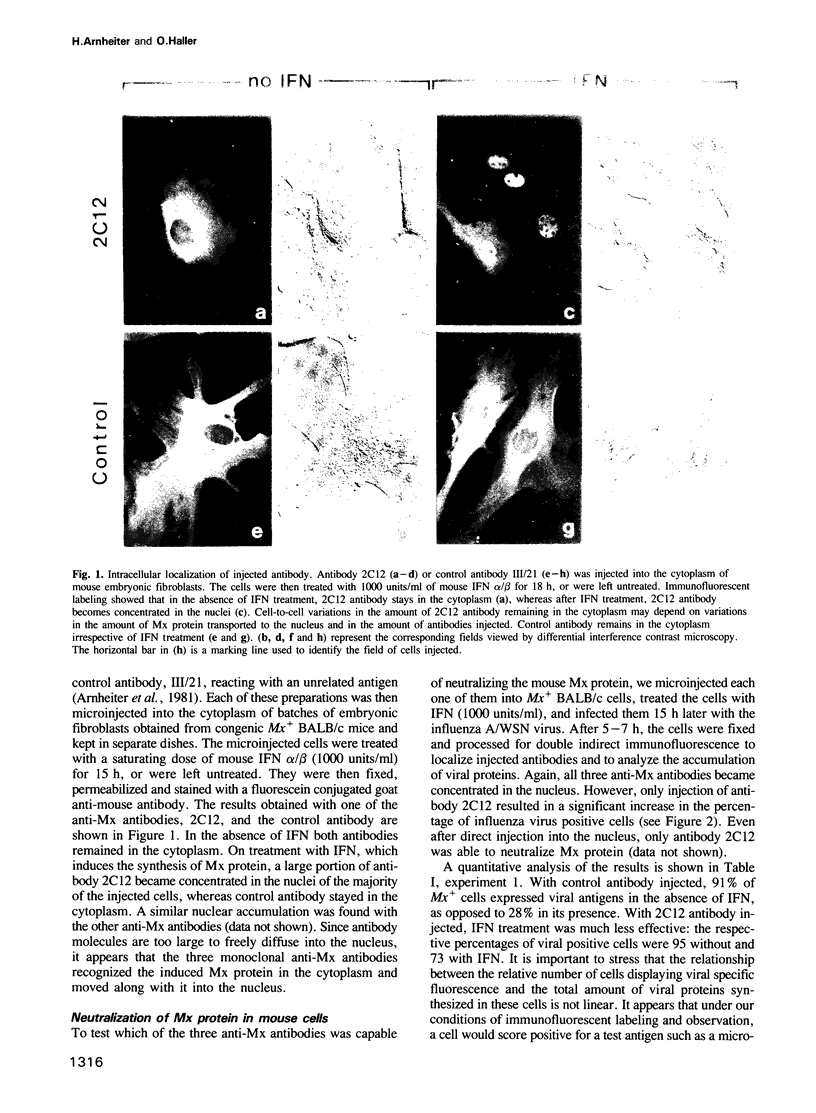
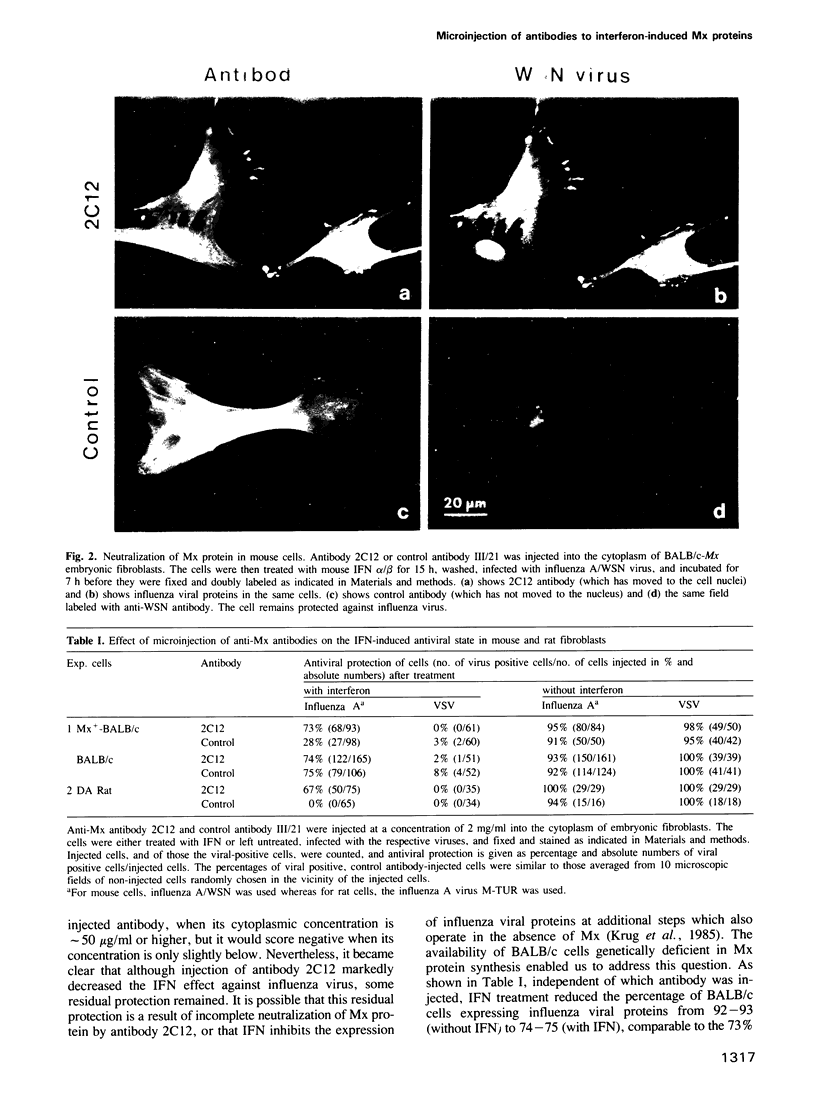
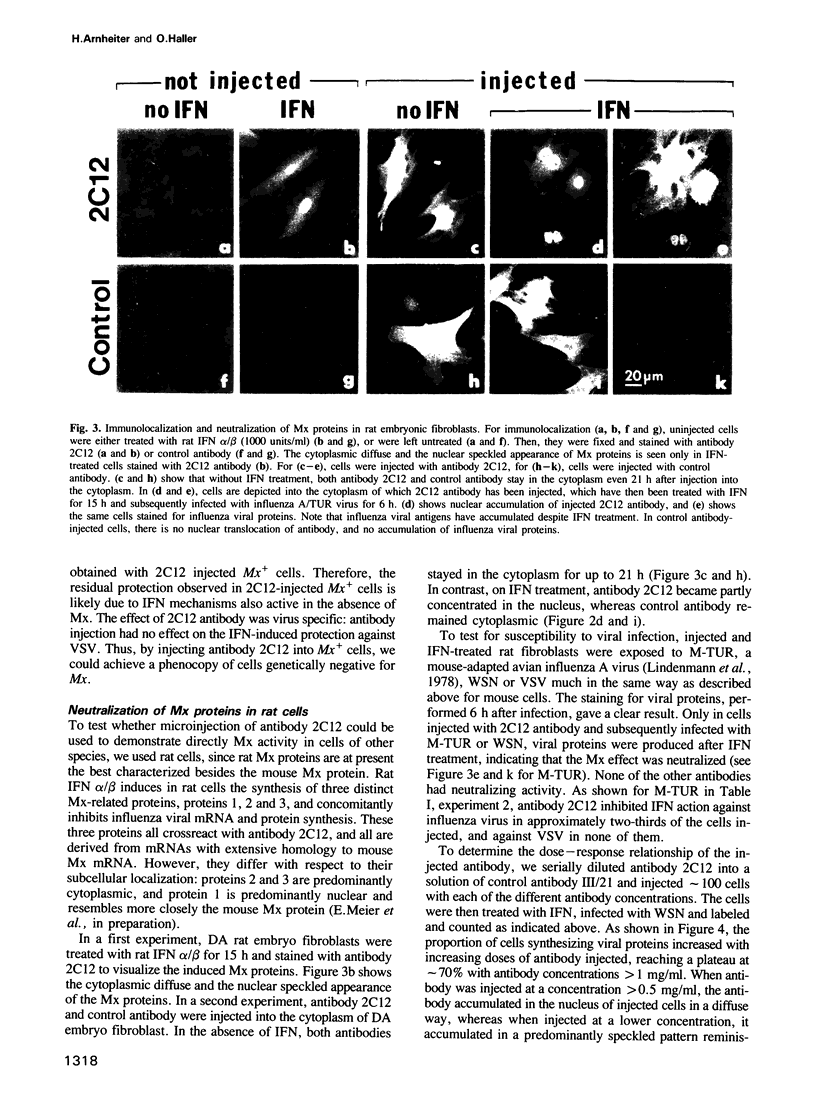
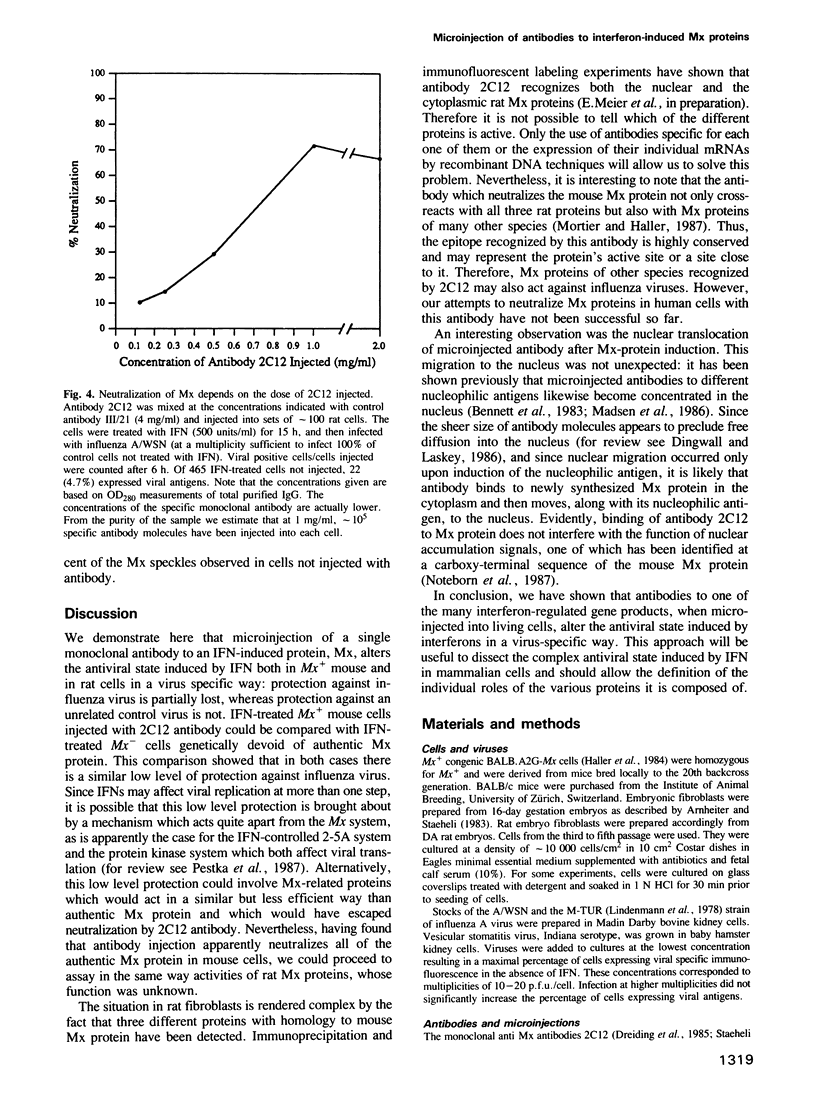
In mouse Mx+ cells, interferon alpha/beta induces the synthesis of the nuclear Mx protein, whose accumulation is correlated with specific inhibition of influenza viral protein synthesis. When Mx+ mouse cells are microinjected with the monoclonal anti-Mx antibody 2C12, interferon alpha/beta still induces Mx protein, but no longer inhibits efficiently the expression of influenza viral proteins as visualized by immunofluorescent labeling. However, interferon inhibition of an unrelated control virus, vesicular stomatitis virus, remains unchanged. Proteins with homology to mouse Mx protein are found in interferon-treated cells of a variety of mammalian species. In rat cells, for instance, rat interferon alpha/beta induces three Mx proteins which all cross-react with antibody 2C12 but differ in mol. wt and intracellular location, and it protects these cells well against influenza viruses. However, when rat cells are microinjected with antibody 2C12, interferon alpha/beta cannot induce an efficient antiviral state against influenza virus infection, whereas protection against vesicular stomatitis virus is not altered. These results show that both mouse and rat cells require functional Mx proteins for efficient protection against influenza virus. They further demonstrate that microinjection of antibodies is a promising way of elucidating the role of particular interferon-induced proteins in the intact cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Davis N. L., Wertz G., Schubert M., Lazzarini R. A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985 May;41(1):259–267. doi: 10.1016/0092-8674(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Direct visualization of protein transport and processing in the living cell by microinjection of specific antibodies. Cell. 1984 Nov;39(1):99–109. doi: 10.1016/0092-8674(84)90195-8. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Haller O., Lindenmann J. Host gene influence on interferon action in adult mouse hepatocytes: specificity for influenza virus. Virology. 1980 May;103(1):11–20. doi: 10.1016/0042-6822(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Staeheli P. Expression of interferon dependent resistance to influenza virus in mouse embryo cells. Arch Virol. 1983;76(2):127–137. doi: 10.1007/BF01311696. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Thomas R. M., Leist T., Fountoulakis M., Gutte B. Physicochemical and antigenic properties of synthetic fragments of human leukocyte interferon. Nature. 1981 Nov 19;294(5838):278–280. doi: 10.1038/294278a0. [DOI] [PubMed] [Google Scholar]
- Bennett F. C., Busch H., Lischwe M. A., Yeoman L. C. Antibodies to a nucleolar protein are localized in the nucleolus after red blood cell-mediated microinjection. J Cell Biol. 1983 Nov;97(5 Pt 1):1566–1572. doi: 10.1083/jcb.97.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Dreiding P., Staeheli P., Haller O. Interferon-induced protein Mx accumulates in nuclei of mouse cells expressing resistance to influenza viruses. Virology. 1985 Jan 15;140(1):192–196. doi: 10.1016/0042-6822(85)90460-x. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Clark R., Wong G., Arnheim N., Milley R., McCormick F. Transient reversion of ras oncogene-induced cell transformation by antibodies specific for amino acid 12 of ras protein. Nature. 1985 Apr 18;314(6012):639–642. doi: 10.1038/314639a0. [DOI] [PubMed] [Google Scholar]
- Hagag N., Halegoua S., Viola M. Inhibition of growth factor-induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986 Feb 20;319(6055):680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Lindenmann J., Gresser I. Host gene influences sensitivity to interferon action selectively for influenza virus. Nature. 1980 Feb 14;283(5748):660–662. doi: 10.1038/283660a0. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Haller O., Arnheiter H. Interferon-dependent genetic resistance to influenza virus in mice: virus replication in macrophages is inhibited at an early step. J Gen Virol. 1980 Sep;50(1):205–210. doi: 10.1099/0022-1317-50-1-205. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Hochkeppel H. K. IFN-alpha induced human 78 kD protein: purification and homologies with the mouse Mx protein, production of monoclonal antibodies, and potentiation effect of IFN-gamma. J Interferon Res. 1987 Aug;7(4):331–343. doi: 10.1089/jir.1987.7.331. [DOI] [PubMed] [Google Scholar]
- Horisberger M. A., Staeheli P., Haller O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1910–1914. doi: 10.1073/pnas.80.7.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M. A. The action of recombinant bovine interferons on influenza virus replication correlates with the induction of two Mx-related proteins in bovine cells. Virology. 1988 Jan;162(1):181–186. doi: 10.1016/0042-6822(88)90407-2. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Shaw M., Broni B., Shapiro G., Haller O. Inhibition of influenza viral mRNA synthesis in cells expressing the interferon-induced Mx gene product. J Virol. 1985 Oct;56(1):201–206. doi: 10.1128/jvi.56.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDENMANN J. INHERITANCE OF RESISTANCE TO INFLUENZA VIRUS IN MICE. Proc Soc Exp Biol Med. 1964 Jun;116:506–509. doi: 10.3181/00379727-116-29292. [DOI] [PubMed] [Google Scholar]
- Lindenmann J., Deuel E., Fanconi S., Haller O. Inborn resistance of mice to myxoviruses: macrophages express phenotype in vitro. J Exp Med. 1978 Feb 1;147(2):531–540. doi: 10.1084/jem.147.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen P., Nielsen S., Celis J. E. Monoclonal antibody specific for human nuclear proteins IEF 8Z30 and 8Z31 accumulates in the nucleus a few hours after cytoplasmic microinjection of cells expressing these proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2083–2089. doi: 10.1083/jcb.103.