Abstract
Rational
Sweet preference is a marker of vulnerability to substance use disorders, and rats selectively bred for high (HiS) vs. low saccharin (LoS) intake display potentiated drug-seeking behaviors. Recent work indicated that LoS rats were more responsive to the negative effects of drugs in several assays.
Objective
The current study used the intracranial self-stimulation (ICSS) procedure to investigate the anhedonic component of morphine withdrawal in male HiS and LoS rats.
Methods
Rats were administered morphine (10 mg/kg) or saline for 8 days. To evaluate withdrawal effects, reward thresholds were measured 24 and 28 h following the 8th morphine injection (spontaneous withdrawal) and again for 4 days following daily acute morphine and naloxone (1 mg/kg) administration (precipitated withdrawal).
Results
Twenty-four hr following the final morphine injection, reward thresholds in LoS rats were significantly elevated compared to reward thresholds in LoS controls, indicating spontaneous withdrawal. This effect was not observed in HiS rats. LoS rats also showed greater elevations of reward thresholds on several days during naloxone-precipitated withdrawal compared to their HiS counterparts.
Conclusions
LoS rats were more sensitive to morphine withdrawal-mediated elevations in ICSS thresholds than HiS rats. While these differences were generally modest, our data suggest that severity of the negative affective component of opiate withdrawal may be influenced by genotypes related to addiction vulnerability.
Keywords: addiction vulnerability, individual differences, intracranial self-stimulation, morphine withdrawal, selective breeding, sweet preference
1. Introduction
High sweet preference is an indicator of drug abuse liability (Carroll and Holtz, 2014; Holtz and Carroll, 2014). In humans, drug-dependent individuals showed greater preference for sweetened dietary substances than nondependent individuals (Kampov-Polevoy et al., 2001; Pepino and Mennella, 2007), and a history of drug abuse was associated with avidity for higher concentration of sweet substances (Janowsky et al., 2003; Pomerleau et al., 1991). Animal research has also demonstrated that sweet preference is a genetically mediated marker for addiction susceptibility. For instance, rats selectively bred for high saccharin consumption (HiS) drink more ethanol (Dess et al., 1998), demonstrate greater locomotor response to cocaine (Carroll et al., 2007), higher rates of reinstatement of cocaine seeking and greater cocaine intake (Holtz and Carroll, 2011; Holtz and Carroll, 2013; Perry et al., 2006) than rats bred for low saccharin consumption (LoS).
In addition to positive rewarding effects, negative drug effects contribute to the development of addiction (Koob and Volkow, 2010). These effects include heightened stress reactivity, anxiety, and anhedonia elicited during drug withdrawal (Schulteis et al., 1994). HiS and LoS animals show differential responses when withdrawn from ethanol and morphine (Dess et al., 2005; Radke et al., 2013), as well as non-drug reinforcers like glucose (Yakovenko et al., 2011), with most of these studies demonstrating heightened vulnerability in LoS animals. Furthermore, we have shown that LoS (vs. HiS) rats are more sensitive to the effects of i.v. histamine punishment on cocaine self administration (Holtz et al., 2013), suggesting a more general link between phenotypic sensitivity to aversive events and addiction vulnerability.
In the present study, the negative, anhedonic effects of spontaneous and naloxone-precipitated morphine withdrawal were assessed using an intracranial self-stimulation (ICSS) threshold procedure. Cessation of drug exposure elevates the minimal (threshold) electrical stimulation intensity that maintains ICSS, a putative measure of the diminished sensitivity to rewarding stimuli (anhedonia) associated with withdrawal (Schulteis et al., 1994). Given the differential vulnerability of HiS and LoS rats to the negative effects of drugs in other assays, it was hypothesized that LoS compared to HiS rats would demonstrate increased threshold elevations during spontaneous and naloxone-precipitated morphine withdrawal.
2. Results
2.1 Baseline measures (Phase 1)
No differences were found in baseline threshold measures between groups (see Table 3). There was a main effect of phenotype for baseline latency measures [F(1,27) = 4.33, p < .05]. Overall, HiS animals had shorter average baseline latencies (2.7 sec ± 0.2 SEM) compared to LoS animals (3.1 sec ± 0.1 SEM).
Table 3.
