Abstract
The molecular motions of membrane proteins in liquid-crystalline lipid bilayers lie at the interface between motions in isotropic liquids and in solids. Specifically, membrane proteins can undergo whole-body uniaxial diffusion on the microsecond time scale. In this work, we investigate the 1H rotating-frame spin-lattice relaxation (T1ρ) caused by the uniaxial diffusion of the influenza A M2 transmembrane peptide (M2TMP), which forms a tetrameric proton channel in lipid bilayers. This uniaxial diffusion was proved before by 2H, 15N and 13C NMR lineshapes of M2TMP in DLPC bilayers. When bound to an inhibitor, amantadine, the protein exhibits significantly narrower linewidths at physiological temperature. We now investigate the origin of this line narrowing through temperature-dependent 1H T1ρ relaxation times in the absence and presence of amantadine. Analysis of the temperature dependence indicates that amantadine decreases the correlation time of motion from 2.8 ± 0.9 μs for the apo peptide to 0.89 ± 0.41 μs for the bound peptide at 313 K. Thus the line narrowing of the bound peptide is due to better avoidance of the NMR time scale and suppression of intermediate time scale broadening. The faster diffusion of the bound peptide is due to the higher attempt rate of motion, suggesting that amantadine creates better-packed and more cohesive helical bundles. Analysis of the temperature dependence of indicates that the activation energy of motion increased from 14.0 ± 4.0 kJ/mol for the apo peptide to 23.3 ± 6.2 kJ/mol for the bound peptide. This higher activation energy indicates that excess amantadine outside the protein channel in the lipid bilayer increases the membrane viscosity. Thus, the protein-bound amantadine speeds up the diffusion of the helical bundles while the excess amantadine in the bilayer increases the membrane viscosity.
Introduction
Nuclear magnetic resonance has long been used as a tool for measuring molecular dynamics over a broad range of time scales, from the fast picosecond – nanosecond regime (Mandel et al., 1996), to the slow microsecond – millisecond regime (Palmer et al., 2001, Schaefer et al., 1977, Shaw et al., 2000), and to the ultra-slow supra-second regime (deAzevedo et al., 2000, Schmidt et al., 1988). Some of the most interesting applications are to biomolecules, where molecular dynamics has a particularly strong connection to function (Ishima and Torchia, 2000, Kay, 1998, Palmer et al., 1996). In solution NMR studies of biomolecular dynamics, 15N relaxation NMR has been the method of choice and the Lipari-Szabo model-free formalism (Clore et al., 1990, Lipari and Szabo, 1982) has provided a simple theoretical framework to separate the effects of internal anisotropic motions from whole-body isotropic motion and to extract the amplitudes and rates of internal motion.
In the solid state, the lack of isotropic molecular tumbling considerably simplifies studies of internal motions by NMR. Many solid-state NMR techniques directly probe the amplitudes of internal motions, most notably 2H quadrupolar NMR (Jelinski et al., 1980), which has exquisite angular resolution but no chemical resolution, and 1H-X dipolar coupling techniques under magic-angle spinning (MAS), which have chemical site resolution (Hong et al., 2002, Munowitz et al., 1982, Schaefer et al., 1983). Nuclear spin relaxation times (T1, T2 and T1ρ) have also been used to determine the correlation times and activation energies of motional processes such as methyl three-site jumps and aromatic ring flips. Combined, these amplitude and rate measurements have provided detailed information on the dynamics of structural proteins such as collagen and silk (Jelinski et al., 1980, Yang et al., 2000), enzymes (Williams and McDermott, 1995), and lipid membranes (Blume et al., 1982, Smith and Oldfield, 1984). Molecular motions of synthetic polymers (Hagemeyer et al., 1989, Schaefer et al., 1990, Schaefer et al., 1984) and small molecules (Rothwell and Waugh, 1981) have also been investigated extensively using solid-state NMR.
The dynamic environment of liquid-crystalline lipid bilayers lies at the interface between isotropic fluids and rigid solids and hence presents unique challenges to understanding membrane protein dynamics. While membrane protein sidechain motions have been studied by NMR for decades (Huster et al., 2001, Kinsey et al., 1981, Lee et al., 1993, Opella, 1986), whole-body uniaxial rotational diffusion of membrane proteins has been less examined by NMR. As the symmetry axis of the lipid bilayer, the bilayer normal is the axis around which phospholipids undergo nanosecond rotational diffusion (Bloom et al., 1991, Gennis, 1989). Membrane proteins can also undergo such rotational diffusion, because the same principle that underlies the phospholipid motion, which is Brownian diffusion in a two-dimensional fluid (Saffman and Delbruck, 1975), also applies to membrane proteins. A number of examples of this uniaxial diffusion have now been reported for membrane peptides and proteins (Hong, 2007, Hong and Doherty, 2006, Lewis et al., 1985, Macdonald and Seelig, 1988, Pauls et al., 1985, Prosser et al., 1992, Tian et al., 1998, Yamaguchi et al., 2001). Their NMR fingerprints include powder lineshapes with reduced anisotropy and an asymmetry parameter (η) of 0, vanishing intensity at the isotropic chemical shift of non-spinning cross polarization (CP) spectra, and narrow lines in macroscopically aligned samples whose alignment axis deviates from the static magnetic field (Aisenbrey and Bechinger, 2004, Glaser et al., 2004, Park et al., 2006).
The influenza A M2 protein forms a proton channel in the virus envelope that is important for the virus life cycle (Pinto et al., 1992, Pinto and Lamb, 2007). Acidification of the virus interior uncoats the viral RNA and releases it into the host cell. Amantadine binds the M2 proton channel and prevents its opening, thus inhibiting viral replication (Hay et al., 1985, Wang et al., 1993). The protein forms a tetrameric helical bundle in the membranes of both whole cells (Sakaguchi et al., 1997) and synthetic lipids (Luo and Hong, 2006). It undergoes uniaxial diffusion at a rate of ~105 s–1 in DLPC bilayers based on 2H NMR spectra and the 2D Brownian diffusion theory (Cady et al., 2007, Kovacs and Cross, 1997). The motional axis is the bilayer normal, which is also the helical bundle axis. Since the TM helices have tilt angles of ~38° in DLPC bilayers (Cady and Hong, 2008), the rotational diffusion has large amplitudes. Combined with the fact that the motional rate is not orders of magnitude different from the 1H-13C and 1H-15N dipolar couplings, the motion strongly impacts the NMR spectra: the ambient-temperature 1H-decoupled 13C and 15N spectra of the protein in lipid bilayers are severely exchange-broadened under both MAS and static conditions (Cady et al., 2007, Li et al., 2007). Interestingly, upon amantadine binding, the resonances in both MAS and static solid-state NMR spectra of the protein sharpen considerably. This line narrowing was also observed in solution NMR spectra of M2(18-60) bound to DHPC micelles when an excess of the analogous rimantadine was added (Schnell and Chou, 2008).
Previously we have compared the 1H-decoupled 13C T2 relaxation times of the apo and bound M2TMP in DLPC bilayers to understand the amantadine-induced line narrowing. We found that the bound peptide has 30-150% longer 13C T2 than the apo state at 303 K (Cady and Hong, 2008, Cady et al., 2009), indicating that the line narrowing has a significant contribution from dynamic changes of the protein. However, the nature of this amantadine-induced relaxation time increase has not been elucidated.
