Abstract
Rationale
Adolescence marks a period of increased vulnerability to the development of substance use disorders. High sweet preference is a genetically mediated behavioral trait that also predicts vulnerability to substances of abuse. Previous research has shown that while adolescent rats selectively bred for high (HiS) saccharin intake acquire cocaine self-administration at the same rate as adult HiS rats, adolescent rats bred for low saccharin intake (LoS) acquire cocaine self-administration faster than adult LoS rats.
Objectives
To investigate the interaction of the addiction vulnerability factors of peri-adolescence and saccharin preference on cocaine-intake using an animal model of escalation of cocaine intake over 6-h/day sessions.
Methods
Peri-adolescent and adult HiS and LoS female rats self-administered i.v. cocaine (0.4 mg/kg/inf) during short-access (2-h/day) sessions for 2 days. Next, a long-access (6-h/day) period (LgA) commenced and lasted 16 days. Following LgA, session length was returned to 2-h/day for a second short access phase.
Results
LoS peri-adolescent rats escalated cocaine intake over the LgA period and consumed more drug than LoS adult rats; however, peri-adolescent and adult HiS rats consumed similar amounts of cocaine during this period. Additionally, adult HiS rats self-administered more cocaine than adult LoS rats during the LgA period, while there was no phenotypic difference between the rat lines during peri-adolescence for the LgA period. During the first short-access phase, peri-adolescent rats self-administered more cocaine than adult rats.
Conclusions
These results emphasize the importance of adolescent drug abuse prevention by illustrating that phenotypic protection from addiction may not be expressed until adulthood.
Keywords: Addiction, Adolescence, Bingeing, Cocaine, Escalation, Extended Access, Long Access, Saccharin, Sweet Preference, Selective Breeding
Introduction
The transitional period of adolescence is marked by enhanced behavioral (e.g., high impulsivity) and neurobiological indicators of drug use vulnerability, accompanied by increased sensitivity to the rewarding effects and decreased sensitivity to the aversive effects of abused drugs (for reviews, see Laviola et al. 1999; Spear 2011; Spear and Varlinskaya 2010). Preclinical research has associated these features of adolescence with increased drug consumption by showing that adolescent rats self-administer more psychostimulants (Anker et al. 2012; Levin et al. 2003), ethanol (Brunell and Spear 2005; Doremus et al. 2005), and opioids (Doherty et al. 2009; Doherty and Frantz 2012) compared to adults. Adolescent (vs. adult) rats also show greater reinstatement of cocaine-seeking behavior in an animal model of relapse (Anker and Carroll 2010).
Similar to adolescence, higher preference for sweetened dietary substances is an individual characteristic that corresponds with elevated vulnerability to substance abuse. Drug-dependent humans, for instance, display greater preference for sweetened dietary substances compared to non-dependent individuals (Kampov-Polevoy et al. 2001; Pepino and Mennella 2007). Additional animal research has suggested that sweet preference is a genetically mediated marker of addiction vulnerability (for review, see Carroll et al. 2008). For example, rats that have been selectively bred for high saccharin intake (HiS) consume more ethanol and show greater cocaine-primed reinstatement of drug-seeking behavior compared to rats bred for low saccharin intake (LoS) (Dess et al. 1998; Perry et al. 2006). Also corresponding to the drug-vulnerable characteristics of adolescence, adult HiS (vs. adult LoS) rats show less aversion to negative aspects of drug taking behavior, such as withdrawal following extended ethanol exposure (Dess et al. 2005). Moreover, HiS rats are more impulsive than LoS rats as measured by the delay-discounting and go/no-go tasks (Anker et al. 2008; Perry et al. 2007b). Thus, age and saccharin preference contribute to substance abuse vulnerability; however, little is known about how these vulnerability factors add or interact.
