Abstract
Delayed wound healing is one of the major complications in diabetes and is characterized by chronic proinflammatory response, and abnormalities in angiogenesis and collagen deposition. Sirtuin family proteins regulate numerous pathophysiological processes, including those involved in promotion of longevity, DNA repair, glycolysis and inflammation. However the role of sirtuin 6 (SIRT6), a NAD+-dependent nuclear deacetylase, in wound healing specifically under diabetic condition remains unclear. To analyze the role of SIRT6 in cutaneous wound healing, paired 6 mm stented wound were created in diabetic db/db mice and injected siRNA against SIRT6 in the wound margins (transfection agent alone and non-sensed siRNA served as controls). Wound time to closure was assessed by digital planimetry, and wounds were harvested for histology, immunohistochemistry and Western blotting. SIRT6-siRNA treated diabetic wound showed impaired healing, which was associated with reduced capillary density (CD31 staining vessels) when compared to control treatment. Interestingly, SIRT6 deficiency decreased vascular endothelial growth factor (VEGF) expression and proliferation markers in the wounds. Furthermore, SIRT6 ablation in diabetic wound promotes nuclear factor kB (NF-kB) activation resulting in increased expression of proinflammatory markers (intercellular adhesion molecule-1, vascular cell adhesion molecule-1, tumor necrosis factor-α and interleukin-1β) and increased oxidative stress. Collectively, our findings demonstrate that loss of SIRT6 in cutaneous wound aggravates proinflammatory response by increasing NF-kB activation, oxidative stress and decrease in angiogenesis in the diabetic mice. Based on these findings, we speculate that activation of SIRT6 signaling might be a potential therapeutic approach for promoting wound healing in diabetics.
Keywords: Sirtuin 6, diabetes, inflammation, angiogenesis, wound healing
Introduction
The number of people with diabetes is growing rapidly worldwide and likely to reach 591.9 million by the year 2035. Simultaneously, the burden of impaired diabetic wound healing and their complications are expected to grow up as well (1, 2). Impaired wound healing is a well-documented major challenge for the clinicians treating these diabetic wounds. The mechanisms by which increased blood glucose cause tissue injury and impaired wound healing in diabetes are still poorly understood. Chronic wounds are those that fail to heal within an expected period and are characterized by impairment of wound closure and contraction rates, reduced rate of reepithelialization and granular tissue formation (3, 4).
Hyperglycemia is a hallmark of diabetes and results in increased oxidative stress and cellular damage (5, 6). We have demonstrated that hyperglycemia leads to reactive oxygen species (ROS) generation and inflammation in diabetic heart tissue by activating NADPH oxidase and p38 mitogen activated protein kinsae, results in cardiac dysfunction in diabetic mice (7–10). Accumulation of ROS and inflammatory cytokines leads to widespread cellular damage and poor neovascularization (11). Wound healing physiology is a complex and dynamic process, which can be further classified into three interrelated phases, proliferation, inflammation and regeneration (12, 13). It is also well established that an imbalance between pro- and anti-inflammatory mediators in the diabetic wound tissue leads to vascular complications and impaired wound healing (13). Increased inflammatory cytokines, oxidative stress, and nuclear factor-kB (NF-kB) activity through suppression of transforming growth factor (TGF)-β signaling results in impaired wound healing in diabetes (14). Angiogenesis is a vital component for wound healing and the formation of new blood vessels plays an important role in provisional granulation tissue (GT) formation during wound healing process (15). Collaborative expression of angiogenic growth factors, including vascular endothelial growth factor (VEGF), TGF-β, fibroblast growth factor, and angiopoietins is important in wound angiogenesis (16). These observations indicate that a loss of tolerance to diabetes-induced excessive oxidative stress, inflammation and impaired angiogeneis may lead to impairment in wound healing. Therefore, further characterization and better understanding of the mechanisms underlying these processes during diabetes might enhance our therapeutic strategies to treat cutaneous wounds in diabetics.
There is increasing evidence that the sirtuin (SIRT) family proteins regulate multiple genes whose products are putatively involved in many cellular responses to stress, ranging from chromatin modification, genomic stability, metabolism, inflammation, cellular senescence and organismal lifespan and consequently have generated significant interest as potential therapeutic targets (17). The SIRT family consists of seven enzymes (SIRT1–SIRT7) that share a conserved core catalytic domain but differ in their cellular localisation and tissue distribution. SIRT1, the most studied mammalian homologue, has been shown to protect endothelial cells from premature senescence and promotes corneal wound healing in diabetic mice (17).
In SIRT family, SIRT6, is reflected to have a leading role in modulating genomic stability, cellular metabolism, stress response and aging (18, 19). In addition SIRT6 also act as a critical regulator of embryonic stem cell differentiation that involves the core pluripotent genes and ten-eleven translocation enzymes-dependent production of 5-hydroxymethylcytosine (20). Recently, SIRT6 (a NAD+-dependent, nuclear deacetylase) has gained attention due to its involvement in attenuation of inflammation, glucose homeostasis, and inhibition of obesity-induced metabolic dysfunction (21). Also, SIRT6 has been shown to protect against cardiac hypertrophy through attenuation of nuclear factor kB (NF-kB)-dependent transcriptional activity (22). However, its role in wound healing in diabetes has so far not been explored. In this study, we determined the role of SIRT6 in wound healing in diabetic (db/db) mice.