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Horisberger M. A. Combined action of mouse alpha and beta interferons in influenza virus-infected macrophages carrying the resistance gene Mx. J Virol. 1984 Mar;49(3):709–716. doi: 10.1128/jvi.49.3.709-716.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noteborn M., Arnheiter H., Richter-Mann L., Browning H., Weissmann C. Transport of the murine Mx protein into the nucleus is dependent on a basic carboxy-terminal sequence. J Interferon Res. 1987 Oct;7(5):657–669. doi: 10.1089/jir.1987.7.657. [DOI] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Shii K., Roth R. A. Inhibition of insulin degradation by hepatoma cells after microinjection of monoclonal antibodies to a specific cytosolic protease. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4147–4151. doi: 10.1073/pnas.83.12.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Dreiding P., Haller O., Lindenmann J. Polyclonal and monoclonal antibodies to the interferon-inducible protein Mx of influenza virus-resistant mice. J Biol Chem. 1985 Feb 10;260(3):1821–1825. [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986 Jan 17;44(1):147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- Staeheli P., Haller O. Interferon-induced Mx protein: a mediator of cellular resistance to influenza virus. Interferon. 1987;8:1–23. [PubMed] [Google Scholar]
- Staeheli P., Haller O. Interferon-induced human protein with homology to protein Mx of influenza virus-resistant mice. Mol Cell Biol. 1985 Aug;5(8):2150–2153. doi: 10.1128/mcb.5.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Horisberger M. A., Haller O. Mx-dependent resistance to influenza viruses is induced by mouse interferons alpha and beta but not gamma. Virology. 1984 Jan 30;132(2):456–461. doi: 10.1016/0042-6822(84)90050-3. [DOI] [PubMed] [Google Scholar]