Given that drug use leading to addiction often begins in adolescence (Nixon and McClain 2010), it is important to determine whether age is a vulnerability factor in rats that are genetically predisposed to self-administer relatively more (i.e., HiS) or less (i.e., LoS) amounts of abused drugs, especially during a critical, transitory phase of addiction, escalation. A previous experiment conducted by Perry et al. (2007a), for example, showed that adolescent and adult HiS rats acquired cocaine self-administration at the same rate. However, LoS adolescent rats acquired self-administration faster than adult LoS rats. These data suggest that the phenotype of decreased avidity for drug consumption (e.g., LoS) may not be expressed until adulthood.
The aim of the present study was to further explore this relationship between age and saccharin-preferring vs. –resistant endophenotypes under periods of extended access to cocaine. During conditions of extended drug access, animals tend to steadily escalate their drug intake. This bingeing is considered to be an animal model of the transition from regulated to dysregulated drug use and addiction (Koob and Kreek 2007), although recent research suggests that escalation of drug intake reflects the normal process of discrimination learning (Beckmann et al. 2012). Nonetheless, previous research has shown that individual differences influence drug-taking behavior during periods of extended access to drug. For instance, adult HiS rats escalate cocaine self-administration at faster rates than adult LoS rats during conditions of extended drug access (Perry et al. 2006). Considering the results of Perry et al. (2007a or b), it was hypothesized that HiS peri-adolescents and adults would self-administer similar amounts of cocaine during the extended-access period, while LoS peri-adolescents would escalate cocaine self-administration faster and consume more cocaine than LoS adults.
Methods
Subjects
Adult (N = 16) and peri-adolescent (N = 18) female Sprague Dawley rats served as subjects in this study. These rats were selected from a breeding program maintained at the University of Minnesota (Carroll et al 2002), originating with the Occidental HiS and LoS lines (Occidental College, Los Angeles, CA). The lines were established by mating rats based on extreme phenotype scores with no sibling, half-sibling, or first cousin pairings. Phenotype scores were assessed using a 24-h two-bottle test (see Badia-Elder et al. 1996 for details), in which consumption of 0.1% saccharin solution was compared to previously attained 24-h water intake and adjusted for body weight [saccharin score = (saccharin mL – water baseline mL, divided by body weight, X 100)]. HiS and LoS adult and peri-adolescent rats comprised the four experimental groups. Table 1 shows sample sizes saccharin scores, body weights and ages when reaching acquisition criteria for self-administration as well as after completion of the study.
Table 1.
Group Age, Weight and Saccharin Score Averages
| Beginning of study (Pre-LgA) | End of study (Post-LgA) | |||||||
|---|---|---|---|---|---|---|---|---|
| Peri-Adolescent | Adult | Peri-Adolescent | Adult | |||||
| HiS (n=11) | LoS (n=7) | HiS (n=10) | LoS (n=8) | HiS | LoS | HiS | LoS | |
| Age (days) | 41.8 | 42.8 | 135.5 | 141.4 | 67.1 | 66.7 | 155.5 | 166.1 |
| (±SEM) | 2.3 | 2.1 | 5.1 | 11.0 | 3.0 | 2.4 | 5.2 | 8.8 |
| Weight (g) | 128.5 | 145.4 | 272.0 | 281.7 | 171.1 | 182.1 | 260.5 | 273.3 |
| (±SEM) | 11.7 | 12.7 | 5.9 | 4.0 | 11.7 | 8.2 | 6.5 | 4.6 |
| Sacc. Scorea | 38.4b | 20.1 | 24.6b | 13.9 | ||||
| (±SEM) | 5.9 | 5.3 | 6.9 | 4.3 | ||||
Saccharin phenotype score={[24-h saccharin intake (ml) average water intake (ml)/weight (g)]×100} (Badia-Elder et al. 1996)
Indicates that HiS rats had significantly greater scores compared to LoS rats after collapsing saccharin phenotype across age (p < .05)