Experimental Group Information
| HiS MOR | LoS MOR | HiS SAL | LoS SAL | |
|---|---|---|---|---|
| Mean (±SEM) saccharin scorea | 13.2 ± 3.0 | 11.0 ± 2.3 | 14.7 ± 2.2 | 8.7 ± 1.7 |
| Mean (±SEM) ICSS threshold baseline(μA) for phase 2 (morphine dependence induction) | 152.6 ± 27.1 | 133.3 ± 8.9 | 136.6 ± 29.4 | 136.46 ± 10.0 |
| Mean (±SEM) response latency (s) baseline for phase 2 (morphine dependence induction) | 2.7 ± 0.3* | 3.0 ± 0.1 | 2.6 ± 0.1* | 3.1 ± 0.1 |
| Mean (±SEM) ICSS threshold baseline (μA) for phase 4 (precipitated withdrawal) | 152.0 ± 25.5 | 136.4 ± 7.6 | 132.1 ± 22.8 | 136.1 ± 8.1 |
| Mean (±SEM) response latency (s) baseline for phase 4 (precipitated withdrawal) | 2.7 ± 0.3 | 2.7 ± 0.2 | 2.4 ± 0.2 | 3.1 ± 0.1 |
Saccharin phenotype score = {[24-h saccharin intake (mL)–average water intake (mL)/weight (g)]×100}
Main effect of phenotype (HiS<LoS; p < .05)
HiS = high saccharin rats; LoS = low saccharin rats; MOR = morphine; SAL = saline
2.2 Spontaneous Withdrawal (Phase 3)
2.2.1 ICSS Thresholds
Analysis of changes in thresholds in HiS and LoS rats 24 and 28 hr after their last morphine or saline injection revealed a main effect of treatment (Fig. 1) [F(1,22) = 27.2, p < .0001]. Post hoc analysis showed that LoS rats treated with MOR+SAL had a greater increase in thresholds 24 h after their last morphine injection compared to LoS control animals treated with SAL+SAL (p < .05), indicating a significant withdrawal effect. This effect was not observed in HiS rats, nor were there differences HiS and LoS rats treated with MOR+SAL. In contrast, both LoS and HiS rats treated with MOR+SAL showed increases in thresholds 28 h following their last morphine injection compared to their respective control groups (p < .05).
Fig. 1.
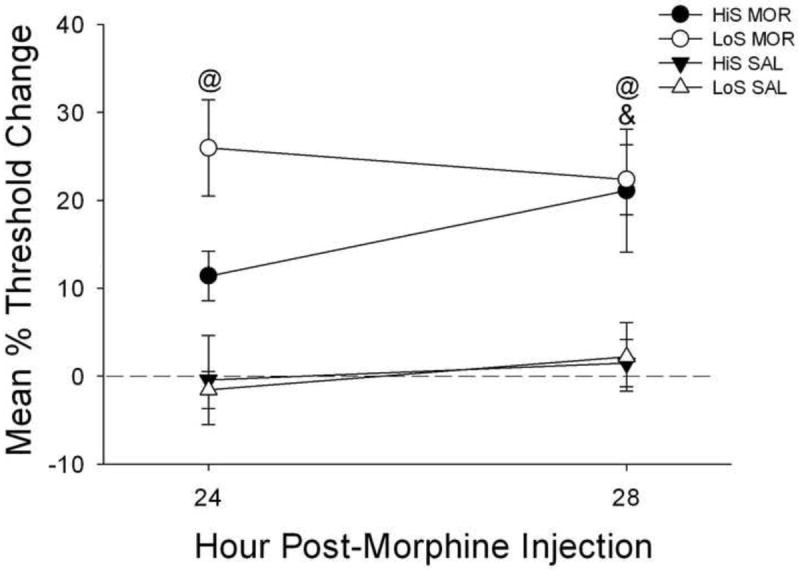
Mean (±SEM) percent changes in intracranial self-stimulation reward thresholds compared to baseline in HiS and LoS rats 24- and 28-h following the last of 8 daily injections of morphine+saline (MOR) or saline+saline (SAL). @ indicates that LoS MOR rats had a greater percent increase in reward threshold compared to LoS rats treated with SAL. & indicates that HiS MOR rats had a greater percent increase in reward thresholds compared to HiS rats treated with SAL.
2.2.2 Latency
There were no differences in ICSS latencies in either HiS or LoS rats during the spontaneous withdrawal phase (data not shown).
2.3 Precipitated Withdrawal (Phase 4)
2.3.1 ICSS Thresholds
No differences were found in baseline thresholds between groups for this phase (see Table 3). Analysis of changes in thresholds across the entire session during the precipitated withdrawal phase indicated only a main effect of treatment (Fig. 2) [F(1,27) = 96.7, p < .0001]. Post hoc comparisons showed that MOR+NAL animals had greater increases in thresholds compared to their SAL+NAL controls for each of the 4 days regardless of phenotype (p < .01).
Fig. 2.