In this work, we have measured and analyzed temperature-dependent 1H T1ρ relaxation times of the apo and amantadine-bound M2TMP to better understand its motional properties. We quantify the rates and activation energies of the M2TMP microsecond motion in the absence and presence of amantadine. We find that amantadine increases the motional rates approximately three fold at 313 K by increasing the attempt rates, thus alleviating intermediate time scale broadening and narrowing the spectral lines. Further, excess amantadine in the bilayer increases the activation energy of the motion, whose physical origin will be discussed.
Material and Methods
Peptides and lipids
FMOC-protected uniformly 13C, 15N-labeled amino acids were either prepared in-house (Carpino and Han, 1972) or purchased from Sigma-Aldrich and Cambridge Isotope Laboratories. The M2 transmembrane domain (residues 22-46) of the Influenza A Udorn strain (Ito et al., 1991) was synthesized by PrimmBiotech (Cambridge, MA) and purified to >95% purity. The amino acid sequence is SSDPL VVAASII GILHLIL WILDRL. Three labeled peptides were used in this work, each with three to four uniformly 13C, 15N-labeled residues. They are LAGI (L26, A29, G34 and I35), VAIL (V27, A30, I33 and L38), and VSL (V28, S31 and L36).
Membrane sample preparation
M2TMP was reconstituted into 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine (DLPC) bilayers by detergent dialysis (Luo and Hong, 2006). The lipid vesicle solution was prepared by suspending dry DLPC powder in 1 mL phosphate buffer (10 mM Na2HPO4/NaH2PO4, 1 mM EDTA, 0.1 mM NaN3) at pH 7.5, vortexing and freeze-thawing 6 times to create uniform vesicles (Traikia et al., 2000). M2TMP was dissolved in octyl-β-D-glucopyranoside (OG) in 2 mL phosphate buffer, then mixed with an equal volume of DLPC vesicles, and dialyzed against the phosphate buffer at 4 °C for 3 days. The final peptide/lipid molar ratio was 1:15. The dialyzed peptide-DLPC solution was centrifuged at 150,000 g to give a pellet containing ~50 wt% water. For the amantadine-bound samples, 10 mM amantadine hydrochloride was added to the phosphate buffer. After pelleting, the amount of amantadine remaining in the supernatant was quantified by 1H solution NMR, and the bound fraction indicates a peptide : amantadine molar ratio of ~1 : 8 (Cady et al., 2009). All membrane-bound M2 samples were thus studied at pH 7.5, corresponding to the closed state of the channel.
Solid-state NMR spectroscopy
NMR experiments were carried out on a Bruker AVANCE-600 (14.1 Tesla) spectrometer (Karlsruhe, Germany) using a 4 mm MAS probe. 1H T1ρ measurements were carried out using a Lee-Goldburg (LG) spin lock sequence shown in Figure 1 (Huster et al., 2001). The initial two 90° and 35° 1H pulses prepare the 1H magnetization to be alternately parallel and antiparallel to the 1H spin lock axis, which lies at the magic angle from the static magnetic field in the yz plane. The spin-lock effective field strength, ωe, was fixed at 2π × 61.2 kHz in all experiments, since T1ρ depends on ωe (equations 1-2). The samples were spun at 7000 Hz during all experiments. Six or seven spin lock time points τSL were measured between 0 and 8 ms to obtain the 1H T1ρ at each temperature, and six or seven temperatures were measured per peptide to provide a sufficient number of data points in the versus inverse temperature plot (Figure 5) to extract the dynamic parameters. The NMR probe and samples were equilibrated for 30 minutes at each temperature before tuning and data acquisition. The 1H LG-CP period had the same field strength as the LG spin lock period, but had a constant contact time of 300 μs. Typical radiofrequency (rf) pulse lengths were 5 μs for 13C and 3.5-4.0 μs for 1H. 13C chemical shifts were referenced to the α-Gly 13C’ signal at 176.49 ppm on the TMS scale.
Figure 1.

13C-detected 1H Lee-Goldburg spin lock pulse sequence used to measure 1H T1ρ relaxation times. The 1H magnetization is spin-locked for a variable time τSL. The LG spin-lock and LG-CP have the same field strength.
Figure 5.
versus 1000/T curves for representative Cα sites of M2TMP. (a) L26. (b A30. (c) S31. (d) G34. (e) I35. (f) L36. The apo data are shown as open squares and fit by dashed lines, and the amantadine-bound data are shown as filled squares and fit by solid lines.
The 1H T1ρ's were measured between 313 K and 243 K on six membrane samples with and without amantadine. The activation energy Eα, correlation time prefactor τ0, and correlation time were extracted by least-square linear fits of the to 1000/T curves on the high temperature side using equation 8. For the apo VAIL sample, artificially high T1ρ values were observed at 313 K, thus the apo and amantadine-bound VAIL-M2TMP data analysis did not include the 313 K data.
C-H dipolar order parameters were measured at 313 K under 7000 Hz MAS using a dipolar-doubled DIPSHIFT experiment (Hong et al., 1997) where 1H homonuclear decoupling was achieved by the frequency-switched Lee-Goldburg sequence (Bielecki et al., 1990). The dipolar-doubled sequence suppresses the apparent T2 relaxation by making the total homonuclear decoupling time constant at one rotor period. The t1 evolution time was controlled by the position of a 13C π pulse in the rotor period.
Theory
The membrane protein rotational diffusion of interest generally occurs on the microsecond time scale based on the 2D Brownian diffusion theory (Saffman and Delbruck, 1975). Motions on this time scale can be probed by 1H-decoupled S-spin spin-spin relaxation times (T2) and rotating-frame spin-lattice relaxation times (T1ρ). The T2 relaxation times of an S spin dipolar coupled to an I spin under conditions of random isotropic motion and I spin rf decoupling depends on the spectral density at the decoupling field strength (Rothwell and Waugh, 1981), which is typically 50-100 kHz. However, measurement of 13C T2 by the Hahn-echo experiment in uniformly 13C-labeled residues in proteins has the shortcoming that 13C-13C scalar coupling contributes to the time-dependent intensity decay, unless a selective 13C π pulse is applied to remove the scalar interaction. Given the small chemical shift dispersion among the aliphatic carbons in proteins, very soft π pulses, which necessitate multiple experiments with shifted 13C offsets, would be required to obtain homonuclear and heteronuclear decoupled 13C T2's of all sites. A simpler alternative, then, for probing microsecond time scale motion is to measure the T1ρ, since it is primarily sensitive to spectral densities at the frequency of the spin-lock field (Schaefer et al., 1977), which is also 50 – 100 kHz.
To obtain site-specific relaxation times, we measure the 1H T1ρ through the directly bonded 13C sites by transferring the 1H magnetization to 13C, and by using spin-diffusion-free Lee-Goldburg spin lock instead of transverse spin lock (van Rossum et al., 2000). The effective spin lock field is thus tilted at the magic angle, , from the static magnetic field. The 1H magnetization, prepared along the direction of the spin-lock field (Figure 1), can only undergo spin-lattice relaxation in the rotating frame.