Materials and Methods
Animals
All animal research in this study was conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Northwestern University (Chicago, IL). Twelve-weeks-old male C57BLKS/J db/db (diabetic) mice were purchased from The Jackson Laboratories (Bar Harbor, ME).
Cutaneous wound model
Experiments utilized a stented-wound healing model described previously (23, 24). Animals were anesthetized, shaved, and prepared according to the standard sterile procedures. A six millimeter (6 mm) punch biopsy tool was used to create two circular, full-thickness cutaneous wounds (which extended through the panniculus carnosus) bilaterally on the shaved dorsal skin of diabetic mice. A donut-shaped silicone splint (Grace Bio-Labs, Bend, OR), with an external diameter of 12 mm and an internal diameter of 8 mm, was centred on the wound and affixed using cyanoacrylate adhesive (Elmer’s Inc., Columbus, OH) and interrupted 6-0 nylon sutures (Ethicon, Somerville, NJ). A semiocclusive dressing (Tegaderm, 3M, St. Paul, MN) was applied to cover the wound and splint. The animals were monitored daily.
RNA Interference
Small interfering RNA (siRNA) against SIRT6 (Qiagen Inc., Valencia, CA) was transfected into animal wounds using a previously described agarose delivery system (25). Briefly, siRNA against SIRT6 was complexed with liposomal transfection reagent and incorporated into a cooling (< 37 °C) 0.4% (w/v) liquid agarose mixture. The optimal formulation was determined to be the least concentration of siRNA necessary for efficacy and the most favorable carrier matrix handling properties. Ultimately, 20 pmol siRNA was complexed with 0.5 mL Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The agarose gel containing siRNA was applied at postwounding day 1, and postwounding day 8, based on the previous delivery studies (25).
Wound closure analysis and histology
Digital photographs were recorded on the day of surgery and every other day after wounding. A reference ruler was placed alongside to permit correction for the distance between the camera and the animals. The digital photographs obtained were analyzed photometrically for wound closure and granulation using Adobe Photoshop photometric software (Adobe Systems Incorporated, San Jose, CA).
At the time of sacrifice, the wounds were excised, bisected, and fixed in 10% formalin for 6 hours. The samples underwent routine histological processing with hematoxylin and eosin (H&E) (26). Photomicrographs were taken of the histologic sections. Digital analysis software was used to histologically determine the total area of GT from photomicrographs of these sections.
Protein isolation and Western blot analysis
Western blotting analyses were performed as previously described (7, 9, 10, 27). For Western blotting experiments, 30 μg of total protein was loaded and proteins were separated by SDS-PAGE (200 V for 40 min) and electrophoretically transferred to a nitrocellulose filters (semi-dry transfer at 10 V for 30 min). Filters were then blocked with 5% non-fat dry milk in Tris buffered saline (20 mM Tris, pH 7.6, 137 mM NaCl) with 0.1% Tween 20, washed, and then incubated with primary antibody. The blots were incubated with antibodies against rabbit polyclonal p47phox and p67phox, (Santa Cruz Biotechnology Inc., CA, USA) and beta-actin (Cell signaling). After incubation with the primary antibody, the bound antibody was visualized with horseradish peroxidase-coupled secondary antibodies (Santa Cruz Biotechnology) and chemiluminescence developing agents (Amersham Biosciences, Buckinghamshire, UK). The expression levels of each protein were quantified by densitometric analysis of corresponding band using Scion image software (28).
Wound angiogenesis
Capillary density in the healing wounds was quantified by immunohistological analysis. Sections were deparaffinized and hydrated, then placed in Tris-Buffered Saline (pH 7.5) for 5 minutes for pH adjustment. Endogenous peroxidase was blocked by 3% hydrogen peroxide/methanol bath for 20 minutes, followed by distilled water rinses. Slides were blocked with normal rabbit serum (Vector Laboratories) for 30 minutes, then incubated for 60 minutes at room temperature with an anti-CD31 antibody (1:50; BD Bioscience) and Vascular endothelial growth factor (VEGF, abcam). Slides were counterstained with Gill 2 Hematoxylin for 10 seconds, differentiated in 1% aqueous glacial acetic acid, and rinsed in running tap water. Twenty random microscopic fields (×200 magnification) were counted to determine the number of capillaries per wound.
Immunohistochemistry
Paraffin embedded tissue sections (5 μm) were deparaffinized, hydrated and washed in Tris-buffered saline (TBS; 10 mM/L Tris HCl, 0.85% NaCl, pH 7.5) containing 0.1% bovine serum albumin (BSA). Endogenous peroxidase activity was quenched by incubating the slides in methanol and 0.6% H2O2 in methanol. In all cases the primary antibody namely, SIRT6, pNF-kB, TNFα, IL-1β, intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, proliferating cell nuclear antigen (PCNA), Ki-67 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was left overnight at 4 °C. The slides were washed in TBS and horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was then added and the slides were further incubated at room temperature for 45 min. The slides were washed in TBS and incubated with diaminobenzidine tetrahydrochloride as the substrate, and counterstained with hematoxylin and observed under light microscope.