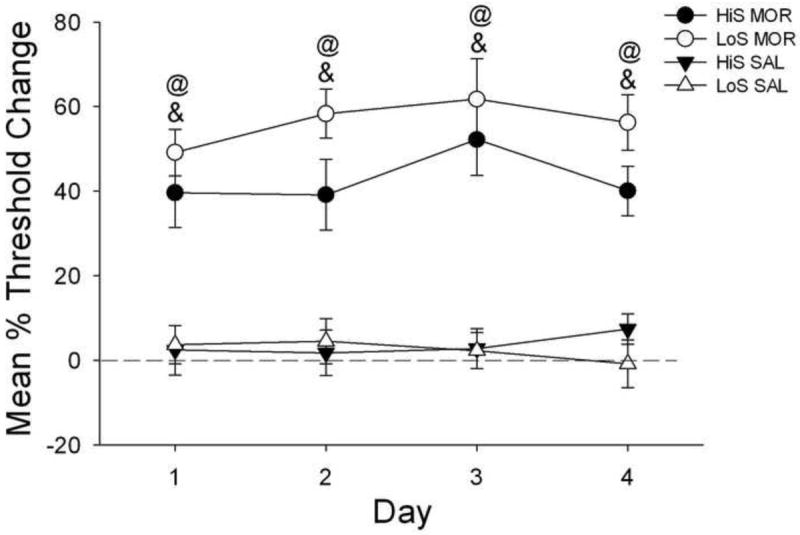
Mean (±SEM) percent changes in intracranial self-stimulation reward thresholds compared to baseline in HiS and LoS rats treated with either morphine+naloxone (MOR+NAL) or saline+naloxone (SAL+NAL) over 4 days. @ indicates that LoS MOR+NAL rats had a greater percent increase in reward threshold compared to LoS SAL+NAL. & indicates that HiS MOR+NAL had a greater percent increase in reward threshold compared to HiS SAL+NAL rats.
Data from the first and second halves of session during this phase were also analyzed separately (see Data Analysis section). There were main effects for treatment [F(1,27) = 77.1, p < .0001] and day [F(3,81) = 2.8, p < .05] following the analysis of threshold changes during the first half of session (Fig. 3A). Post hoc comparisons showed that all MOR+NAL groups had greater threshold increases than their SAL+NAL treated control groups for each day (p < .01). There were main effects for treatment [F(1,27) = 47.7, p < .0001] and day [F(3,81) = 3.7, p < .05], as well as interaction effects between treatment and phenotype [F(1,93) = 10.0, p < .005] and treatment, phenotype and day [F(3,123) = 2.9, p < .05] following the analysis of threshold changes for the second half of session (Fig. 3B). Post hoc comparisons showed that LoS rats treated with MOR+NAL had greater threshold increases on Days 2 (p < .05) and 4 (p < .01) compared to HiS rats treated with MOR+NAL. In contrast, these groups did not differ on Days 1 and 3. Compared to their SAL+NAL controls, LoS rats treated with MOR+NAL showed greater threshold increases across all days (p < .01), while HiS rats treated with MOR+NAL only showed greater threshold increases on Days 1 and 3 (p < .01). HiS rats treated with SAL+NAL showed greater threshold increases on Days 2 (p < .05) and 4 (p < .01) compared to LoS rats treated with SAL+NAL.
Fig. 3.
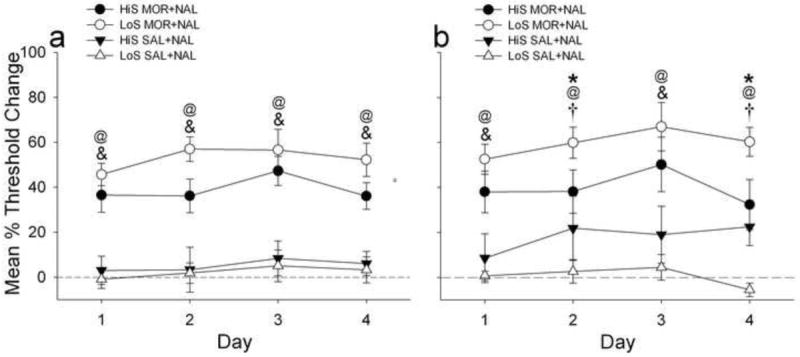
Mean (±SEM) percent changes in intracranial self-stimulation reward thresholds compared to baseline in HiS and LoS rats treated with either morphine+naloxone (MOR+NAL) or saline+naloxone (SAL+NAL) over 4 days divided into the first (panel A) and second (panel B) halves of the session (see text for details). @ indicates that LoS MOR+NAL rats had a greater percent increase in reward threshold compared to LoS SAL+NAL. & indicates that HiS MOR+NAL had a greater percent increase in reward threshold compared to HiS SAL+NAL rats. * indicates that LoS MOR+NAL rats had a greater percent increase in reward threshold compared to HiS MOR+NAL rats. † indicates that HiS SAL+NAL rats had a greater percent increase in reward threshold compared to LoS SAL+NAL rats
2.3.2 Latency
No differences were found in baseline latency measures for this phase between groups (see Table 3). There was a main effect of phenotype [F(1,27) = 13.2, p < .005] following the analysis of change in latency from baseline, wherein HiS animals showed a greater increase in latency (17.6% ± 4.7 SEM) compared to LoS rats (3.3% ± 1.3 SEM). Post hoc analyses revealed no further differences.