The 1H T1ρ relaxation in 13C-labeled molecules is driven by fluctuating 1H-1H and 1H-13C dipolar couplings due to random molecular motions. The orientation-dependent relaxation rate depends on spectral densities, J(ω), at the spin lock field ωe, 2ωe, Larmor frequencies ωH, ωO, and the sum and difference of ωH and ωC (Huster et al., 2001, Mehring et al., 1983). Since the Larmor frequencies are three to four orders of magnitude larger than ωe (ωH = 2π × 600 MHz, ωC = 2π × 150 MHz, and ωe = 2π × 61.2 kHz in our experiments), for motions in the tens to hundreds of kilohertz regime, one can safely ignore the spectral density terms at the Larmor frequencies. Taking into account magic-angle spinning at a frequency ωR, the powder averaged T1ρ for the rotating-frame relevant part is (Fares et al., 2005):
| (1) |
Here ΔM2 is the product of the dynamic portion of the dipolar second moment and orientational terms that transform spin interaction tensors from their molecule-fixed principal axis frames to the rotor frame (Fares et al., 2005). The dynamic portion of the dipolar second moment is approximately (1 – S2) times the rigid-limit dipolar second moment, where the order parameter S represents the orientation-invariant part of the interaction. In solid-state NMR, the order parameter can be independently measured by various dipolar chemical-shift correlation experiments (Hong et al., 2002, Huster et al., 2001, Schmidt-Rohr et al., 1992). The dependence of ΔM2 on the C-H (δCH) and H-H (δHH) dipolar couplings in equation (1) reflects the fact that the 13C-detected 1H T1ρ relaxation is driven by C-H and H-H dipolar couplings. β is the angle between B0 and the spin-lock field and is in our experiments.
For motions with a single correlation time τ, the spectral density is given by (Lipari and Szabo, 1982):
| (2) |
Explicitly writing the spectral density terms in equation (1), we obtain
| (3) |
The dependence of T1ρ on correlation time τ can be considered in three regimes. In the long correlation time or strong collision limit where 2ωeτ >> 1, equation 3 is approximated as:
| (4) |
In the short correlation time or weak collision regime, equation (3) is simplified to:
| (5) |
In the intermediate motional regime where 2ωeτ = 1, for ωe >> ωR, the relaxation rate is the fastest, corresponding to a T1ρ minimum.:
| (6) |
For an activated motional process, the correlation time is given by the Arrhenius law:
| (7) |
where Ea is the activation energy and τ0 is the prefactor describing the attempt rate of motion. The larger the attempt rate, the smaller the τ0, and the shorter the correlation time τ. Substituting τ into equation (5), we find that Ea can be extracted from a plot of versus 1000/T in the short τ limit:
| (8) |
Equation (8) indicates that the natural logarithm of relaxation rates is linear with 1000/T with a slope of Ea/1000R in the short correlation time regime. Moreover, the pre-exponential factor τ0 can be extracted from the intercept of the linear fit. For this purpose, we use the second moment expression from Mehring, which takes into account both C-H and H-H dipolar relaxation as well as the LG spin lock factor (Huster et al., 2001, Mehring et al., 1983):
| (9) |
The motional correlation time is fully determined once the activation energy Ea and the pre-exponential factor τ0 are obtained from the slope and the intercept, respectively.
In principle, an alternative method to determine the pre-exponential factor τ0 is to exploit the T1ρ minimum temperature, where 2ωeτ = 1. However, as we show below, the T1ρ minima observed for membrane-bound M2TMP result from the lipid phase transition, and are thus not the true minima of a single motional process. On the other hand, the lipid-induced T1ρ minima should not affect the high-temperature slopes or intercepts, thus the activation energy Ea and τ0 can still be extracted reliably from these features.
The T1ρ in the long correlation time limit can in principle also be used to extract Ea and τ. Equations (4) and (5) indicate that the slope of the plot with 1/T on the low temperature side has the same magnitude but the opposite sign from that of the high temperature side. But this scenario is true only if a single motional process with the same Ea persists throughout the temperature range. Thus, the lipid phase transition makes this assumption invalid. Since our purpose is to understand the physiological temperature dynamics of M2TMP, below we will analyze only the high temperature regime of the T1ρ data to extract M2TMP motional parameters.
Results
Site-specific 1H T1ρ relaxation times were measured on six membrane samples with three sets of labeled residues (LAGI, VAIL, and VSL) without and with amantadine. The eleven labeled residues are distributed from position 26 to 38 in the transmembrane domain, with I32 and H37 being the only residues not measured in this range.
Figure 2 shows representative 13C spectra of LAGI-M2TMP in the apo- and amantadine-bound states at 313 K, 273 K and 243 K. At 313 K, the amantadine-bound peptide has narrower linewidths than the apo peptide, whereas at 243 K the peptide signals of the two samples have similar intensities and linewidths, indicating immobilization of both states of the peptide. At 273 K, both the apo and bound peptide spectra exhibit significant exchange broadening with very low 13C intensities. This broadening coincides with the gel to liquid-crystalline phase transition temperature (271 K) of the DLPC membrane, indicating that the M2TMP motion responsible for the exchange broadening is intimately associated with the phase property of the lipid bilayer. The VAIL and VSL spectra exhibit similar temperature dependences and are given in the Supporting Information (Figures S1, S2). The fact that the amantadine-bound samples show narrower and higher backbone Cα signals than the apo samples at high temperature (Cady and Hong, 2008) indicates dynamic differences induced by amantadine, the nature of which are analyzed below.
Figure 2.
1D 13C CP-MAS spectra of LAGI M2TMP in DLPC bilayers at 313 K, 273 K, and 243 K. (a) With amantadine. (b) Without amantadine.
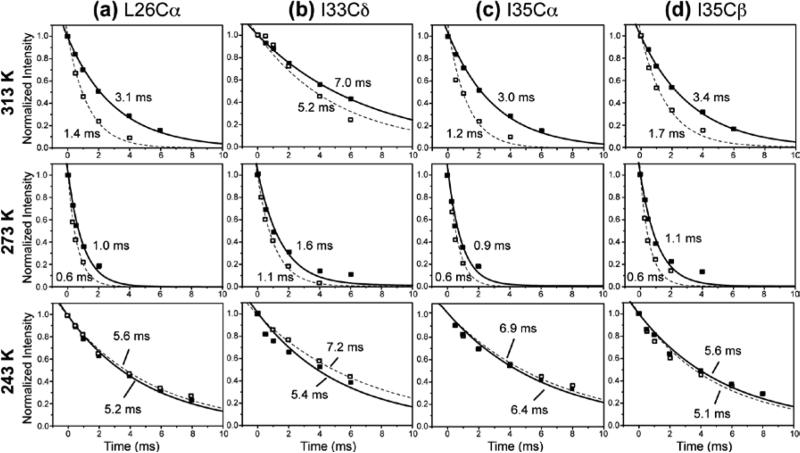
1H T1ρ were measured at six to seven temperatures between 313 and 243 K. Figure 3 displays representative T1ρ decay curves at 313 K, 273 K, and 243 K as a function of spin-lock time. The decay curves are well fit by single exponential functions, where the decay constants correspond to T1ρ. At 313 K, the T1ρ decays are slower for the amantadine-bound peptide than for the apo peptide for all residues, indicating the bound peptide has longer T1ρ's. At 273 K, the 1H T1ρ's are significantly shorter than the high-temperature values for both the apo and bound peptides, and the bound peptide has marginally longer T1ρ's than the apo peptide. At 243 K, the T1ρ values increase significantly over the high temperature values, and there is no longer a consistent difference between the apo and bound peptide. Supporting information Table S1-S3 lists all T1ρ values for backbone Hα, sidechain Hβ, and sidechain methyl protons in the apo and bound M2TMP at all temperatures.
Figure 3.
Representative 1H T1ρ relaxation decays of M2TMP in DLPC bilayers at 313 K, 273 K, and 243 K. (a) L26 Cα, (b) I33 Cδ, (c) I35 Cα, and (d) I35 Cβ. The apo data are shown as open squares and fit by dashed lines. The amantadine-bound data are shown as filled squares and fit by solid lines. The decay constants are indicated for all sites.
