Abstract
Background
The antifungal agent, voriconazole, is associated with phototoxicity and photocarcinogenicity. Prior work has indicated that voriconazole and its hepatic N-oxide metabolite do not sensitize keratinocytes to ultraviolet B (UVB). Clinical observations have suggested ultraviolet A (UVA) may be involved.
Objectives
To determine the photochemistry and photobiology of voriconazole and its major hepatic metabolite, voriconazole N-oxide.
Methods
Voriconazole and voriconazole N-oxide were spectrophotometrically monitored following various doses of UVB. Cultured human keratinocytes were treated with parental drugs or with their UVB photoproducts, and survival following UVA irradiation was measured by thiazolyl blue metabolism. Reactive oxygen species and 8-oxoguanine were monitored by fluorescence microscopy.
Results
Voriconazole and voriconazole N-oxide have varying ultraviolet B (UVB) absorption but do not acutely sensitize cultured human keratinocytes following UVB exposure. However, sustained UVB exposures produced notable dose- and solvent-dependent changes in the absorption spectra of voriconazole N-oxide which in aqueous solution acquires a prominent ultraviolet A (UVA) absorption band, suggesting formation of a discrete photoproduct. Neither the parental drugs nor their photoproducts sensitized cells to UVB though all but voriconazole N-oxide were moderately toxic to cells in the dark. Notably, both voriconazole N-oxide and its UVB photoproduct, but not voriconazole or its photoproduct, additionally sensitized cells to UVA by >3-fold relative to controls in association with UVA-induced reactive oxygen species and 8-oxoguanine levels.
Conclusions
Voriconazole N-oxide and its UVB-photoproduct act as UVA-sensitizers that generate reactive oxygen species and that produce oxidative DNA damage. These results suggest a mechanism for the phototoxicity and photocarcinogenicity observed with voriconazole treatment.
Introduction
Voriconazole is a triazole antifungal agent that is widely used as prophylaxis for and treatment of fungal infections in solid organ and hematopoietic cell transplant recipients and other immunosuppressed patients. The drug is generally well-tolerated but, among its side-effects, photosensitivity occurs in approximately 8% of patients, typically after 120 days of treatment 1. The incidence of phototoxicity has been reported to be one-third of children receiving voriconazole 2.
Starting in 2007, reports emerged that European patients receiving voriconazole were developing aggressive squamous cell carcinomas 3,4. Shortly thereafter, cases emerged in the United States of both non-melanoma and less commonly melanoma in situ skin cancers 5,6. Most subsequent reports have further confirmed the risk of skin cancer in voriconzaole-treated patients 7,8, particularly in those receiving long-term treatment 9–12. The squamous cell carcinomas associated with voriconazole have been on sun-exposed skin and associated with a pigmentation pattern that has been described as reminiscent of the lentigines caused by psoralen plus UVA photochemotherapy or of freckling in the nucleotide excision repair-deficiency syndrome, xeroderma pigmentosum 5. Further supporting the idea that these skin cancers are related to sun exposure, patients in geographic locations with higher levels of ultraviolet radiation were at increased risk of squamous cell carcinomas 8. Most of the reported patients have been immunosuppressed, as many of the larger epidemiological studies have been on the lung transplant population 7–11, although it has been argued that such studies are confounded 13. Interestingly, squamous cell carcinomas tend to occur only after chronic exposure to voriconazole, with a mean time of 41 weeks until photosensitivity occurs, and a mean of 36 months from the onset of treatment before onset of the cancer 1. The latency of voriconazole photosensitivity is in contrast to many photosensitizing drugs that typically produce symptoms much sooner following their administration. A recent study found that the onsets of photosensitivity, actinic keratoses and squamous cell carcinomas following initiation of voriconazole therapy were temporally sequenced, consistent with a multi-step process 14.