2.4 Saccharin Scores
Overall, HiS rats had greater saccharin preference scores compared to LoS rats; however this difference was not significant (see Table 3).
3. Discussion
This study examined whether HiS and LoS rats, a selectively bred model of high and low drug abuse vulnerability, differed in their motivational response during morphine withdrawal as measured with the ICSS procedure. Twenty-four hours following the final morphine injection, reward thresholds in LoS rats were significantly elevated compared to reward thresholds in the LoS saline-treated group, indicating spontaneous withdrawal. In contrast, this effect was not observed in the HiS line. Spontaneous withdrawal was observed in both lines 28 h after drug exposure. Subsequently, following naloxone-precipitated withdrawal, LoS rats exhibited higher thresholds than HiS rats during the second half of the session on some test days. Overall, the aversive effects of spontaneous and precipitated morphine withdrawal were more pronounced in LoS rats than in HiS rats, although these differences were generally modest.
Our data may corroborate a general trend in which LoS rats are more sensitive to aversive or punishing events than HiS rats. For example, in previous studies, LoS rats showed greater ethanol withdrawal-induced anxiety and heightened withdrawal effects from discontinuation of a glucose solution, as well as greater latency of emergence and increased defecation in the novel open field (Dess et al., 2005; Yakovenko et al., 2011). Dess and co-workers have also noted more stress-induced anorexia and analgesia (Dess and Minor, 1996) than HiS rats (Dess et al., 2000b), and LoS rats also exhibited more emotional reactivity (e.g., subordination, hyperthermia) than HiS rats when competing with weight-matched HiS rats for food (Eaton et al., 2012). Recently, we found that i.v. histamine had a greater enduring suppressant effect on cocaine self-administration in LoS (vs. HiS) rats (Holtz et al., 2013) and that LoS, but not HiS, rats exhibit a sustained anhedonic state during withdrawal from chronic, continuous cocaine exposure (Radke et al., 2014). Notably, the latter study revealed a pattern of withdrawal severity similar to that observed here (LoS > HiS) using a different measure of anhedonia (responding for a sucrose reward) and a different drug of abuse. This phenotypic increase in sensitivity to aversive events may predict withdrawal severity and could help explain lower levels of opiate self-administration in LoS rats compared to HiS rats (e.g., Carroll et al., 2002). Specifically, increased anhedonia 24 h after drug exposure could decrease motivation to take drug or punish further drug-taking behavior (Doremus et al., 2003; O’Dell et al., 2006; O’Dell et al., 2007).
Our results using ICSS measures differ from previous work investigating morphine withdrawal in the HiS and LoS lines using potentiation of the acoustic startle reflex (a measure of anxiety-like behavior) and conditioned place aversion (CPA) to examine spontaneous and precipitated withdrawal from the same dose of morphine (10 mg/kg) (Radke et al., 2013). In these studies, in contrast to the greater sensitivity to the aversive effects of withdrawal measured using ICSS in the current study, LoS rats exhibited reduced spontaneous withdrawal-induced increases in the startle response following 7 days of morphine exposure, and reduced precipitated withdrawal-induced CPA following one exposure. The differences between this work and our recent studies (i.e., the present study and Radke et al., 2014) may be because different behavioral measures, such as ICSS, potentiated startle, and CPA, reflect different underlying emotional states and neural systems. For example, anhedonia as measured with ICSS is mediated by reduced responsiveness of the mesolimbic dopamine (reward) system, while anxiety as measured through startle is produced primarily through increased activity in extended amygdala (threat) circuitry (Lang et al., 2000; Nestler and Carlezon, 2006). Changes in measures of anxiety generally do not affect ICSS performance (Carlezon and Chartoff, 2007). Further work with the HiS and LoS lines should seek to confirm this distinction between withdrawal-induced anxiety and anhedonia through the use of additional behavioral measures and drugs of abuse.
Also of note, naloxone alone increased thresholds in HiS vs. LoS rats that were morphine-naïve. Threshold-elevating effects of naloxone have previously been reported in drug-naïve animals with higher doses (2.0 mg/kg) (Watkins et al., 2000). The difference in response to naloxone suggests possible basal differences in endogenous opioid systems between the two lines, a prospect that warrants further investigation. The general threshold-elevating effects of naloxone in HiS rats may also have inflated the apparent withdrawal effect in HiS rats receiving morphine and naloxone, such that the difference between the lines during precipitated withdrawal may have been larger than observed. We also found the HiS rats had a nearly 3-fold greater variability in baseline reward thresholds compared to LoS rats. HiS rats are generally more variable than LoS rats across many different measures (see Carroll et al., 2008), and thus differences in variability in the present study were controlled for by using percent change in thresholds from baseline within each animal as the primary independent measure. Furthermore, the modest phenotype differences in ICSS response latency increases (HiS>LoS) under certain conditions (e.g., precipitated withdrawal phase) suggest that ICSS threshold data could have been influenced by non-specific motor effects. However, the discrete-trial ICSS threshold procedure used in this study is relatively response rate-independent, as numerous treatments that alter ICSS latencies, such as nicotine, have little or no effect on ICSS thresholds and vice versa (Harris et al., 2010b; Harrison and Markou, 2001; Markou and Koob, 1992).