Figure 4 shows the 1H T1ρ's as a function of 1000/T (K−1) for representative backbone and sidechain sites. All curves have a V shape, indicative of passage through the fastest relaxing intermediate motional regime. Between the apo and bound peptides, the minimum T1ρ positions match well, with relatively similar T1ρ values as well as the transition temperature T0. On the high temperature side of the minimum, the bound peptide has progressively longer T1ρ's than the apo peptide with increasing temperature, indicating that M2TMP has shorter correlation times in the presence of amantadine (equation 5). On the low temperature side, the apo and bound peptides have smaller T1ρ differences for the small number of temperatures measured.
Figure 4.
1H T1ρ versus 1000/T for representative M2TMP Cα sites in the apo (open squares and dashed line) and amantadine-bound (closed squares and solid line) states in DLPC bilayers. (a) L26 Cα, (b) S31 Cα, (c) G34 Cα, (d) L36, (e) V27 Cγ2, (f) I35 Cδ.
To quantify the amantadine-induced dynamic differences of M2TMP, we convert the T1ρ plots to and extract the activation energy and correlation time prefactor τ0 using equations (8-9). Figure 5 shows as a function of 1000/T for a number of Hα sites. As expected, the shapes of the plots are roughly inverted from those of Figure 4, with the amantadine-bound peptide showing smaller relaxation rates than the apo peptide at physiological temperature. On the high temperature side of the T1ρ minimum, is roughly linear with inverse temperature for most sites, thus supporting the assumption that the rotational diffusion can be regarded as an activated process (vide infra). Moreover, the amantadine-bound peptide has more positive slopes than the apo peptide, indicating larger Ea. Least-square linear fits of the high-temperature side of the curves yielded the activation energies, listed in Table 1.
Table 1.
Ea, τ0, and τ313K of M2TMP in DLPC bilayers in the absence and presence of amantadine extracted from Hα T1ρ's. The 313 K T1ρ and SCH are also listed along with their uncertainties (superscript).
| Residue | Amantadine-bound peptide | Apo peptide | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | τ0 (ns) | τ313K (μs) | (ms) | S CH | Ea (kJ/mol) | τ0 (ns) | τ313K (μs) | (ms) | S CH | |
| L26 | 20.91.3 | 0.25 | 0.76 | 3.10.1 | 0.420.02 | 13.51.3 | 10.3 | 1.9 | 1.30.1 | 0.440.02 |
| V27 | 25.17.8 | 0.03 | 0.40 | 4.00.3 | 0.330.04 | 17.1– | 3.8 | 2.8 | 1.40.1 | 0.310.02 |
| V28 | 32.45.4 | 0.004 | 1.07 | 2.60.3 | 0.460.04 | 18.36.2 | 2.1 | 2.4 | 1.20.1 | 0.460.04 |
| A29 | 21.71.3 | 0.18 | 0.76 | 3.50.1 | 0.440.02 | 17.51.3 | 1.7 | 1.4 | 1.70.1 | 0.460.02 |
| A30 | 15.94.7 | 2.32 | 4.3 | 1.90.2 | 0.600.02 | 11.0– | 62.9 | 1.1 | 1.20.1 | 0.600.07 |
| S31 | 30.77.8 | 0.0086 | 1.1 | 2.30.3 | 0.500.02 | 13.67.2 | 15.0 | 2.8 | 0.90.1 | 0.400.02 |
| I33 | 25.11.8 | 0.027 | 0.40 | 4.00.3 | 0.460.02 | 17.1– | 4.4 | 3.2 | 1.40.1 | 0.310.02 |
| G34 | 12.10.4 | 17.5 | 1.8 | 1.70.1 | 0.600.02 | 5.40.9 | 514 | 4.1 | 0.90.1 | 0.690.09 |
| I35 | 22.21.3 | 0.16 | 0.79 | 3.10.1 | 0.420.02 | 12.01.3 | 18.0 | 1.9 | 1.30.1 | 0.420.02 |
| L36 | 30.22.1 | 0.010 | 1.1 | 2.20.2 | 0.480.04 | 18.68.1 | 1.8 | 2.3 | 1.20.1 | 0.440.02 |
| L38 | 20.69.9 | 0.18 | 0.48 | 2.90.2 | 0.350.02 | 9.6– | 96.6 | 3.8 | 1.30.1 | 0.330.07 |
| Mean | 23.3±6.2 | 0.89±0.41 | 2.8±0.8 | 14.0±4.2 | 2.8±0.9 | 1.3±0.2 | ||||
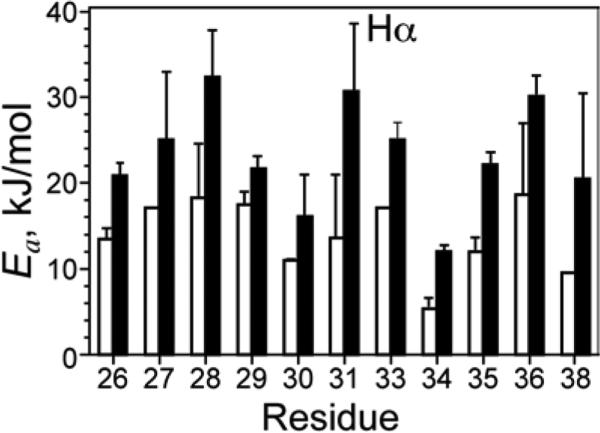
The amantadine-bound peptide has an average Ea of 23.3 kJ/mol, which is 66% larger than the average Ea of 14.0 kJ/mol for the apo peptide. The average experimental uncertainty of the Ea is 17%. The standard deviation of the Ea distributions is 6.2 kJ/mol for the bound peptide and 4.2 kJ/mol for the apo peptide. Figure 6 compares the Ea values of the apo and bound peptides for each Hα site. With the exception of G34, the Ea's are relatively uniform across all residues, given the experimental uncertainty. The small Ea distribution is consistent with the approximation that the main motional process driving T1ρ relaxation is whole-body uniaxial diffusion rather than segmental motion. In addition, the Ea ratio between the bound and apo peptides for each site is relatively uniform, with an average ratio of 1.73 and a standard deviation of 0.35.
Figure 6.
Activation energy Ea (kJ/mol) extracted from the high-temperature slopes of the Hα versus 1000/T curves. The amantadine-bound M2TMP (filled bars) has larger activation energies than the apo peptide (open bars).
To obtain the motional correlation times, we need the C-H order parameters and Ea (equations 7 and 9). The Cα-Hα order parameters (Table 1) are found to be similar between the apo and bound peptides, indicating that the amantadine-induced spectral line narrowing is not due to amplitude changes but due to rate changes. Using the high-temperature intercepts and SCH, we calculated τ0 and τ313K for the apo and amantadine-bound M2TMP (Table 1). The bound peptide exhibits smaller τ0 values, indicating higher attempt rates. As a result, the average τ313 K is shorter for the bound peptide (0.89 ± 0.41 μs) than the apo peptide (2.8 ± 0.9 μs). The three-fold reduction of τ313 K in the presence of amantadine is qualitatively consistent with the two-fold longer T1ρ, since is linear with τ in the short τ regime (equation 5). The magnitude of τ313 K is also reasonable, as it is similar to or shorter than the inverse of the spin lock field of 2.7 μs. In other words, the amantadine-bound M2TMP has a shorter correlation time than the characteristic time scale of the spin-lock field, thus alleviating exchange broadening, while the apo peptide correlation time is closer to the NMR time scale, thus giving rise to broader lines. Decreasing the temperature to 273 K increased the τ to 6.0 μs and 3.4 μs for the apo and bound peptides, respectively.