Statistical analysis
All experiments were repeated at least twice. Data are expressed as the mean ± SEM for the indicated number of independently performed duplicated experiments. Statistical significance between the means was analyzed by two-tailed Student t test using the GraphPad Prism 5 software. A value of p <0.05 was considered statistically significant.
Results
SIRT6-knockdown (KD) delays wound healing in diabetic mice
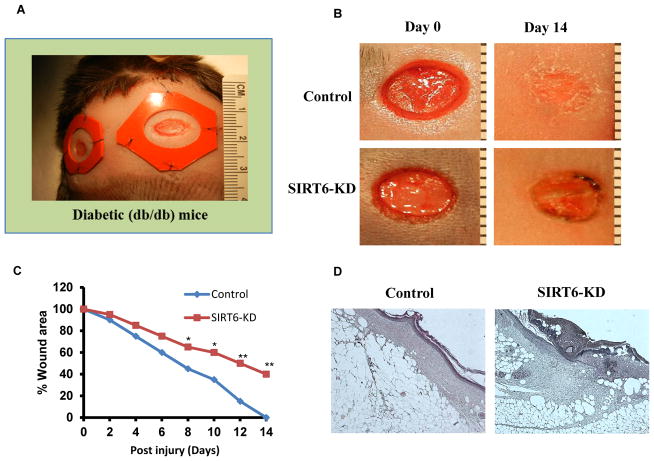
Recent study indicates that SIRT6 regulates inflammatory response in mice (29). Also, previous report has shown that SIRT6 deficiency in endothelial cells is associated with up-regulation of genes involved in inflammation and vascular remodeling (30). These studies suggest that SIRT6 might play an important role in wound healing. To test this, we evaluated if SIRT6 deficiency further aggravates impaired wound healing response in diabetic mice. As shown in Fig. 1A, two circular, full-thickness cutaneous wounds were created bilaterally on the shaved dorsal skin of diabetic mice using 6 mm punch biopsy tool. Wound areas were analyzed throughout the healing process. In nonsense siRNA-treated mice, the diabetic wounds underwent gradual and progressive healing, which reached complete closure by day 14 after injury (Fig. 1B). In contrast, db/db mice that were treated with SIRT6 siRNA (SIRT6-KD) showed marked delay in wound closure time. On day 14 after wounding, the control wounds had already lost their eschars and appeared completely epithelialized, whereas the SIRT6-KD wounds showed only partial epithelialization and still carried scab (Fig. 1B). Statistical analysis indicated that SIRT6-KD significantly delayed diabetic wound closure as compared with nonsense siRNA treatment (Fig. 1C; P<0.05 starting day 8 through day 14 after injury). Histological assessment showed that the SIRT6-KD diabetic wounds had thinner and shorter neo-epidermal sheets on the edge of the wound (Fig. 1D; H&E stained sections). Furthermore, on the day 14 postinjury, the re-epithelialization and GT formation were decreased, and the epithelial gap was increased in the SIRT6-KD diabetic wounds as compared with nonsense siRNA treatment (Fig. 1D). Our data demonstrates that SIRT6 deficiency further delays wound healing in diabetes.
Fig 1. Delayed cutaneous wound healing after SIRT6 knockdown in db/db mice.
(A) Full-thickness skin wounds were created in diabetic db/db mice. (B) Representative photographs showing the macroscopic wound closure on day 14 postinjury from Control and SIRT6-KD diabetic wounds. (C) Microphotographs of wounds was analyzed at indicated time intervals to determine closure in control and SIRT6-KD. (D) Histopathology (H–E staining) of control and SIRT6-KD wound on day 14 postinjury. SIRT6-KD wounds showed less re-epithelialization and granulation formation. A stratified neoepidermis was visible on the edge of the wounds in control, whereas the epithelial tissue was disorganized in SIRT-KD wounds. Representative results from three independent experiments with three-four animals in each group are shown. All values represent the mean ± SEM. *p < 0.05 indicates significantly and **p < 0.01 indicates very significantly higher values observed in SIRT6-KD compared with control wound.
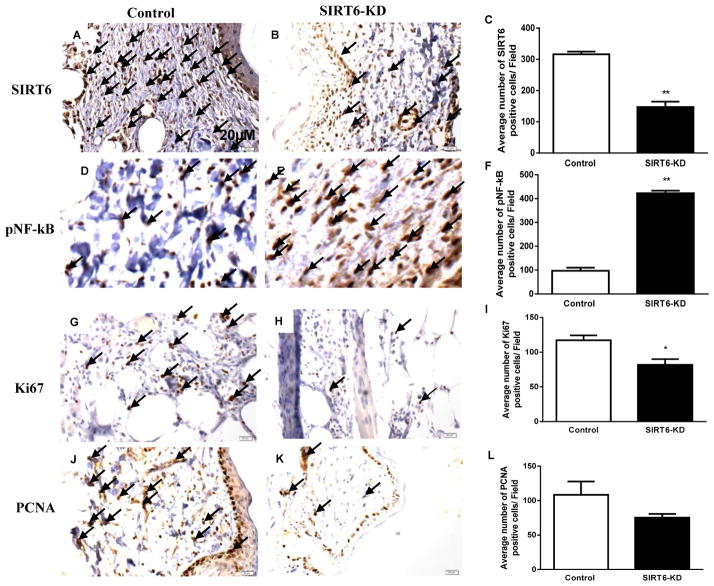
Furthermore, we confirmed SIRT6 knockdown in siRNA transfected murine wounds at day 14 postwounding. SIRT6 protein levels were measured in transfected diabetic wounds by immunohistochemsitry. The diabetic wound demonstrated a significant reduction in SIRT6-treated wounds compared with nonsense siRNA-treated wounds (P < 0.01; Fig. 2A–C).