A limitation of this study is that ICSS thresholds were only measured twice during spontaneous withdrawal, making it difficult to determine whether the magnitude of withdrawal was actually larger in the LoS rats, or if it was merely shifted temporally; however, differences in withdrawal magnitude during precipitated withdrawal support the former scenario. We also cannot rule out the latter possibility as we lack data on pharmacokinetic differences between the two lines. Future studies could resolve this by characterizing a more complete time course of threshold increases during spontaneous withdrawal. While we chose drug doses that allowed comparison with our previous studies, future studies should also examine dose-response relationships and whether similar effects can be seen with other classes of abused drugs. An examination of sex differences would also be important as sweet preference is greater in females (Carroll et al., 2008; Valenstein et al., 1967), and our previous studies found effects of sex on withdrawal-induced CPA in HiS and LoS rats (Radke et al., 2013). Additionally, while HiS rats had higher saccharin preferences scores than LoS rats, these results were not statistically different. Typically, saccharin scores are more divergent between HiS and LoS female rats (Carroll et al., 2008), but only males were used in the present study. Furthermore, opioid antagonists have been shown to decrease saccharin consumption, even following protracted periods of withdrawal from chronic drug exposure (Biggs and Myers, 1998; Lynch and Libby, 1983; Lynch, 1986). Thus, naloxone exposure may have reduced saccharin preference scores in the HiS rats to bring their scores closer to LoS (i.e., floor) levels.
To our knowledge, no direct relationship between sweet preference and withdrawal severity has been reported in human populations, but further research in this area is warranted. Differences in sweet preference in drug dependent individuals could be used as a predictive tool for those attempting abstinence. For example, alcoholics who rated highest in sweet preference were also most likely to relapse after six months (Krahn et al., 2006), and sweet-preferring subjects achieved fewer days of abstinence than sweet-nonpreferring subjects over a 12-week treatment period (Garbutt et al., 2009). Understanding how the withdrawal syndrome varies with sweet preference or other individual differences could lead to more individualized treatments and better success rates.
In summary, the purpose of this study was to examine differences in morphine withdrawal between rats selectively bred for high or low saccharin consumption. While differences between HiS and LoS rats were generally modest, the greater vulnerability in LoS rats, vs. HiS rats during both spontaneous and precipitated withdrawal agree with previous studies suggesting that the anhedonic effects of withdrawal may be more pronounced in the LoS line (Radke et al., 2014). These results contribute to our understanding of the interaction between sweet-preference and drug addiction.
4. Experimental Procedures
4.1 Subjects
Adult male rats selectively bred at the University of Minnesota (Carroll et al. 2002) from Occidental HiS and LoS lines (Occidental College, Los Angeles, CA) were used in this study. They began as outbred lines, defined as no mating closer than second cousins (Dess and Minor, 1996). To maintain outbred status of the lines, we avoided sibling, half-sibling, and first cousin mating. Also, every four to six generations, rats were purchased from the founding stock (i.e., Sprague-Dawley from Harlan Laboratories, Madison, WI) and tested for saccharin preference. Male and female rats expressing high or low saccharin preferences were then mated with our selectively bred HiS and LoS lines, respectively. Thus, the rats were selectively outbred from the Sprague-Dawley founding stock (Carroll et al., 2008; Dess et al., 2000a)
Rats were bred and pair-housed in plastic cages prior to the experiments, and singly housed in hanging metal cages during the experiments. The animals had ad libitum access to rat pellet chow (Purina Mills, Minneapolis, MN, USA) and water except during experimental sessions. The humidity, temperature (21–23 °C), and light–dark cycle (12 h–12 h; lights on at 6:00 AM) were all regulated. All behavioral testing occurred during the light (inactive) phase of the light-dark cycle. All procedures conformed to the eighth edition of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academies Press 2011) and were approved by the University of Minnesota Institutional Animal Care and Use Committee under protocol number 1008A87755. Laboratory facilities were approved by the American Association for the Accreditation of Laboratory Animal Care.
4.2 Drugs
Morphine sulfate was purchased from Mallinckrodt (Hazelwood, MO). Naloxone hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). Systemically administered drugs were dissolved in 0.9% saline and injected subcutaneously in a volume of 1 mL/kg. Drug doses are expressed as the weight of the salt.