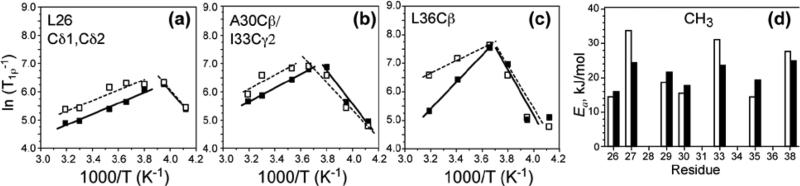
It is also interesting to examine the sidechain 1H T1ρ trends. Table S3 shows that the methyl proton T1ρ's are much longer than the backbone Hα T1ρ's and are relatively similar between the apo and bound peptides at high temperatures. Figure 7 shows representative plots of two methyl groups and one methine Cβ site. The methyl protons have more similar high-temperature slopes between the apo and bound peptides, with an apparent activation energy difference of only 20% between the two states. The similarities can be attributed to three-site jumps of the methyl groups that are additional to the uniaxial diffusion, which reduce the dynamic difference between the apo and bound peptide. In comparison, the Cβ sites show more distinct activation energies between the apo and bound states, consistent with the Hα T1ρ behavior.
Figure 7.
(a-c) versus 1000/T curves for several M2TMP sidechains. (a) L26 methyl Cδ1/ Cδ2. (b) A30 Cβ and I33 Cγ2 methyl groups. (c) L36 Cβ. Apo data: open squares and dashed lines. Amantadine-bound data: filled squares and solid lines. (d) Activation energy (kJ/mol) of the methyl protons. The average Ea difference between the apo and bound peptides is ~20%.
Discussion
M2TMP uniaxial diffusion is the main motion driving T1ρ relaxation
The motivation for this work is to quantify the motion of M2TMP that is responsible for exchange broadening of its NMR spectra in phosphocholine bilayers at physiological temperatures, and to understand the origin of amantadine-induced line narrowing.
The exchange broadening is due to microsecond motion of the peptide that interferes with 13C-1H and 15N-1H dipolar decoupling and cross polarization. We assign the motion to whole-body uniaxial rotational diffusion of the M2 helical bundle. The presence of this uniaxial diffusion has been previously shown based on the lineshapes of 2H quadrupolar spectra, 15N static powder spectra, and 15N-1H and 13C-1H dipolar couplings (Cady et al., 2007). Two lines of evidence support the assignment of the T1ρ relaxation mechanism to this uniaxial diffusion. First, all eleven measured residues exhibit rapid T1ρ relaxation at high temperatures, and all T1ρ values are increased by amantadine. Such across-the-board effects can only result from a whole-body motion. Second, the two-dimensional Brownian diffusion theory of Saffman and Delbrück predicts a rate of 105 s–1 for the uniaxial diffusion of the M2 helical bundle, which agrees well with the time scale probed by the T1ρ experiments.
The presence of whole-body motion does not exclude additional internal motions. Sidechain motions are certainly present, although they do not interfere with the extraction of the rates and activation energy of uniaxial diffusion from the backbone Hα T1ρ data. The clearest manifestation of sidechain motions is the fact that the methyl protons have longer T1ρ's than the backbone protons, and the methyl 1H T1ρ's are similar between the apo and bound peptides at high temperatures (Table S3). These observations are not surprising, since the fast methyl rotation on the nanosecond time scale pre-averages the dipolar second moment, and thus reduce the relaxation rates (equation 1). The local nature of the methyl rotation also makes the motion less sensitive to amantadine binding. The full T1ρ dependence includes spectral densities at the 1H and 13C Larmor frequencies, which are more relevant time scales for the methyl rotation. Thus, equation 1 does not apply fully to methyl groups. Since the main purpose of this study is to understand the motion that causes exchange broadening of the NMR spectra, but sidechain methyl 13C signals do not suffer from exchange broadening, we do not consider the combined motion of three-site jumps and uniaxial diffusion experienced by the methyl groups further. Another manifestation of possible segmental motions is the distribution of Ea. Specifically, the G34 Ea is two standard deviations lower than the average, suggesting the local motion at this residue (vide infra).
Correlation time of M2TMP diffusion and origin of spectral line narrowing by amantadine
The three-fold shorter correlation time of the amantadine-bound M2TMP (Table 1) explains the amantadine-induced line narrowing of the peptide spectra. The faster motional rates better avoid the intermediate time scale condition, thus alleviating line broadening. The faster diffusion rates result from the one to two orders of magnitude reduction of the prefactor τ0, as obtained from the high-temperature intercepts of the curves. The intercept depends both on τ0 and the dipolar second moment (equation 9), whose exact magnitude differs somewhat between different theories, depending on how many spin interactions are included and whether the MAS or the static condition is operative (Fares et al., 2005, Huster et al., 2001, Mehring et al., 1983). However, the ratio of the intercepts between the apo and bound peptide depends only on τ0. Thus, the relative size of τ0 between the apo peptide (long τ0) and the bound peptide (short τ0) is unambiguous.
The shorter τ0 of the amantadine-bound M2TMP indicates higher attempt rates of motion, which suggest that the M2 helices form better-packed tetramers in the presence of amantadine. Amantadine may interact with all four helices of the tetramer, thus serving as a non-covalent linker that brings the four helices together to form a more cohesive tetrameric bundle. A more cohesive helical bundle can diffuse faster, and would also have structurally and dynamically more homogeneous individual helices. This interpretation is consistent with the relative lack of correlation time distribution for the bound peptide (vide infra), as manifested by the sharpness of the T1ρ minima, and is also consistent with disulfide cross linking data that indicate increased tetramer association in the presence of amantadine (Cristian et al., 2003). Thus, the reduced τ0 of the bound M2TMP suggests that amantadine is centrally located in the pore of the channel, shared by all four helices, consistent with the binding site seen in a recent crystal structure (Stouffer et al., 2008). In comparison, a solution NMR study (Schnell and Chou, 2008) found four rimantadine molecules at the lipid-facing surface of each channel. It is difficult to imagine how this surface binding motif would homogenize the peptide conformation and speed up its motion.
A question that is beyond the scope of the current study is the correlation time distribution of apo M2TMP, which is manifested by the broad T1ρ minima of the apo peptide. The τ distribution suggests that without amantadine, the M2 helical bundles are floppier, with more internal degrees of freedom, and may exhibit dynamic heterogeneities between different tetramers. Such dynamic heterogeneity may be functionally relevant, as it may allow the apo peptide to adopt appropriate conformations to achieve its many functions, including channel activation, gating, and inhibition.
The activation energy of M2TMP uniaxial diffusion is related to membrane viscosity
A basic assumption in our dynamic analysis is that the rotational diffusion of a membrane protein in lipid bilayers can be considered an activated process that follows an Arrhenius law (equation 7). The linearity of the observed with respect to 1000/T at high temperatures (Figure 5) validates this assumption, but it is of interest to consider the physical basis for the activated diffusion. Membrane protein diffusion in lipid bilayers requires the availability of free volume in the vicinity of the protein, which is achieved by discrete hopping of the lipid molecules. Thus, activated lateral and rotational diffusion of membrane proteins has its molecular origin in the free volume theory of liquids (Cohen and Turbill, 1959, Galla et al., 1979). Considered in this light, the activation energy essentially reflects the macroscopic viscosity of the membrane. Higher activation energies indicate higher viscosities. Thus, the higher Ea of the amantadine-bound peptide indicates that excess amantadine increases the membrane viscosity. If this is true, then the lipid 1H T1ρ values should be affected by the excess amantadine outside the channel in the bilayer, in the same manner as the protein.