The mechanism by which voriconazole produces its photosensitizing and photocarcinogenic effects is not known. Voriconazole itself has relatively weak absorbance in the UVB (290–320 nm) and UVA (320–400 nm) spectral regions which are relevant for terrestrial solar exposure, so speculation has focused on voriconazole N-oxide (VNO), the major hepatic metabolite of voriconazole that constitutes 72% of circulating metabolites and which possesses stronger UVB and UVA absorbance (Fig. 1) 15,16. Cytochrome P450 enzymes, principally CYP2C19 and CYP3A4, metabolize voriconazole to the N-oxide as well as hydroxyl derivatives, and CYP2C19 polymorphisms have been speculated to be one factor governing the risk of phototoxicity 5,17,18. Since keratinocytes also express many of the same cytochrome P450 enzymes, it is possible that VNO, rather than solely traveling from liver to skin, is locally generated in skin epidermis where voriconazole appears to accumulate 19–21. However, our initial studies have shown that neither voriconazole nor VNO are sensitized by UVB in vitro, suggesting that these molecules themselves are not direct UVB photosensitizing agents 16.
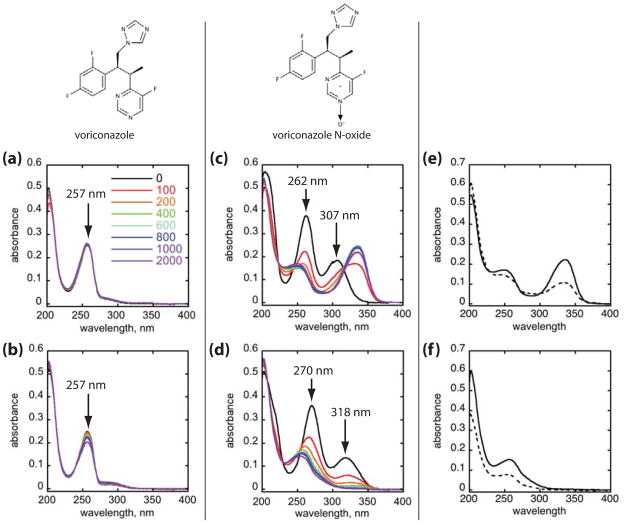
Figure 1.
UVB photoconversion of voriconazole and voriconazole N-oxide. Chemical structures of each compound are shown at top. Absorption spectra of voriconazole (a, b) and voriconazole N-oxide (c, d) in either PBS (a, c) or ethanol (b, d). Compounds at a concentration of 30 μM were UVB irradiated in increasing doses from 0–2,000 J/m2 (0–200 mJ/cm2). Color legend in panel (a) applies to all panels (a–d), with black indicating the pre-irradiation spectrum, and progressively increasing cumulative UVB doses in units of J/m2 are denoted by the color change from red to violet. Voriconazole N-oxide originally dissolved in either PBS (e) or ethanol (f) was irradiated with 2000 J/m2 (200 mJ/cm2) UVB and the reaction mixtures were dried and redissolved in either PBS or ethanol for absorption spectrophotometry. Solid lines represent resuspension of the photoreaction mixture in the same solvent, and dashed lines represent resuspension in the other solvent.
UVA sensitivity, however, has been reported in patients 22, and untested is the possibility that UVA acts on voriconazole or its metabolites, generating reactive oxygen species (ROS) much as other photosensitizing molecules do 23–25. In this report, we demonstrate that UVB leads to changes in VNO, including acquisition of significant UVA absorption. In aqueous media, both VNO and its UVB photoproduct prove to be UVA-photosensitizers, leading to generation of ROS and 8-oxoguanine.
Materials and Methods
Chemicals
Voriconazole and VNO (Santa Cruz Biotechnology, Inc., Dallas, TX) were dissolved in either phosphate-buffered saline (PBS) or 95% ethanol as 30 mM stock solutions and were used immediately or stored at −20°C until used. All manipulations involving these drugs or their photoproducts were performed under dim yellow light (General Electric F32T8 GO, Fairfield, CT).