4.3 Surgery
For all surgical procedures, rats were anesthetized with a combination of ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and they were administered doxapram (5 mg/kg, i.p.) and atropine (0.4 mg/mL, 0.15 mL, s.c.) to facilitate respiration. For electrode implantation, rats were secured in a Kopf stereotaxic instrument, and the skull was exposed. With the incisor bar set at +5.0 mm above the interaural line, bipolar stainless steel electrodes (#MS303-2-B-SPC, Plastics One, Roanoke, VA) were lowered into the medial forebrain bundle at the level of the lateral hypothalamus (AP: -0.5, ML: ±1.7 mm from bregma, DV: -8.3 mm from dura) (Pellegrino et al., 1979). Rats were randomly selected to have the electrode implanted in either the left or right hemispheres. Jeweler screws were anchored to the skull, and the entire assembly was cemented into place using Loctite 444 Tak Pak Instant Adhesive (Henkel Corporation, Düsseldorf, Germany) and Perm Reline & Repair Resin (Hygenic Corporation, Akron, OH). Animals were allowed to recover for at least 5 days prior to ICSS training, during which time they were administered enrofloxacin (10 mg/kg, s.c.) and ibuprofen (5 mg/kg, P.O.) daily.
4.4 Intracranial Self-Stimulation
Rats were tested in custom-made operant conditioning chambers consisting of alternating stainless steel and Plexiglas walls each enclosed in a sound-attenuating box (for details, see Carroll et al., 2001). Each chamber contained a 5 cm wide metal wheel (model # ENV-113M, Med Associates, St. Albans, VT) on one wall. Brain stimulation was administered with constant current stimulators (model #PHM-152, Med Associates). Rats were connected to the stimulation circuit through bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (Plastics One). MED-PC IV software was used to control stimulation parameters and for data collection.
Following recovery from surgery, animals were trained on a modified version of the Kornetsky and Esposito (1979) discrete-trial current threshold procedure (Harris et al., 2010a; Markou and Koob, 1992). Each trial was initiated with presentation of a non-contingent stimulus (0.1 ms cathodal square wave pulses at a frequency of 100 Hz for 500 ms) followed by a 7.5 s window, during which a positive response on the wheel produced a second contingent stimulation identical to the first. Lack of responding during the 7.5 s window was considered a negative response. Each positive or negative response was followed by a variable intertrial interval averaging 10 s (range, 7.5–12.5 s), during which time additional responses delayed the onset of the subsequent trial by 12.5 s. Stimulus intensities were presented in four alternating descending and ascending series (step size, 5 μA), with five trials presented at each current intensity step. The current threshold for each series was defined as the midpoint between two consecutive intensity steps that yielded three or more positive responses and two consecutive intensity steps that yielded three or more negative responses. The overall ICSS threshold for the session was defined as the mean of the current thresholds from the four alternating series. To assess performance effects (e.g., motor disruption), response latencies (seconds between onset of the non-contingent stimulus and a positive response) were averaged across all trials in which a positive response was made. ICSS sessions were conducted once a day, 7 days a week, during training and testing unless stated otherwise.
4.5 Saccharin Preference Testing
Phenotype score was derived from a 24 h two-bottle test (see Badia Elder et al. 1996 for details) in which consumption of 0.1% saccharin solution was assessed relative to previously attained 24 h water intake, divided by body weight [saccharin score = (saccharin mL − water baseline mL)/ body weight × 100]. Saccharin preference was measured two weeks after drug exposure, which is a standard procedure in our laboratory because it allows adequate time for drug washout and environmental acclimation (Carroll et al., 2008).
4.6 Experimental Design
Table 1 illustrates the experimental design. Test sessions began when thresholds were stable (≤ 10% coefficient of variation over 3 consecutive days and no apparent trend). Each test day consisted of an injection of saline (SAL) or morphine (MOR, 10 mg/kg) followed 4 h later by an injection of saline or naloxone (NAL, 1 mg/kg; see Table 2 for treatment groups). Drug doses were based on those previously used to study morphine withdrawal in several rat strains including HiS and LoS rats (Harris and Gewirtz, 2005; Radke et al., 2013). Further, it is well established that this naloxone dose does not influence ICSS thresholds in drug-naïve rats (Schulteis et al., 1994; Watkins et al., 2000). Test phases were as follows: phase 1) 5 stable days of SAL+SAL (baseline for phase 2), phase 2) 8 days of MOR+SAL or SAL+SAL (morphine dependence induction), phase 3) at least 5 days of SAL+SAL and until stable for 3 consecutive days (spontaneous withdrawal/baseline for phase 4), and phase 4) 4 days of MOR+NAL or SAL + NAL (precipitated withdrawal). ICSS thresholds were measured twice on the first day of phase 3 (spontaneous withdrawal), once immediately following the first injection, and once following the second injection. Thus, animals were assessed 24-h and 28-h following the last MOR+SAL/SAL+SAL treatment. Following completion of testing, all rats were moved to plastic housing and saccharin scores were assessed 2 weeks later.