Examination of several resolved lipid 13C signals confirms that there is indeed an amantadine-induced increase of the lipid Ea. Figure 8 shows the curves of the lipid glycerol G3 and the acyl chain C2 signals. The excess-amantadine-containing membrane clearly has larger slopes than the amantadine-free membrane, similar to the peptide T1ρ behavior. The amantadine-bound samples in our experiments contain 8-fold molar excess of amantadine to the peptide, or 32-fold excess amantadine to the channel. The excess amantadine partitions into the bilayer (Subczynski et al., 1998, Wang et al., 2004). Paramagnetic relaxation enhancement NMR data indicate that amantadine is located at the interfacial region of the DMPC bilayer with the amine group pointing to the surface (Li et al., 2008). This depth and orientation are consistent with the amphipathic nature of amantadine, with its hydrophobic adamantane cage close the hydrocarbon core and its polar ammonium moiety pointing to the polar region of the bilayer.
Figure 8.
versus 1000/T curves for several lipid 13C signals in M2TMP-containing DLPC bilayers. (a) Glycerol backbone G3. (b) Acyl chain C2. Apo data: open squares and dashed lines; Amantadine-bound data: filled squares and solid lines.
Our recently measured lipid 13C T2 relaxation times at 313 K in peptide-free DLPC bilayers showed no discernible difference between amantadine-containing and amantadine-free bilayers (Cady et al., 2009), in contrast to the current lipid 1H T1ρ data. This discrepancy is likely due to the presence of the peptide in the current measurements but the lack of the peptide in the previous T2 experiments. Membrane viscosity is a function of molecular crowding: peptide-containing bilayers have much smaller free volumes for amantadine than pure lipid bilayers, thus amantadine may cause detectable fluidity changes only in M2-containing bilayers.
The viscosity origin of the activation energy of M2TMP diffusion also explains the asymmetric slope of the curves on the two sides of the T1ρ minimum. Both the apo and bound peptides have steeper slopes or larger apparent activation energies on the low temperature side. Since this T1ρ minimum is associated with phase transition of the DLPC bilayer, the slope increase at low temperature is caused by the significant viscosity increase of the gel-phase membrane compared to the liquid-crystalline phase. Thus, the M2TMP rotational diffusion is not a single motional process above and below the lipid phase transition temperature, in contrast to most other motions that have been characterized by NMR (Douglass and Jones, 1966, Rothwell and Waugh, 1981).
The viscosity origin of the activation energy may also partly explain the Ea distribution among the residues. Since a perfectly rigid-body motion should have a single Ea, the observed Ea distribution beyond the experimental uncertainty must reflect additional motions, which may result from varying membrane viscosity across the bilayer thickness. The abnormally low Ea of G34 may then be partly due to the location of G34 at the center of the TM helix and thus at the center of the bilayer, which has the lowest viscosity due to large-amplitude motions of the lipid chain ends. While the exact nature of the local G34 motion is unknown, the conclusion of increased conformational flexibility is consistent with a large number of NMR parameters for this site: its 15N anisotropic chemical shifts, N-H dipolar coupling (Hu et al., 2007), and 15N isotropic chemical shifts were all found to change with drug binding (Cady et al., 2009, Wang et al., 2009) and membrane composition (Luo et al., 2009).
The lipid-mediated influences of excess amantadine on the diffusion dynamics of M2TMP do not change the previous conclusion that the protein-bound amantadine causes site-specific conformational changes through direct amantadine-protein interactions. The structural changes are manifested as chemical shift perturbations that are highly site specific, with residues S31, G34, and V28 exhibiting large chemical shift perturbations while other residues showing little changes (Cady and Hong, 2008, Cady et al., 2009).
Conclusion
The temperature-dependent 1H T1ρ relaxation times shown here indicate that the uniaxial diffusion of the M2TMP in liquid-crystalline DLPC bilayers causes efficient 1H T1ρ relaxation, and the diffusion rates are increased by amantadine through an increase in the attempt frequencies. The average motional correlation time at 313 K is 0.89 μs for the bound peptide and 2.8 μs for the apo peptide. The faster diffusion of the bound peptide suggests that amantadine induces more homogeneous and better-packed M2TMP tetramers by coordinating with all four helices. Thus, the well documented amantadine-induced NMR line narrowing is due to suppression of the intermediate time scale broadening.
From the linear relation of with 1/T at high temperature, we extracted the activation energy of the M2 uniaxial diffusion. The average Ea is 23.3 kJ/mol for the bound peptide and 14.0 kJ/mol for the apo peptide. The higher Ea of the bound peptide is attributed to larger membrane viscosity by excess amantadine, which is confirmed by the lipid T1ρ data.
Similar exchange broadening of the apo protein and line narrowing of the bound protein have also been reported for solution NMR spectra of M2(18-60) in DHPC micelles (Schnell and Chou, 2008). There, the exchange broadening of the apo protein spectra was alleviated by the addition of 40 mM rimantadine. At 53-fold molar excess to the protein (0.75 mM), the excess rimantadine is expected to significantly increase the viscosity of the micelle interior, which may affect the dynamics of the micelle-bound rimantadine in a way that facilitates the observation of its NOEs with the protein.
The present study illustrates the rich information that can be obtained from temperature-dependent relaxation studies of membrane proteins. Compared to most relaxation NMR studies so far, which were targeted to either small molecules with a single motion (Rothwell and Waugh, 1981) or macromolecules with a well-defined local motion (Batchelder et al., 1982), the present study examines the rates and energetics of the global motion of a membrane protein (Reuther et al., 2006). It gives a glimpse into the energetic driving force behind membrane protein uniaxial diffusion, which is not easily obtained by other biophysical techniques (Gennis, 1989).