Rats were pair-housed and bred in plastic cages with ad libitum access to rat pellet chow (Purina Mills, Minneapolis, MN, USA) and water before the experiment. Temperature (21–23°C), light-dark cycle (lights on at 6:00 a.m.; 12-h on, 12-h off), and humidity were all regulated. Following surgery, and throughout housing in operant conditioning chambers, adult rats were given access to 16 g of ground rat chow (Purina Mills) between 3:00 p.m. and 8:00 a.m. the following day. Peri-adolescent rats had ad libitum access to ground rat chow between 3:00 p.m. and 8:00 a.m. the following day until their daily amount of food consumption reached 16 g. At that point, they were restricted to 16 g/day of chow for the remainder of the study. All rats had continuous access to water throughout the experiment. Experimental procedures were approved by The University of Minnesota Institutional Care and Use Committee under Protocol #1007A85632, and they complied with the Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al. 2011)
Surgery
Peri-adolescent rats were implanted with intravenous catheters before adolescence on postnatal day (PND) 21, while adult rats received catheters on approximately PND 100. Intravenous catheters were surgically implanted using procedures previously described (Anker et al. 2009). Rats were allowed three days to recover from surgery (including day of surgery) and received four total subcutaneous buprenorphine injections (one on the day of surgery, two the following day, and one the day after that; 0.05 mg/kg) for analgesia, as well as heparinized saline (20 USP units/mL, 0.3 mL/rat) and baytril (22.7 mg/mL, 0.1 mL/rat) to prevent infection and catheter occlusion. Catheter patency was assessed weekly with a combination of ketamine (100 mg/mL), midazolam (5 mg/mL), and saline (3:3:14 ratio, 0.10 mL/adult rats, 0.50 mL/adolescent rat), and was documented by loss of the righting reflex following infusion of this combination. Failing patency, a second catheter was implanted in the opposite jugular vein following the surgical procedures described above, and the rats were allowed to recover for three days before resuming experimental sessions. Two animals from each experimental group received a second surgery to reimplant a catheter.
Apparatus
Following surgery and throughout the experiment, rats were housed in custom-made, octagon-shaped operant conditioning chambers described elsewhere (Carroll et al. 1981). Briefly, chambers contained two levers (one active, one inactive) and two stimulus lights above each lever, as well as access to food and a drinking spout. Levers for peri-adolescent and adult rats were situated approximately 3.5 and 7 cm from the floor of the cage, respectively. This differential placement of levers was an effort to equate the performance demands of lever pressing between the age groups. Programming, data collection, and data storage for all experimental sessions were handled by Med-PC software and PCs equipped with a Med-PC interface (Med Associates). The cocaine solution (1.6 mg/mL) was prepared by dissolving cocaine HCL (National Institute of Drug Abuse, Research Triangle Institute, Research Triangle Park, NC) in sterile saline (0.9% NaCl) and adding heparin (5 USP units/mL) to prevent catheter thrombus formation. The cocaine infusion rate was 0.025 mL/s, and the solution was dispensed at an interval of 1 s/100 g body weight resulting in 0.4 mg/kg per infusion (i.v.). Thus, while the infusion rate was the same for all animals, the infusion duration was positively related to animal weight.
Procedure
Cocaine self-administration training
Rats were trained to self-administer cocaine (0.4 mg/kg/infusion) during daily 2-h sessions (9:00 a.m.–11:00 a.m.) using a fixed ratio (FR1) schedule of reinforcement. During training, the infusion-paired lever was baited with a small amount of peanut butter and rats were given three experimenter-delivered priming infusions until they acquired stable cocaine self-administration behavior. The criterion for stable cocaine self-administration was ≥25 infusions per session for two sessions without lever baiting or priming infusions. Rats were required to have an active to inactive lever response ratio of at least 2:1. The ratio requirement was established to confirm drug-reinforced operant responding as opposed to non-drug conditioned reinforcement (i.e., responding for stimulus lights) or incidental lever responses due to cocaine-induced locomotor activity.