Fig 2. SIRT6, NF-kB and proliferation markers expression in diabetic wounds with SIRT6-deficiency.
(A–B) Immunohistochemical staining for SIRT6 demonstrate reduced expression of SIRT6 within SIRT6-KD wound beds compared with nonsense-treated wounds at postwounding day 14. (C) Bar graph showing the number of SIRT6 positive cells (20 fields) in SIRT6-KD and control diabetic wounds. (D–E) Immunohistochemical staining of p-NF-kB showed increased expression of p-NF-kB positive cells in the SIRT6-KD treated diabetic wounds compared to non sense treated. (F) Bar graph showing number of p-NF-kB positive cells in SIRT6-KD and control diabetic wounds. (G–H) Immunohistochemical staining for Ki67 demonstrate reduced expression of Ki67 within SIRT6-KD wound beds compared with nonsense-treated wounds at postwounding day 14. (I) Bar graph showing the number of Ki67 positive cells (20 fields) in SIRT6-KD and control diabetic wounds. (J–K) Immunohistochemical staining of PCNA shows reduced expression of PCNA positive cells in the SIRT6-KD treated diabetic wounds compared to non sense treated. (L) Bar graph showing number of PCNA positive cells in SIRT6-KD and control diabetic wounds. Representative results from three independent experiments with three-four animals in each group are shown. Values are means ± SEM. *p; **p < 0.01 vs Control.
Silencing SIRT6 increases pNF-kB expression in cutaneous diabetic wound
Previous study has demonstsrated that SIRT6 interacts with RelA subunit of NF-kB and deacetalytes histone H3K9 at NF-kB target gene promoters, which leads to their repression (29). Also, our earlier studies indicated that hyperglycemia leads to hyperactivation of NF-kB signaling and is, thus, the likely cause for increased inflammatory response (7). These reports allowed us to speculate that the protective function of SIRT6 might be exerted through regulation of NF-kB signaling pathway during the process of wound healing in diabetes. In order to determine the effects of SIRT6-KD on NF-kB expression during diabetic wound healing, stented wounds in db/db mice were harvested for immunohistochemsitry following the application of SIRT6 siRNA or nonsense siRNA. At 14 days postwounding, delayed wound healing was associated with a significant increase in pNF-kB positive stained cells in SIRT6 siRNA treated wounds compared with nonsense siRNA-treated diabetic wounds (P < 0.01; Fig. 2D–F). These data suggest that SIRT6 defeciency leads to hyperactivation of NF-kB and might promote inflammatory response in diabetic wound.
Knockdown of SIRT6 aggravates oxidative stress in cutaneous wounds of diabetic mice
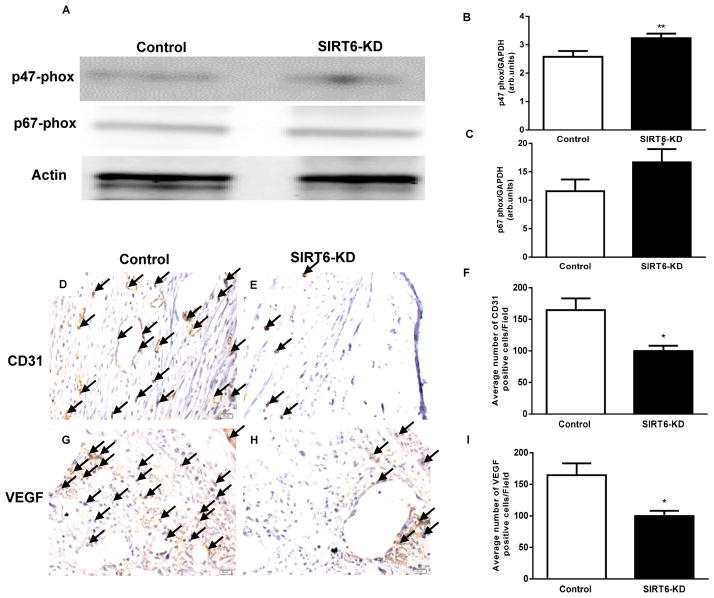
Oxidative stress is known to be a major cause of complications in diabetes and leads to delayed wound healing (31). Previous report has shown that antioxidant therpy reverses the complications of diabetes via SIRT1 signaling (32), therefore suggestive of an important role for SIRT6 in diabetes-induced, oxidative stress-mediated complications(32). Recently, we have reported that diabetic heart is associated with increased oxidative stress via upegulation of NADPH oxidase subunits expression (9). To determine the effect of SIRT6-KD on oxidative stress, we assessed NADPH oxidase subunits (p47 phox and p67 phox) in the wounds using Western blotting. SIRT6-KD significantly increased p47 phox and p67 phox levels as compared to control siRNA-treated diabetic wounds (P < 0.01; Fig 3A–C), suggesting that SIRT6 deficiency promotes oxidative stress in diabetic wound.