Table 1.
Experimental Design
| Phase 1: Baseline | Phase 2: Morphine dependence induction | Phase 3: Spontaneous withdrawal | Phase 4: Precipitated withdrawal | |
|---|---|---|---|---|
| # of sessions | ~5 | 8 | ~6a | 4 |
| Pre-session injection (4 h) | SAL | MOR or SAL | SAL | MOR or SAL |
| Pre-session injection (5 min) | SAL | SAL | SAL | NAL |
Sessions were conducted once daily except during the first day of phase 3, during which sessions were conducted twice (i.e., 24 and 28 h following the last MOR injection of Phase 2)
MOR = morphine (10 mg/kg); NAL = naloxone (1 mg/kg); SAL = saline
Table 2.
Experimental Treatments
| HiS MOR (n = 9) | LoS MOR (n = 11) | HiS SAL (n = 6) | LoS SAL (n = 6) | |
|---|---|---|---|---|
| Phase 2 (morphine dependence induction) treatments | MOR+SAL | MOR+SAL | SAL+SAL | SAL+SAL |
| Phase 4 (precipitated withdrawal) treatments | MOR+NAL | MOR+NAL | SAL+NAL | SAL+NAL |
HiS = high saccharin rats; LoS = low saccharin rats; MOR = morphine (10 mg/kg); NAL = naloxone (1 mg/kg); SAL = saline
4.7 Data Analysis
Throughout the text and figures, all data are expressed as mean ± SEM. Data from each phase were analyzed separately. Threshold and latency data are expressed as percent of baseline (average of last 3 days of baseline responding). A two-way ANOVA (phenotype × treatment) was conducted on baseline threshold averages to assess if there were initial differences in baseline thresholds or latencies between groups. For all phases, threshold data were analyzed using overall threshold session averages (the average currents from all four current series in a session). For the precipitated withdrawal phase, within-session analyses suggested that averaging threshold data across the entire 45 min ICSS session obscured differences in reward thresholds due to the onset of naloxone’s effect occurring during experimental testing. Therefore, mean threshold data from the first two alternating current series (obtained approximately from 0-25 min of the session) and the mean thresholds from the last two alternating current series (obtained approximately from 25– 45 min of the session) were analyzed in addition to overall session data for the precipitated withdrawal phase (Roiko et al., 2009). Latency data were collected and analyzed as trial averages from the entire session. Due to a minor change in the initial protocol, 2 HiS and 4 LoS rats received only 7 days of morphine-saline during the morphine dependence induction phase. Data from these rats during the spontaneous withdrawal phase were excluded from analyses. In general, data were analyzed using multi-factor ANOVA (phenotype × treatment × time point) with time point (day or hour) serving as a repeated measure. ANOVA were followed by Fisher’s LSD or Tukey Kramer post hoc tests as appropriate. All analyses were conducted with GB Stat software (Dynamic Microsystems, Silver Spring, MD), and results were considered significant if p < .05.
Rats bred for high (HiS) vs. low (LoS) saccharin intake consume more drugs of abuse
Reward thresholds were measured in HiS vs. LoS rats during morphine withdrawal
LoS vs. HiS rats showed greater effects of morphine withdrawal
Acknowledgments
We thank Seth Johnson for his technical assistance. This research was supported by NIDA/NIH grants R01 DA003240, K05 DA 015267 (MEC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biggs TA, Myers RD. Naltrexone and amperozide modify chocolate and saccharin drinking in high alcohol-preferring P rats. Pharmacol Biochem Behav. 1998;60:407–13. doi: 10.1016/s0091-3057(97)00598-4. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Campbell UC, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: sex differences. Exp Clin Psychopharmacol. 2001;9:307–16. doi: 10.1037//1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–13. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav. 2007;88:94–104. doi: 10.1016/j.pbb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–60. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Holtz NA. The relationship between feeding and drug-seeking behaviors. In: Brewerton TD, Dennis AB, editors. Eating Disorders, Addictions and Substance Use Disorders: Research, Clinical and Treatment Aspects. Springer-Verlag; New York: 2014. [Google Scholar]
- Dess NK, Minor TR. Taste and emotionality in rats selectively bred for high versus low saccharin intake. Learning and Behavior. 1996;24:105–115. [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–8. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Dess NK, Arnal J, Chapman CD, Sidebel S, VanderWeele DA, Green KF. Exploring Adaptations to Famine: Rats Selectively Bred for Differential Intake of Saccharin Differ on Deprivation-Induced Hyperactivity and Emotionality. International Journal of Comparative Psychology. 2000a;13:34–52. [Google Scholar]
- Dess NK, Arnal J, Chapman CD, Siebal S, VanderWeele DA, Green KF. Exploring adaptations to famine: Rats selectively bred for differential intake of saccharin differ on deprivation-induced hyperactivity and emotionality. International Journal of Comparative Psychology. 2000b;13:34–52. [Google Scholar]