Pre-LgA short-access period
Once stability criteria were met, rats maintained cocaine self-administration during daily sessions (9:00 a.m.–11:00 a.m.) for two days (2-h/day), comprising the first short-access period preceding the long-access (LgA) period. Previous clinical research has shown that the initiation of cocaine use and transition into dependence in humans occurs primarily in late adolescence/early adulthood (Ridenour et al. 2006). Although estimates vary, adolescence in the female rat has been suggested to span PND 28–42 (Spear 2000). The present study included multiple phases of the addiction process, including the transitional phases of initiation and bingeing (e.g., acquisition, maintenance, escalation). To be included in the study, rats catheterized on PND 21 were considered to be peri-adolescent if they reached the pre-LgA phase on or before PND 53. The average age of peri-adolescent rats when reaching the first short access period was PND 42 (see Table 1 for mean rat ages).
Long-access (LgA) period
Following the pre-LgA phase, session length was extended to 6-h/day (9:00 a.m.–3:00 p.m.), and rats continued to self-administer cocaine (0.4 mg/kg/infusion) for 16 days. While LgA sessions typically last for 21 or more days (Koob and Kreek 2007), Anker et al. (2012) demonstrated that adolescent rats escalated methamphetamine intake over 15 days of extended access while adults did not. Therefore, the present study employed a similarly abbreviated procedure to accommodate the narrow window of adolescence/peri-adolescence in the rat.
Post-LgA short-access period
Following the LgA period, a second short-access phase began in which session length was decreased to 2-h (9:00 a.m.–11:00 a.m.) for 2 days.
Saccharin test
Following the post-LgA phase, animals were removed from their operant conditioning chambers and individually housed in plastic cages. After 14 days, they were tested for saccharin phenotype scores using the same procedure described above.
Data analysis
Pre- and Post-LgA phases
For the pre- and post-LgA phases, responses on the infusion-paired (active) and non-infusion paired (inactive) levers and cocaine infusions served as the dependent measures. These data were averaged between the two days of each short-access phase, and compared using mixed-factorial three-way analyses of variance (ANOVA). Between-subjects factors consisted of phenotype (HiS vs. LoS), age (peri-adolescent vs. adult), and phase (pre-LgA vs. post-LgA) was the repeated measure (phenotype × age × phase). Post-hoc analyses were conducted using the Tukey-Kramer procedure. All statistical analyses were conducted using GB Stat software (Dynamic Microsystems, Silver Spring, MD), and results were considered significant if p≤.05.
LgA phase
For the LgA phase, responses on the active and inactive levers and cocaine infusions served as the dependent measures. Within each dependent measure type, data were averaged into 4 blocks of 4 days and analyzed using 4 separate mixed-factorial, two-way ANOVA (illustrated by the separate panels in Figs. 2 and 3). Age (HiS peri-adolescent vs. HiS adult; LoS peri-adolescent vs. LoS adult) or phenotype (HiS peri-adolescent vs. LoS peri-adolescent; HiS adult vs. LoS adult) served as the between-subjects factors, and session block served as the within-subjects factor. Post-hoc analyses were conducted using the Tukey-Kramer procedure.
Fig. 2.