Fig 3. Deficiency of SIRT6 aggravates oxidative stress and markedly impairs wound vascularity in diabetic mice.
(A–C) Representative western immunoblots and densitometry analysis using Image Quant software for p47 phox and p67 phox in SIRT6-KD and control diabetic wounds; Blots were normalized against Actin. White and black bars represent Control and SIRT6-KD diabetic wounds respectively. (D–E) Immunohistochemical staining for CD31 demonstrate reduced expression of CD31 within SIRT6-KD wound beds compared with nonsense-treated wounds at postwounding day 14. (F) Bar graph showing the number of CD31 positive cells (20 fields) in SIRT6-KD and control diabetic wounds. (G–H) Immunohistochemical staining of VEGF shows reduced expression of VEGF positive cells in the SIRT6-KD treated diabetic wounds compared to non sense treated. (I) Bar graph showing number of VEGF positive cells in SIRT6-KD and control diabetic wounds. Representative results from three independent experiments with three-four animals in each group are shown. Values are means ± SEM. *p < 0.05 ; **p < 0.01 vs. Control diabetic wounds on the same day.
SIRT6 knockdown markedly impairs wound vascularity in db/db diabetic mice
Poorly healing diabetic wounds are characterized by inflammation and impaired angiogenesis (33). To determine vascularity, cutaneous wound in db/db diabetic mice were transfected with either control or SIRT6 siRNA, wounds were harvested at day 14 post-wounding, and the sections were stained with CD31 and VEGF specific antibodies to assess vasculature and proangiogenic factor. As shown in Fig. 3D–F, SIRT6-KD significantly reduced CD31-positive vascular structures (P < 0.01) as compared to control siRNA-treated wounds. Furthermore, reduction in CD31 in the wounds was associated with a significant decrease in VEGF staining in SIRT6-KD wounds (P < 0.01; Fig 3G–I). These data suggest that SIRT6 might play an important role in angiogenesis and vasculogenesis in cutaneous wounds of db/db mice.
SIRT6-deficiency increases inflammatory response in wounds of diabetic mice
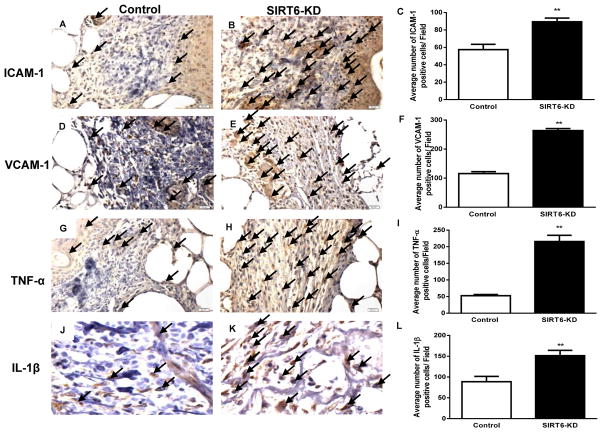
Inflammatory cytokines play a pivotal role in delayed wound healing (33). We assessed the effect of SIRT6-KD on inflammatory response in cutaneous diabetic wounds. Immunohistochemsitry on histological sections of wound demonstrated that ICAM-1 and VCAM-1 positive cells were significantly increased in SIRT6 siRNA-treated diabetic wounds compared to control siRNA treated wounds (Fig 4A–F). Interestingly, SIRT6-KD also significantly increased proinflammatory cytokines, TNFα and IL-1β in diabetic wounds (P < 0.01; Fig 4G–L). These data suggest that SIRT6 deficiency promotes proinflammatory response in diabetic wound, which might be possibly due to increased NFkB activity.
Fig 4. SIRT6 knockdown increases cellular adhesion markers and inflammatory cytokines in wounds of diabetic mice.
(A–B) Immunohistochemical staining for ICAM-1 demonstrate increased expression of ICAM-1 within SIRT6-KD wound beds compared with nonsense-treated wounds at postwounding day 14. (C) Bar graph showing the number of ICAM-1 positive cells (20 fields) in SIRT6-KD and control diabetic wounds. (D–E) Immunohistochemical staining of VCAM-1 shows increased expression of VCAM-1 positive cells in the SIRT6-KD treated diabetic wounds compared to non sense treated. (F) Bar graph showing number of VCAM-1 positive cells in SIRT6-KD and control diabetic wounds. (G–H) Immunohistochemical staining for TNF-α demonstrate increased expression of TNF-α within SIRT6-KD wound beds compared with nonsense-treated wounds at postwounding day 14. (I) Bar graph showing the number of TNF-α positive cells (20 fields) in SIRT6-KD and control diabetic wounds. (J–K) Immunohistochemical staining of IL-1β shows increased expression of IL-1β positive cells in the SIRT6-KD treated diabetic wounds compared to non sense treated. (L) Bar graph showing number of IL-1β positive cells in SIRT6-KD and control diabetic wounds. Representative results from three independent experiments with three-four animals in each group are shown. Values are means ± SEM. **p < 0.01 vs Control.
Furthermore, HE staining was performed for analysis of infiltration of inflammatory cells. The numbers of mononuclear cells were assessed in H&E stained sections. SIRT6-KD resulted in a significant increase in the number of inflammatory cells on day 14 in diabetic wounds compared to control siRNA-treated diabetic wounds (P < 0.01, Fig. 1D).