Cell culture
Normal human epidermal keratinocytes were obtained from neonatal foreskin as previously described 26. Keratinocytes were used within one passage and were cultured in Medium 154 supplemented with Human Keratinocyte Growth Supplement and 0.07 mM CaCl2 (Invitrogen, Grand Island, NY). GM637, an SV40-transformed human fibroblast cell line, and XP12RO-SV40, an SV40-transformed fibroblast cell line from a xeroderma pigmentosum complementation group A individual, were originally obtained from J.E. Cleaver and P.C. Hanawalt, respectively, and were both grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum.
Irradiation
The UVB irradiation device was a parallel array of two UVB lamps (General Electric FS20T12) whose output was filtered through Kodacel film, resulting in emission throughout the UVB range 27,28. Although these lamps also emit in the UVA, at the maximal UVB doses delivered in this study (708 J/m2 or 70.8 mJ/cm2), the UVA output was measured to be less than 0.003 J/cm2 (3 mJ/cm2) or approximately three orders of magnitude lower than the lowest dose delivered by the true UVA lamps used in this study’s experiments. The UVA irradiation device was a parallel array of six UVA lamps (Sylvania F15T8/350BL, Danvers, MA) whose output was filtered through common plate glass, resulting in irradiance from 320 nm into the visible range 29. Pure chemical solutions were irradiated through 1 cm pathlength quartz fluorescence cuvettes; photoproducts of voriconazole and VNO were generated from voriconazole and VNO 30 mM stock solutions in PBS or ethanol, and confirmed spectrophotometrically. The voriconazole and VNO photoproducts (denoted vori’ and VNO’, respectively) subsequently used for experiments with cells were generated by irradiating 30 mM voriconazole or VNO in PBS with 2000 J/m2 (200 mJ/cm2) UVB and were stored at −20°C if not used immediately. Cells were irradiated in PBS from above with lids removed. All dosimetry was monitored by an IL1400 photometer equipped with a SEL240 UVB or SEL033 UVA detector (International Light, Newburyport, MA)x. Unirradiated controls were sham irradiated but otherwise physically and temporally handled identically to irradiated samples, both during incubation of cells as well as in manipulation outside the incubator.
Spectrophotometry
Spectroscopic studies were conducted at room temperature (~20°C) on solutions in 3 mL quartz cuvettes with 1 cm pathlength. Absorption spectra were recorded on a Beckman DU530 spectrophotometer with a scan rate of 1 nm/s. Emission spectra were recorded on a SpectraFluorophotometer RF-5301PC fluorimeter (Shimadzu, Columbia, MD) with an excitation wavelength of 250 nm, and excitation and emission slit widths of 5 nm and a scan rate of 1 nm/s.
Thiazolyl blue survival assay
For survival experiments, cells were plated in triplicate and allowed to incubate in growth medium containing 30 μM voriconazole, voriconazole N-oxide or their corresponding UVB-induced photoproducts for 24 hours prior to irradiation. Cells treated with PBS served as controls. After 5–7 days following irradiation, cells were washed with PBS, and incubated in 0.5 mg/mL thiazolyl blue tetrazolium bromide (Sigma-Aldrich, St. Louis, MO) in medium for up to 4 hours, washed in PBS again, and the resulting formazan salt was dissolved in dimethylsulfoxide and absorbance was measured at 540 nm on a microplate spectrophotometer (Thermo Scientific, Waltham, MA).