Supplementary Material
References
- Aisenbrey C, Bechinger B. Investigations of polypeptide rotational diffusion in aligned membranes by 2H and 15N solid-state NMR spectroscopy. J. Am. Chem. Soc. 2004;126:16676–16683. doi: 10.1021/ja0468675. [DOI] [PubMed] [Google Scholar]
- Batchelder LS, Sullivan CE, Jelinski LW, Torchia DA. Characterization of leucine side-chain reorientation in collagen-fibrils by solid-state 2H NMR. Proc. Natl. Acad. Sci. U. S. A. 1982;79:386–389. doi: 10.1073/pnas.79.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki A, Kolbert AC, de Groot HJM, Griffin RG, Levitt MH. Frequency-switched Lee-Goldburg sequences in solids. Adv. Magn. Reson. 1990;14:111–124. [Google Scholar]
- Bloom M, Evans E, Mouritsen OG. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q. Rev. Biophys. 1991;24:293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Blume A, Rice DM, Wittebort RJ, Griffin RG. Molecular dynamics and conformation in the gel and liquid-crystalline phases of phosphatidylethanolamine bilayers. Biochemistry. 1982;21:6220–6230. doi: 10.1021/bi00267a030. [DOI] [PubMed] [Google Scholar]
- Cady SD, Goodman C, Tatko CD, DeGrado WF, Hong M. Determining the orientation of uniaxially rotating membrane proteins using unoriented samples: a 2H, 13C, AND 15N solid-state NMR investigation of the dynamics and orientation of a transmembrane helical bundle. J. Am. Chem. Soc. 2007;129:5719–5729. doi: 10.1021/ja070305e. [DOI] [PubMed] [Google Scholar]
- Cady SD, Hong M. Amantadine-induced conformational and dynamical changes of the influenza M2 transmembrane proton channel. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1483–1488. doi: 10.1073/pnas.0711500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady SD, Mishanina TV, Hong M. Structure of Amantadine-Bound M2 Transmembrane Peptide of Influenza A in Lipid Bilayers from Magic-Angle-Spinning Solid-State NMR: The Role of Ser31 in Amantadine Binding. J. Mol. Biol. 2009;385:1127–1141. doi: 10.1016/j.jmb.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino LA, Han GY. The 9-Fluorenylmethoxycarbonyl Amino-Protecting Group. J. Org. Chem. 1972;37:3404–3409. [Google Scholar]
- Clore GM, Szabo A, Bax A, Kay LE, Driscoll PC, Gronenborn AM. Deviations from the simple 2-parameter model-free approach to the interpretation of N-15 nuclear magnetic relaxation of proteins. J. Am. Chem. Soc. 1990;112:4989–4991. [Google Scholar]
- Cohen MH, Turbill D. Molecular transport in liquids and glasses. J. Chem. Phys. 1959;31:1164–1169. [Google Scholar]
- Cristian L, Lear JD, DeGrado WF. Use of thiol-disulfide equilibria to measure the energetics of assembly of transmembrane helices in phospholipid bilayers. Proc. Natl. Acad. Sci. USA. 2003;100:14772–14777. doi: 10.1073/pnas.2536751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deAzevedo ER, Hu WG, Bonagamba TJ, Schmidt-Rohr K. Principles of centerband-only detection of exchange in solid-state nuclear magnetic resonance, and extension to four-time centerband-only detection of exchange. J. Chem. Phys. 2000;112:8988–9001. [Google Scholar]
- Douglass DC, Jones GP. Nuclear magnetic relaxation of n-alkanes in rotating frame. J. Chem. Phys. 1966;45:956–963. [Google Scholar]
- Fares C, Qian J, Davis JH. Magic angle spinning and static oriented sample NMR studies of the relaxation in the rotating frame of membrane peptides. J. Chem. Phys. 2005;122:194908. [Google Scholar]
- Galla HJ, Hartmann W, Theilen U, Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J. Membr. Biol. 1979;48:215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Gennis RB. Biomembranes: Molecular Structure and Function. Springer; New York: 1989. [Google Scholar]
- Glaser RW, Sachse C, Durr UH, Wadhwani P, Ulrich AS. Orientation of the antimicrobial peptide PGLa in lipid membranes determined from 19F-NMR dipolar couplings of 4-CF3-phenylglycine labels. J. Magn. Reson. 2004;168:153–163. doi: 10.1016/j.jmr.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Hagemeyer A, Schmidt-Rohr K, Spiess HW. 2D NMR experiments for studying molecular order and dynamics in static and rotating solids. Adv. Magn. Reson. 1989;13:85–130. [Google Scholar]
- Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M. Structure, topology, and dynamics of membrane peptides and proteins from solid-state NMR spectroscopy. J. Phys. Chem. B. 2007;111:10340–10351. doi: 10.1021/jp073652j. [DOI] [PubMed] [Google Scholar]
- Hong M, Doherty T. Orientation determination of membrane-disruptive proteins using powder samples and rotational diffusion: a simple solid-state NMR approach. Chem. Phys. Lett. 2006;432:296–300. doi: 10.1016/j.cplett.2006.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Gross JD, Rienstra CM, Griffin RG, Kumashiro KK, Schmidt-Rohr K. Coupling Amplification in 2D MAS NMR and Its Application to Torsion Angle Determination in Peptides. J. Magn. Reson. 1997;129:85–92. doi: 10.1006/jmre.1997.1242. [DOI] [PubMed] [Google Scholar]
- Hong M, Yao XL, Jakes K, Huster D. Investigation of molecular motions by Lee-Goldburg cross-polarization NMR spectroscopy. J. Phys. Chem. B. 2002;106:7355–7364. [Google Scholar]
- Hu J, Asbury T, Achuthan S, Li C, Bertram R, Quine JR, Fu R, Cross TA. Backbone structure of the amantadine-blocked trans-membrane domain M2 proton channel from Influenza A virus. Biophys. J. 2007;92:4335–4343. doi: 10.1529/biophysj.106.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster D, Xiao LS, Hong M. Solid-state NMR investigation of the dynamics of the soluble and membrane-bound colicin Ia channel-forming domain. Biochemistry. 2001;40:7662–7674. doi: 10.1021/bi0027231. [DOI] [PubMed] [Google Scholar]
- Ishima R, Torchia DA. Protein dynamics from NMR. Nat. Struct. Biol. 2000;7:740–743. doi: 10.1038/78963. [DOI] [PubMed] [Google Scholar]
- Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J. Virol. 1991;65:5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinski LW, Sullivan CE, Torchia DA. 2H NMR study of molecular motion in collagen fibrils. Nature. 1980;284:531–534. doi: 10.1038/284531a0. [DOI] [PubMed] [Google Scholar]
- Kay LE. Protein dynamics from NMR. Nature Struct. Biol. supplement. 1998:513–517. doi: 10.1038/755. [DOI] [PubMed] [Google Scholar]
- Kinsey RA, Kintanar A, Tsai MD, Smith RL, Janes N, Oldfield E. First observation of amino acid side chain dynamics in membrane proteins using high field deuterium nuclear magnetic resonance spectroscopy. J. Biol. Chem. 1981;256:4146–4149. [PubMed] [Google Scholar]
- Kovacs FA, Cross TA. Transmembrane four-helix bundle of influenza A M2 protein channel: structural implications from helix tilt and orientation. Biophys. J. 1997;73:2511–2517. doi: 10.1016/S0006-3495(97)78279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-C, Hu W, Cross TA. 2H NMR determination of the global correlation time of the gramicidin channel in a lipid bilayer. Biophys. J. 1993;65:1162–1167. doi: 10.1016/S0006-3495(93)81150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BA, Harbison GS, Herzfeld J, Griffin RG. NMR structural analysis of a membrane protein: bacteriorhodopsin peptide backbone orientation and motion. Biochemistry. 1985;24:4671–4679. doi: 10.1021/bi00338a029. [DOI] [PubMed] [Google Scholar]
- Li C, Qin H, Gao FP, Cross TA. Solid-state NMR characterization of conformational plasticity within the transmembrane domain of the influenza A M2 proton channel. Biochim. Biophys. Acta. 2007;1768:3162–3170. doi: 10.1016/j.bbamem.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yi M, Hu J, Zhou HX, Cross TA. Solid-state NMR and MD simulations of the antiviral drug amantadine solubilized in DMPC bilayers. Biophys. J. 2008;94:1295–1302. doi: 10.1529/biophysj.107.112482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari G, Szabo A. Model-Free Approach to the Interpretation of Nuclear Magnetic-Resonance Relaxation in Macromolecules.1. Theory and Range of Validity. J. Am. Chem. Soc. 1982;104:4546–4559. [Google Scholar]