SIRT6-KD impairs cell proliferation in wounds of diabetic mice
To explore the effect of SIRT6-KD on cell proliferation in the wound tissues, we assessed the expression of PCNA and Ki67 (markers of cell proliferation) by immunohistochemical staining. As shown in Fig. 2(G – L), the expression of PCNA and Ki67 was significantly lower in SIRT6 siRNA-treated wounds compared with the control siRNA-treated diabetic wounds (P < 0.05). These data suggest that SIRT6-KD negatively impairs cell proliferation in diabetic wound.
Discussion
Diabetes imposes a large economic burden on individuals and families and national healthcare systems. Expenditures on diabetes care in the US alone surpassed $263 billion in 2013 (2). Diabetes complications, such as impaired wound healing, represent a significant medical problem, with the annual treatment cost of diabetic lower extremity ulcers alone exceeding 1.5 billion dollars (34), highlighting the importance of this problem. In addition, an ulcer of the lower extremity precedes 84% of all diabetic lower extremity amputations and is the primary cause for hospitalization among diabetics (30). Currently there are no fully effective strategies for the prevention or treatment of these complications.
This study evaluated the effect of SIRT6 knockdown on wound healing response in diabetic mice, with specific focus on inflammation, angiogenesis and oxidative stress. The major new findings were as follows: SIRT6-KD in cutaneous wounds- (1) impaired diabetic wound closure; (2) diminishes wound vascularity; (3) increases the expression of pNF-kB; (4) aggravates oxidative stress; (5) increased inflammatory response; and (6) impairs cellular proliferation. Taken together, these findings demonstrate that SIRT6 deficiency impairs wound healing in diabetes and we speculate that activation of SIRT6 signaling might be a potential approach for promoting wound healing in diabetics. Evidence from our study suggests that targeting SIRT6/NF-kB pathway might have a potential positive impact on clinical therapy of diabetes-induced impaired wound healing.
Recent study has shown that SIRT6 plays a role in inflammation in mice (29) and previous report on endothelial cells showed that SIRT6 deficiency is associated with up-regulation of genes involved in inflammation and vascular remodeling (30). To date, there is no studies to indicate a possible role for SIRT6 in wound healing in diabetes and the present study is novel in that direction. Angiogenesis is vital during the proliferation phase of wound closure and involves the formation of new blood vessels in order to stimulate granulation tissue formation and remodeling (35). Diminished production of VEGF and decreased angiogenesis are thought to contribute to impaired tissue repair in diabetic patients (35). In the present study, we show evidence that SIRT6-KD in the diabetic wounds markedly down-regulated VEGF and is associated with decreased CD31 positive cells and increased inflammatory cytokines as compared to control-siRNA treated wounds. These results suggest that SIRT6 might play an important role in chronic inflammatory reaction and reduced angiogenesis in cutaneous wounds of diabetics. In line with the above evidence, we noticed lower expression of SIRT6 and concomitantly higher levels of oxidative stress, inflammatory cytokine and increased NF-kB activation in diabetic wounds. These data are consistent with our previous findings that inflammatory response and oxidative stress are higher in diabetic tissue (7–9, 36, 37). Furthermore, our findings in the present study are in agreement with previous studies demonstrating that loss of SIRT6 in endothelial cells increases expression of proinflammatory transcription factor NF-kB (30). Conversely, the authors show that overexpression of SIRT6 is associated with a downregulation of NF-kB activity and target gene expression (30). Taken together, these findings suggest that SIRT6, a critical enzyme in the maintenance of genomic stability, could also play a key role in angiogenesis by acting in the pathway(s) controlling for vascular oxidative stress, inflammation and cellular damage (38). Furthermore, our observation that reduced SIRT6 levels in diabetic wound provides novel understanding of the signaling pathways involved in impaired wound healing in diabetes. However, although our findings suggest that SIRT6 loss in diabetes further exacerbates oxidative stress, inflammation and NF-kB activation, the mechanism is unclear.
Oxidative stress has been shown to be critical in several pathological and physiological processes, including the progression of wound healing (39). One of the leading causes of impaired wound healing in diabetes is oxidative stress (40). ROS and oxidative stress arise from inflammatory cells, which are strongly implicated in the pathogenesis of several diseases including chronic ulcers (40). Hyperglycemia can induce the activation of p22 phox, p47 phox and p67 phox, components of the NADPH oxidase by regulating mitogen activated protein kinase/NF-kB signaling (8). ROS are thought to act as cellular messengers to activate key processes associated with wound healing, including, angiogenesis and cytokine activity (39). Recently, Balestrieri ML et al (41) reported that impairment in SIRT6 signaling in diabetic patient atherosclerotic lesions (as compared with non-diabetic lesions) was associated with increased oxidative stress and higher NF-κB and pro-inflammatory cytokines. Here, we report that loss of SIRT6 increased oxidative stress (NAPDH oxidase subunits) and NF-kB expression in association with increased inflammatory response and reduced angiogenesis and proliferation in diabetic wounds, suggesting the involvement of oxidative stress in SIRT6-KD-mediated effects on wound repair in diabetes. However, the role of SIRT6 in diabetes-induced NADPH oxidase activation needs further clarification. These findings suggest that SIRT6 plays a crucial role in limiting NF-kB mediated inflammation and oxidative stress in the diabetic wound. Recently approved wound treatment gels for diabetic chronic ulcers have been shown to increase oxidative stress in the early phase of wound healing followed by a decrease in the late phase (40). However, further studies are necessary to characterize the time- and dose-dependent effects of exogenous SIRT6 administration or endogenous SIRT6 overexpression on oxidative stress during cutaneous wound healing.