Mean (±SEM) active lever responses made during the period of long access (LgA) to cocaine. Responses were compared between HiS adults and peri-adolescents (panel a), HiS peri-adolescents and LoS peri-adolescents (panel b), LoS adolescents and adults (panel c), and HiS adults and LoS adults (panel d). The * indicates that LoS adolescents made significantly more responses during the last block of 4 days compared to LoS adults (p<.01)
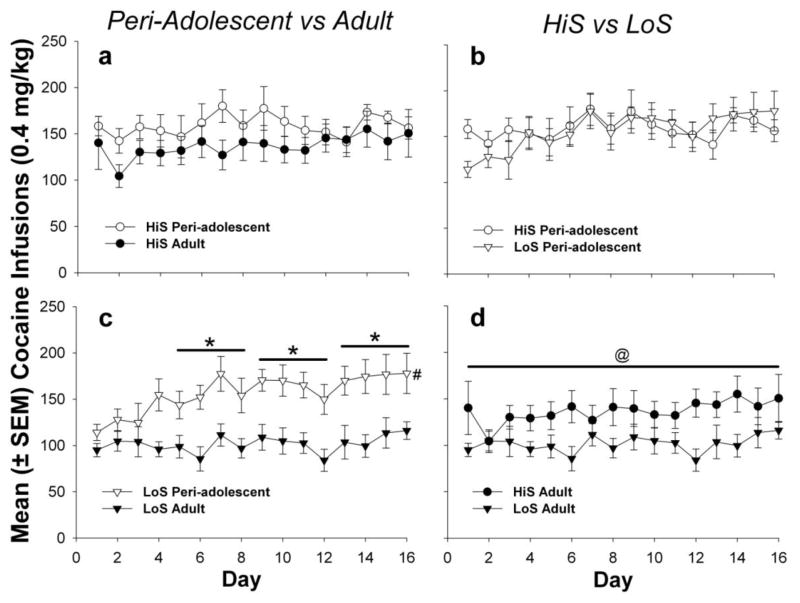
Fig. 3.
Mean (±SEM) cocaine infusions self-administered during the period of long access (LgA) to cocaine. Infusions were compared between HiS adults and adolescents (panel a), HiS adolescents and LoS adolescents (panel b), LoS adolescents and adults (panel c), and HiS adults and LoS adults (panel d). The * indicates that LoS adolescents self-administered more infusions during the second, third, and fourth block of 4 days than LoS adults (p<.01). The @ indicates a main effect of phenotype such that and that HiS adults earned more infusions than LoS adults during the LgA phase (p<.05). The # indicates that the LoS adolescent group self-administered more cocaine on the last block of 4 days compared to the first, indicating an escalation effect
Saccharin Phenotype Scores
Saccharin phenotype scores were analyzed using 2-way ANOVA (age × phenotype). Post-hoc analysis was conducted using the Student’s t-test.
Results
Pre- and Post-LgA phases
For responses on the active lever (see Fig. 1, panel a), there was only a main effect for age (F(1,33) =5.49; p<.05); thus, data were collapsed across phenotype and pre-/post-LgA phases. A Student’s t-test showed that peri-adolescent rats made more active lever responses than adults rats (t(59)=2.00; p<.05). For infusions self-administered during the pre-/post-LgA access phases (see Fig. 1, panel b), there was a main effect for age (F(1,33)= 11.0; p<.01) and short-access period (F(1,29)=4.4; p<.05), so data were collapsed across phenotype. Post hoc analyses indicated that peri-adolescent rats self-administered more cocaine infusions during pre-LgA compared to adult rats (p<.01). For inactive lever presses, there was a main effect of age (F(1,33)=5.76; p<.05) (data not shown). However, when data were collapsed across phenotype and short-access periods, Student’s t-test revealed no significant differences between peri-adolescents and adults.
Fig. 1.

Mean (±SEM) active lever responses (panel a) and infusions (panel b) during periods of short access before and after the long-access period (pre- and post-LgA). The @ indicates a main effect such that peri-adolescent rats made more responses than adults when data were collapsed across phenotype and short-access period (p<.05). The * indicates that peri-adolescent rats self-administered more cocaine infusions than adults during the pre-LgA period (p<.01)
LgA phase
When comparing active lever responses between LoS peri-adolescent and LoS adult rats, there was a main effect of age (F(1,45)=4.78; p<.05) and an interaction between age and block (F(3,59)=3.20; p<.05). Post-hoc analyses showed that LoS peri-adolescent rats made significantly more active lever responses than LoS adults on the last block of 4 days (p<.01) (see Fig. 2, panel c). There were also main effects for age (F(1,45)=13.22; p<.01), block (F(3,59)=7.19; p<.01), and an interaction between age and block (F(3,59)=3.98; p<.05) when comparing infusions between LoS peri-adolescent and LoS adult rats. Post-hoc analyses showed that LoS peri-adolescent rats self-administered more infusions than LoS adults during the 2nd, 3rd, and 4th block of days (p<.01) (see Fig. 3, panel c). LoS peri-adolescent rats also self-administered more infusions during the last compared to the first block of days (p<.01). Additionally, there was a main effect of phenotype (F(1,48)=4.55; p=.05) when comparing infusions between HiS adults and LoS adults. (see Fig. 3, panel d). No significant differences were found in inactive responses in the LgA phase (data not shown).