- Luo W, Cady SD, Hong M. Immoblization of the influenza A M2 transmembrane peptide in virus-envelope mimetic lipid membranes: a solid-state NMR investigation. Biochemistry. 2009 doi: 10.1021/bi900716s. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hong M. Determination of the oligomeric number and intermolecular distances of membrane protein assemblies by anisotropic (1)H-driven spin diffusion NMR spectroscopy. J. Am. Chem. Soc. 2006;128:7242–7251. doi: 10.1021/ja0603406. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Seelig J. Dynamic properties of gramicidin A in phospholipid membranes. Biochemistry. 1988;27:2357–2364. doi: 10.1021/bi00407a017. [DOI] [PubMed] [Google Scholar]
- Mandel AM, Akke M, Palmer AG. Dynamics of ribonuclease H: Temperature dependence of motions on multiple time scales. Biochemistry. 1996;35:16009–16023. doi: 10.1021/bi962089k. [DOI] [PubMed] [Google Scholar]
- Mehring M, Mehring M, Diehl P. e. Principles of high resolution NMR in solids. Springer-Verlag; Berlin: New York: 1983. Berlin; New York. [Google Scholar]
- Munowitz M, Aue WP, Griffin RG. Two-dimensional separation of dipolar and scaled isotropic chemical shift interactions in magic angle NMR spectra. J. Chem. Phys. 1982;77:1686–1689. [Google Scholar]
- Opella SJ. Protein dynamics by solid state nuclear magnetic resonance. Methods in Enzym. 1986;131:327–361. doi: 10.1016/0076-6879(86)31048-6. [DOI] [PubMed] [Google Scholar]
- Palmer AG, Kroenke CD, Loria JP. Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Meth. Enzymol. Part B: NMR of Biol. Macromolecules. 2001;339:204–238. doi: 10.1016/s0076-6879(01)39315-1. [DOI] [PubMed] [Google Scholar]
- Palmer AG, Williams J, McDermott A. Nuclear magnetic resonance studies of biopolymer dynamics. J. Phys. Chem. 1996;100:13293–13310. [Google Scholar]
- Park SH, Mrse AA, Nevzorov AA, De Angelis AA, Opella SJ. Rotational diffusion of membrane proteins in aligned phospholipid bilayers by solid-state NMR spectroscopy. J. Magn. Reson. 2006;178:162–165. doi: 10.1016/j.jmr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Pauls KP, MacKay AL, Söderman O, Bloom M, Tanjea AK, Hodges RS. Dynamic properties of the backbone of an integral membrane polypeptide measured by 2H-NMR. Eur. Biophys. J. 1985;12:1–11. doi: 10.1007/BF00254089. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Pinto LH, Lamb RA. Controlling influenza virus replication by inhibiting its proton flow. Mol. BioSyst. 2007;3:18–23. doi: 10.1039/b611613m. [DOI] [PubMed] [Google Scholar]
- Prosser RS, Davis JH, Mayer C, Weisz K, Kothe G. Deuterium NMR relaxation studies of peptide-lipid interactions. Biochemistry. 1992;31:9355–9363. doi: 10.1021/bi00154a005. [DOI] [PubMed] [Google Scholar]
- Reuther G, Tan KT, Vogel A, Nowak C, Arnold K, Kuhlmann J, Waldmann H, Huster D. The lipidated membrane anchor of full length N-Ras protein shows an extensive dynamics as revealed by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2006;128:13840–13846. doi: 10.1021/ja063635s. [DOI] [PubMed] [Google Scholar]
- Rothwell WP, Waugh JS. Transverse Relaxation of Dipolar Coupled Spin Systems under Rf-Irradiation - Detecting Motions in Solids. J. Chem. Phys. 1981;74:2721–2732. [Google Scholar]
- Saffman PG, Delbruck M. Brownian motion in biological membranes. Proc. Natl. Acad. Sci. U. S. A. 1975;72:3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Tu Q, Pinto LH, Lamb RA. The active oligomeric state of the minimalistic influenza virus M2 ion channel is a tetramer. Proc. Natl. Acad. Sci. USA. 1997;94:5000–5005. doi: 10.1073/pnas.94.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer D, Spiess HW, Suter UW, Fleming WW. 2D solid-state NMR studies of ultraslow chain motion: glass transition in aPP versus helical jumps in iPP. Macromolecules. 1990;23:3431–3439. [Google Scholar]
- Schaefer J, Mckay RA, Stejskal EO. Dipolar rotational spin-echo 13C NMR of polymers. J. Magn. Reson. 1983;52:123–129. [Google Scholar]
- Schaefer J, Stejskal EO, Buchdahl R. Magic-Angle C-13 NMR Analysis of Motion in Solid Glassy Polymers. Macromolecules. 1977;10:384–405. [Google Scholar]
- Schaefer J, Stejskal EO, Mckay RA. Phenylalanine ring dynamics by solid-state 13C NMR. J. Magn. Reson. 1984;57:85–92. [Google Scholar]
- Schmidt C, Blumich B, Spiess HW. Deuteron two-dimensional exchange NMR in solids. J. Magn. Reson. 1988;79:269–290. [Google Scholar]
- Schmidt-Rohr K, Clauss J, Spiess HW. Correlation of structure, mobility, and morphological information in heterogeneous polymer materials by two-dimensional wideline-separation NMR spectroscopy. Macromolecules. 1992;25:3273–3277. [Google Scholar]
- Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WJ, Long JR, Campbell AA, Stayton PS, Drobny GP. A solid state NMR study of dynamics in a hydrated salivary peptide adsorbed to hydroxyapatite. J. Am. Chem. Soc. 2000;122:7118–7119. [Google Scholar]
- Smith RL, Oldfield E. Dynamic structure of membranes by deuterium NMR. Science. 1984;225:280–288. doi: 10.1126/science.6740310. [DOI] [PubMed] [Google Scholar]
- Stouffer AL, Acharya R, Salom D, Levine AS, Di Costanzo L, Soto CS, Tereshko V, Nanda V, Stayrook S, DeGrado WF. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451:596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subczynski WK, Wojas J, Pezeshk V, Pezeshk A. Partitioning and localization of spin-labeled amantadine in lipid bilayers: an EPR study. J. Pharm. Sci. 1998;87:1249–1254. doi: 10.1021/js970381n. [DOI] [PubMed] [Google Scholar]
- Tian F, Song Z, Cross TA. Orientational constraints derived from hydrated powder samples by two-dimensional PISEMA. J. Magn. Reson. 1998;135:227–231. doi: 10.1006/jmre.1998.1544. [DOI] [PubMed] [Google Scholar]
- Traikia M, Warschawski DE, Recouvreur M, Cartaud J, Devaux PF. Formation of unilamellar vesicles by repetitive freeze-thaw cycles: characterization by electron microscopy and P-31-nuclear magnetic resonance. Eur. Biophys. J. 2000;29:184–195. doi: 10.1007/s002490000077. [DOI] [PubMed] [Google Scholar]
- van Rossum BJ, de Groot CP, Ladizhansky V, Vega S, de Groot HJM. A method for measuring heteronuclear (H-1-C-13) distances in high speed MAS NMR. J. Am. Chem. Soc. 2000;122:3465–3472. [Google Scholar]
- Wang C, Takeuchi K, Pinto LH, Lamb RA. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cady SD, Balannik V, Pinto LH, DeGrado WF, Hong M. Discovery of spiro-piperidine inhibitors and their modulation of the dynamics of the M2 proton channel from influenza A virus. J. Am. Chem. Soc. 2009 doi: 10.1021/ja900063s. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Schnell JR, Chou JJ. Amantadine partition and localization in phospholipid membrane: a solution NMR study. Biochem. Biophys. Res. Commun. 2004;324:212–217. doi: 10.1016/j.bbrc.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Williams JC, McDermott AE. Dynamics of the flexible loop of triosephosphate isomerase: the loop motion is not ligand gated. Biochemistry. 1995;34:8309–8319. doi: 10.1021/bi00026a012. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Huster D, Waring A, Lehrer RI, Tack BF, Kearney W, Hong M. Orientation and Dynamics of an Antimicrobial Peptide in the Lipid Bilayer by Solid-State NMR. Biophys. J. 2001;81:2203–2214. doi: 10.1016/S0006-3495(01)75868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liivak O, Seidel A, LaVerde G, Zax DB, Jelinski LW. Supercontraction and backbone dynamics in spider silk: 13C and 2H NMR studies. J. Am. Chem. Soc. 2000;122:9019–9025. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.