Wound healing is a dynamic and synchronized molecular process that sequentially involves cell recruitment, proliferation, extracellular matrix deposition and remodeling. Inflammation plays a critical role in tissue regeneration and timely initiation and resolution of inflammation are equally important for tissue repair mechanisms (42, 43). NF-kB might participate in some of the downstream effects of NADPH oxidase on diabetic wound and also regulates the expression of inflammatory genes, including TNF-α and IL-6 (8, 42). Impairment in diabetic wound is associated with increased levels of the NF-kB and pro-inflammatory cytokines (42). Recent report demonstrated that SIRT6 had an inhibitory effect on NF-kB inflammatory signaling pathways by decreasing mRNA levels of the NF-kB downstream target genes TNF-α, ICAM-1, IL-2, and IL-6 (44). Moreover, atherosclerotic plaque from diabetes patients had more inflammation and oxidative stress, along with reduced SIRT6 and increased NF-kB expression (41). In the present study, we found that SIRT6 deficiency leads to impaired wound healing in diabetic mice and were associated with reduced wound closure, proliferation and angiogenesis, and increased TNF-α, IL-1β and cellular adhesion molecules in diabetic wound. These results suggest that SIRT6-KD-associated impairment in wound healing is possibly mediated through NF-kB signaling. However, to enhance the translational relevance of the findings, analysis of clinical specimens based on this concept is warranted.
In summary, our study demonstrates the critical role of SIRT6 in cutaneous wound healing under diabetic condition. Therefore, targeting of the SIRT6/NF-kB pathway may be a valuable therapeutic strategy to treat impaired wound healing in diabetics.
Acknowledgments
This work was supported, in part, by the National Institutes of Health (NIH) grants 1R01HL116729 (to P.K.); HL091983, HL053354, HL108795 and HL108806 (to R.K.); and American Heart Association National-The Davee Foundation SDG Grant 0530350N (to P.K.).
Footnotes
Author contributions
RAT and PK study conception and design; VKSG, SVK and RAT performed experiments and drafting manuscript; DJ, SSB, MK and PJ analysis and interpretation of data; RAT, SA, KW, RJ, ARM and PK revising article, critically for important intellectual content; All the authors approved the final version to be published. All authors discussed the results and implication and commented on the manuscript at all stages.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Ethics approval for animal care
All animal research in this study was conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Northwestern University (Chicago, IL).
References
- 1.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, et al. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Cho NH, Tajima N, et al. Diabetes in the Western Pacific Region--past, present and future. Diabetes research and clinical practice. 2014;103:244–255. doi: 10.1016/j.diabres.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Bitar MS, Labbad ZN. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. The Journal of surgical research. 1996;61:113–119. doi: 10.1006/jsre.1996.0090. [DOI] [PubMed] [Google Scholar]
- 4.Yue DK, Swanson B, McLennan S, et al. Abnormalities of granulation tissue and collagen formation in experimental diabetes, uraemia and malnutrition. Diabetic medicine : a journal of the British Diabetic Association. 1986;3:221–225. doi: 10.1111/j.1464-5491.1986.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 6.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicology and applied pharmacology. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Thandavarayan RA, Giridharan VV, Sari FR, et al. Depletion of 14-3-3 protein exacerbates cardiac oxidative stress, inflammation and remodeling process via modulation of MAPK/NF-kB signaling pathways after streptozotocin-induced diabetes mellitus. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;28:911–922. doi: 10.1159/000335805. [DOI] [PubMed] [Google Scholar]
- 8.Thandavarayan RA, Giridharan VV, Watanabe K, et al. Diabetic cardiomyopathy and oxidative stress: role of antioxidants. Cardiovascular & hematological agents in medicinal chemistry. 2011;9:225–230. doi: 10.2174/187152511798120877. [DOI] [PubMed] [Google Scholar]
- 9.Thandavarayan RA, Watanabe K, Ma M, et al. Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. American journal of physiology Heart and circulatory physiology. 2009;297:H911–919. doi: 10.1152/ajpheart.00124.2009. [DOI] [PubMed] [Google Scholar]
- 10.Thandavarayan RA, Watanabe K, Ma M, et al. 14-3-3 protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochemical pharmacology. 2008;75:1797–1806. doi: 10.1016/j.bcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Tie L, Li XJ, Wang X, et al. Endothelium-specific GTP cyclohydrolase I overexpression accelerates refractory wound healing by suppressing oxidative stress in diabetes. American journal of physiology Endocrinology and metabolism. 2009;296:E1423–1429. doi: 10.1152/ajpendo.00150.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 13.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Frontiers in bioscience : a journal and virtual library. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 14.Dasu MR, Thangappan RK, Bourgette A, et al. TLR2 expression and signaling-dependent inflammation impair wound healing in diabetic mice. Lab Invest. 90:1628–1636. doi: 10.1038/labinvest.2010.158. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 16.Schweigerer L, Neufeld G, Friedman J, et al. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Zhao X, Shi D, et al. Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1R/AKT pathway. Invest Ophthalmol Vis Sci. 54:3806–3814. doi: 10.1167/iovs.13-12091. [DOI] [PubMed] [Google Scholar]