- Dess NK, O’Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol. 2005;37:9–22. doi: 10.1016/j.alcohol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Eaton J, Dess N, Chapman C. Sweet Success, Bitter Defeat: A Taste Phenotype Predicts Social Status in Selectively Bred Rats. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0046606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Osborne M, Gallop R, Barkenbus J, Grace K, Cody M, Flannery B, Kampov-Polevoy AB. Sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol Alcohol. 2009;44:293–300. doi: 10.1093/alcalc/agn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Acute opioid dependence: characterizing the early adaptations underlying drug withdrawal. Psychopharmacology (Berl) 2005;178:353–66. doi: 10.1007/s00213-005-2155-0. [DOI] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol Biochem Behav. 2010a;96:217–27. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacology, biochemistry, and behavior. 2010b;96:217–27. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Markou A. Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. The Journal of pharmacology and experimental therapeutics. 2001;297:316–25. [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Baclofen has opposite effects on escalation of cocaine self-administration: increased intake in rats selectively bred for high (HiS) saccharin intake and decreased intake in those selected for low (LoS) saccharin intake. Pharmacol Biochem Behav. 2011;100:275–83. doi: 10.1016/j.pbb.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Anker JJ, Regier PS, Claxton A, Carroll ME. Cocaine self-administration punished by i.v. histamine in rat models of high and low drug abuse vulnerability: effects of saccharin preference, impulsivity, and sex. Physiol Behav. 2013;122:32–8. doi: 10.1016/j.physbeh.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Escalation of i.v. cocaine intake in peri-adolescent vs. adult rats selectively bred for high (HiS) vs. low (LoS) saccharin intake. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-012-2958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Carroll ME. Animal Models of Addiction: Genetic Influences. In: Kim Y-K, Gewirtz JC, editors. Animal Models for Behavior Genetics Researc. Vol. 7. Springer; London: 2014. [Google Scholar]
- Janowsky DS, Pucilowski O, Buyinza M. Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiatr Res. 2003;37:35–41. doi: 10.1016/s0022-3956(02)00063-8. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol. 2001;36:165–70. doi: 10.1093/alcalc/36.2.165. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–6. [PubMed] [Google Scholar]
- Krahn D, Grossman J, Henk H, Mussey M, Crosby R, Gosnell B. Sweet intake, sweet-liking, urges to eat, and weight change: relationship to alcohol dependence and abstinence. Addict Behav. 2006;31:622–31. doi: 10.1016/j.addbeh.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–59. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lynch WC, Libby L. Naloxone suppresses intake of highly preferred saccharin solutions in food deprived and sated rats. Life Sci. 1983;33:1909–14. doi: 10.1016/0024-3205(83)90675-6. [DOI] [PubMed] [Google Scholar]
- Lynch WC. Opiate blockade inhibits saccharin intake and blocks normal preference acquisition. Pharmacol Biochem Behav. 1986;24:833–6. doi: 10.1016/0091-3057(86)90420-x. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–9. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–9. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum Press; New York: 1979. [Google Scholar]
- Pepino MY, Mennella JA. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891–9. doi: 10.1111/j.1530-0277.2007.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology (Berl) 2006;186:235–45. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Garcia AW, Drewnowski A, Pomerleau OF. Sweet taste preference in women smokers: comparison with nonsmokers and effects of menstrual phase and nicotine abstinence. Pharmacol Biochem Behav. 1991;40:995–9. doi: 10.1016/0091-3057(91)90118-l. [DOI] [PubMed] [Google Scholar]
- Radke AK, Holtz NA, Gewirtz JC, Carroll ME. Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Psychopharmacology (Berl) 2013;227:117–26. doi: 10.1007/s00213-012-2945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Zlebnik NE, Carroll ME. Cocaine withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Pharmacol Biochem Behav. 2014 doi: 10.1016/j.pbb.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol Biochem Behav. 2009;93:105–11. doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–8. [PubMed] [Google Scholar]
- Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156:942–3. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Stinus L, Koob GF, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther. 2000;292:1053–64. [PubMed] [Google Scholar]
- Yakovenko V, Speidel ER, Chapman CD, Dess NK. Food dependence in rats selectively bred for low versus high saccharin intake. Implications for “food addiction”. Appetite. 2011;57:397–400. doi: 10.1016/j.appet.2011.06.002. [DOI] [PubMed] [Google Scholar]