Saccharin phenotype scores
Saccharin scores confirmed the selective breeding phenotype. For saccharin phenotype scores, there was only a main effect for phenotype (F(1,24)=6.55; p<.05); thus, data were collapsed across age. Student’s t-test showed that HiS rats had significantly higher saccharin scores than LoS rats (t(26)=2.67; p<.05) (see Table 1).
Discussion
The results from the present study indicate that LoS peri-adolescent rats self-administered the same amount of cocaine as HiS peri-adolescent rats during the LgA phase. In contrast, HiS adult rats self-administered more cocaine than LoS adult rats during this period. Furthermore, the LoS peri-adolescent rats escalated their cocaine intake over the LgA phase. HiS peri-adolescents, however, did not escalate their cocaine intake during LgA, most likely due to ceiling effects indicated by the HiS peri-adolescent rats’ greater initial rate of self-administration during this phase compared to the LoS peri-adolescents. Although a previous study has shown that HiS adult rats escalate their cocaine self-administration at faster rates than LoS adult rats over 21 days of 12-h sessions (Perry et al. 2006), neither the HiS nor the LoS adults escalated cocaine self-administration across the 6-h/day sessions, a result also found in a previous study using the 6-h/day session length (Holtz and Carroll 2011). Additionally, the LoS peri-adolescents consumed more cocaine than the LoS adults, although there was no age difference in the HiS rats.
While the present study used female animals, we did not monitor their estrous cycles for several reasons. Importantly, we did not want to introduce any untoward stress-related confounds as a result of vaginal cytological sample collection, particularly given the developmental differences between adult and adolescent female rats. Additionally, while the effects of estrous cycle appear to influence progressive ratio responding for drug (Roberts et al. 1989) and reinstatement of drug-seeking behavior (Feltenstein et al. 2011), previous research has not established a relationship between self-administration of cocaine during an FR1 schedule of reinforcement and estrous cycle phase during LgA in female rats (Larson et al. 2007; Perry et al. 2006).
The main finding of the present experiment is that phenotypic variance in cocaine self-administration expected between the HiS and LoS adult rats (HiS>LoS) was not found in adolescent HiS and LoS rats. These results agree with a study by Perry et al. (2007a), which showed age effects in acquisition rates of cocaine self-administration in LoS rats (adolescents>adults) but not HiS rats. Since the acquisition and escalation phases are preclinical models of the critical human phases of initiation of drug use and the transition binge-level drug consumption and dependence, these results may help us understand the scope of adolescent addiction vulnerability. That is, with regard to these critical phases, latent phenotypes that engender decreased risk for compulsive drug consumption in some individuals may have less influence during adolescence, a finding that has also been established in human research. For instance, Ridenour et al. (2006) showed no relationship between paternal cocaine addiction and the rate of transition from first use of the drug to problem drug use in offspring. Insofar as paternal addiction serves as a proxy for genetic influence on addiction vulnerability (without accounting for environmental factors), these data and results from the present study suggest that the age of cocaine use initiation may be a useful predictor of subsequent cocaine dependence. However, clinical research has robustly illustrated that a substantial portion of the variance in drug use and severity of dependence between individuals can be accounted for by genetic variance (Agrawal and Lynskey 2008; Ducci and Goldman 2008; Mayfield et al. 2008). Future work with models in which animals are selected or selectively bred based on extremely high or low measures of substance abuse-related criteria (Bell et al. 2006; Blanchard et al. 2009; Giorgi et al. 2007; Kosten and Ambrosio 2002; Riley 2011; Sommer et al. 2006) may help us better understand how the factors of genes and age ultimately manifest substance dependence and direct its trajectory. Depending on the research question being addressed, the translational value of these models may be strengthened with the use of adolescent or periadolescent animals in addition to adult animals. Importantly, future research may investigate the neurobiological mechanisms responsible for compromising the drug-resilient phenotype particular to adult LoS rats. Likely loci of interest are the mesolimbic dopamine system, prefrontal cortex and hypothalamic-pituitary-adrenal axis (Doremus-Fitzwater et al. 2010; Sinha 2008). Such data could inform personalized treatment strategies that account for age as well as phenotype.