- 18.Kanfi Y, Peshti V, Gil R, et al. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging cell. 2010;9:162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 19.Michishita E, McCord RA, Berber E, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etchegaray JP, Chavez L, Huang Y, et al. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nature cell biology. 2015;17:545–557. doi: 10.1038/ncb3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardus A, Uryga AK, Walters G, et al. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res. 97:571–579. doi: 10.1093/cvr/cvs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu SS, Cai Y, Ye JT, et al. Sirtuin 6 protects cardiomyocytes from hypertrophy in vitro via inhibition of NF-kappaB-dependent transcriptional activity. British journal of pharmacology. 2013;168:117–128. doi: 10.1111/j.1476-5381.2012.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galiano RD, Michaels Jt, Dobryansky M, et al. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 24.Michaels Jt, Churgin SS, Blechman KM, et al. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen. 2007;15:665–670. doi: 10.1111/j.1524-475X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 25.Thanik VD, Greives MR, Lerman OZ, et al. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14:1305–1308. doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 26.Karuppagounder V, Arumugam S, Thandavarayan RA, et al. Modulation of HMGB1 translocation and RAGE/NFkappaB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Experimental dermatology. 2015 doi: 10.1111/exd.12685. [DOI] [PubMed] [Google Scholar]
- 27.Thandavarayan RA, Watanabe K, Sari FR, et al. Modulation of doxorubicin-induced cardiac dysfunction in dominant-negative p38alpha mitogen-activated protein kinase mice. Free radical biology & medicine. 2010;49:1422–1431. doi: 10.1016/j.freeradbiomed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Sreedhar R, Arumugam S, Thandavarayan RA, et al. Myocardial 14-3-3eta protein protects against mitochondria mediated apoptosis. Cellular signalling. 2015;27:770–776. doi: 10.1016/j.cellsig.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara TL, Michishita E, Adler AS, et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes care. 1999;22:157–162. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- 31.Lan CC, Wu CS, Huang SM, et al. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: new insights into impaired diabetic wound healing. Diabetes. 2013;62:2530–2538. doi: 10.2337/db12-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitada M, Kume S, Imaizumi N, et al. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60:634–643. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen SL, Myers CA, Charboneau A, et al. HoxD3 accelerates wound healing in diabetic mice. The American journal of pathology. 2003;163:2421–2431. doi: 10.1016/S0002-9440(10)63597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington C, Zagari MJ, Corea J, et al. A cost analysis of diabetic lower-extremity ulcers. Diabetes care. 2000;23:1333–1338. doi: 10.2337/diacare.23.9.1333. [DOI] [PubMed] [Google Scholar]
- 35.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Medicinal research reviews. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe K, Thandavarayan RA, Gurusamy N, et al. Role of 14-3-3 protein and oxidative stress in diabetic cardiomyopathy. Acta physiologica Hungarica. 2009;96:277–287. doi: 10.1556/APhysiol.96.2009.3.3. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe K, Thandavarayan RA, Harima M, et al. Role of differential signaling pathways and oxidative stress in diabetic cardiomyopathy. Current cardiology reviews. 2010;6:280–290. doi: 10.2174/157340310793566145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etchegaray JP, Zhong L, Mostoslavsky R. The histone deacetylase SIRT6: at the crossroads between epigenetics, metabolism and disease. Current topics in medicinal chemistry. 2013;13:2991–3000. doi: 10.2174/15680266113136660213. [DOI] [PubMed] [Google Scholar]
- 39.Lin YT, Chen JS, Wu MH, et al. Galectin-1 accelerates wound healing by regulating the neuropilin-1/Smad3/NOX4 pathway and ROS production in myofibroblasts. The Journal of investigative dermatology. 2015;135:258–268. doi: 10.1038/jid.2014.288. [DOI] [PubMed] [Google Scholar]
- 40.Lee YH, Chang JJ, Chien CT, et al. Antioxidant sol-gel improves cutaneous wound healing in streptozotocin-induced diabetic rats. Experimental diabetes research. 2012;2012:504693. doi: 10.1155/2012/504693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balestrieri ML, Rizzo MR, Barbieri M, et al. Sirtuin 6 Expression and Inflammatory Activity in Diabetic Atherosclerotic Plaques: Effects of Incretin Treatment. Diabetes. 2014 doi: 10.2337/db14-1149. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Wu W, Zhang L, et al. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes. 2012;61:2906–2912. doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niebuhr M, Muhlradt PF, Wittmann M, et al. Intracutaneous injection of the macrophage-activating lipopeptide-2 (MALP-2) which accelerates wound healing in mice--a phase I trial in 12 patients. Experimental dermatology. 2008;17:1052–1056. doi: 10.1111/j.1600-0625.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Sun T, Wu J, et al. Icariin Intervenes in Cardiac Inflammaging through Upregulation of SIRT6 Enzyme Activity and Inhibition of the NF-Kappa B Pathway. BioMed research international. 2015;2015:895976. doi: 10.1155/2015/895976. [DOI] [PMC free article] [PubMed] [Google Scholar]