In conclusion, we found that the drug-prone and -protected phenotypes particular to the HiS and LoS animals were expressed in adult but not peri-adolescent rats during a period of extended access to cocaine. Since the initiation of most human substance dependence disorders occurs in adolescence, these results underscore the importance of age as a vulnerability factor. Future preclinical work further examining the additive or interactive effects of age and phenotype will broaden our understanding of substance dependence development in humans.
Acknowledgments
We thank Justin Anker, Seth Johnson, Amy Saykao, and Natalie Zlebnik for their technical assistance. This research was supported by NIDA/NIH grants RO1 DA003240, P20 DA024196, K05 DA015627 (MEC).
Footnotes
The authors have no conflicts of interest to report.
References
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–81. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Baron TR, Zlebnik NE, Carroll ME. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–22. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19:615–29. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME. Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 2009;203:63–72. doi: 10.1007/s00213-008-1371-9. [DOI] [PubMed] [Google Scholar]
- Badia-Elder N, Kiefer SW, Dess NK. Taste reactivity in rats selectively bred for high vs. low saccharin consumption. Physiol Behav. 1996;59:749–55. doi: 10.1016/0031-9384(95)02131-0. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Gipson CD, Marusich JA, Bardo MT. Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–54. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–53. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–7. [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–60. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–8. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Dess NK, O’Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol. 2005;37:9–22. doi: 10.1016/j.alcohol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92:164–72. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JM, Frantz KJ. Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology (Berl) 2012;219:763–73. doi: 10.1007/s00213-011-2398-x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–28. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 2011;216:53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi O, Piras G, Corda MG. The psychogenetically selected Roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neurosci Biobehav Rev. 2007;31:148–63. doi: 10.1016/j.neubiorev.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Baclofen has opposite effects on escalation of cocaine self-administration: increased intake in rats selectively bred for high (HiS) saccharin intake and decreased intake in those selected for low (LoS) saccharin intake. Pharmacol Biochem Behav. 2011;100:275–83. doi: 10.1016/j.pbb.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol. 2001;36:165–70. doi: 10.1093/alcalc/36.2.165. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–71. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–9. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. Br J Pharmacol. 2008;154:275–87. doi: 10.1038/bjp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.); 2011. [PubMed] [Google Scholar]; Guide for the care and use of laboratory animals. 8. National Academies Press, National Academies Press; [Google Scholar]
- Nixon K, McClain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry. 2010;23:227–32. doi: 10.1097/YCO.0b013e32833864fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–9. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Anderson MM, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav. 2007a;91:126–33. doi: 10.1016/j.physbeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology (Berl) 2006;186:235–45. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007b;86:822–37. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Lanza ST, Donny EC, Clark DB. Different lengths of times for progressions in adolescent substance involvement. Addict Behav. 2006;31:962–83. doi: 10.1016/j.addbeh.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer W, Hyytia P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–43. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]