ROS and 8-oxoguanine assays
Cells were plated in chambered #1 borosilicate glass slides (Thermo Scientific, Rochester, NY) and treated with drug in growth medium for 30 minutes. For measurement of reactive oxygen species, medium was replaced with PBS containing 10 μM 5-(and 6-)chloromethyl-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, Invitrogen) with or without drug for 30 minutes prior to UVA irradiation after which live cells were visualized. CM-H2DCFDA is non-fluorogenic but becomes so intracellularly and is converted to the fluorescent dichlorofluorescein diacetate upon exposure to reactive oxygen species. For assay of 8-oxoguanine, cells were incubated with drug alone for 30 minutes prior to UVA irradiation, then immediately fixed in 4% paraformaldehyde for 10 min. followed by ice-cold acetone:methanol (1:1) for 20 min. Cells were washed with PBS, blocked in 1% bovine serum albumin (BSA) and 10% fetal bovine serum for 1 hour at 37°C, incubated with mouse monoclonal antibody to 8-oxoguanine (Abcam 2Q2311, 1:75) overnight at 4°C, washed with PBS and then incubated with goat anti-mouse IgM antibody conjugated to AlexaFluor 488 (Life Technologies, Grand Island, NY, 1:1000) for 1 hour at room temperature before mounting in Prolong Gold (Invitrogen) and visualization of both DAPI signals identifying nuclei and the green fluorescence associated with the AlexaFluor 488. DAPI images are not shown to conserve space. For both reactive oxygen species and 8-oxoguanine measurements, cells were visualized and imaged with a Zeiss Axiovert 200 microscope. For quantitative analysis of images, mean nuclear fluorescence intensity was counted in at least 200 randomly chosen cells from multiple images from two independent samples using Axiovision software, version 4.7 (Carl Zeiss, Jena, Germany).
Statistical analysis
Comparisons between replicate experimental groups were performed with ANOVA using the Bonferroni test in Kaleidagraph 4.1 (Synergy Software, Reading, PA). Except where noted, error bars depicted in figures represent the standard error. Differences between groups were regarded as significant when p values were <0.05.
Results
Spectroscopic changes in voriconazole and voriconazole N-oxide following UVB
Voriconazole and voriconazole N-oxide in either PBS or ethanol were irradiated with UVB doses varying from 0 – 2,000 J/m2 (0–200 mJ/cm2), and absorption spectra were recorded at each dose (Fig. 1). Unirradiated voriconazole in both PBS and ethanol exhibited a principal absorption band centered at 257 nm (Fig. 1a, b). With progressive UVB exposures up to 2,000 J/m2 (200 mJ/cm2), only subtle changes in the position and intensity of the absorption band occurred in each solvent.
In contrast, the absorption spectrum of VNO exhibited a strong solvent- and UVB-dependence (Fig. 1c, d). In PBS, in addition to the short wavelength ultraviolet absorption centered at 262 nm, the presence of the N-oxide group significantly enhanced absorption in the UVB range relative to that of voriconazole with the appearance of a new discrete and relatively strong absorption band centered at 307 nm which also extended significantly into the UVA range. The absorption maxima of both of the bands were strongly solvent-dependent, shifting to longer wavelengths in ethanol relative to PBS.
In PBS, UVB irradiation rapidly shifted VNO’s absorption maximum at 262 nm toward 250 nm with a loss of absorption intensity while the longer wavelength maximum at 307 nm shifted to 337 nm and increased in intensity, further increasing absorption within the UVA range (Fig. 1c). The presence of an apparent invariant absorption at 315 nm (isosbestic point) in the series of absorption spectra in PBS suggests that UVB directly photoconverts aqueous VNO to a single discrete photoproduct whose absorption spectrum overlaps with that of VNO at 315 nm. In ethanol, the behavior was different: The absorption maximum at 270 nm shifted to 255 nm and decreased in intensity, while the band centered at 318 nm disappeared almost completely by 400 J/m2 (40 mJ/cm2), resulting in loss of nearly all UVA and UVB absorption present in the parental VNO molecule (Fig. 1d). In both solvents, the absence of additional spectroscopic changes with progressive UVB doses as well as the absence of any detectable residual spectroscopic feature of VNO suggested that the UVB photoproducts are photostable and that their formation from VNO is essentially quantitative by 800 J/m2 (80 mJ/cm2).
The different spectroscopic changes observed in different solvents, particularly for the N-oxide compound, could be explained by a single UVB-induced photoproduct whose absorption spectrum markedly differs in PBS and ethanol, or by the creation of entirely different photoproducts in the different solvents. To distinguish between these possibilities, we obtained spectra of the photoproducts in either solvent following UVB irradiation of VNO (Fig. e, f). Following 2,000 J/m2 (200 mJ/cm2) UVB, the final N-oxide photoproducts from PBS and ethanol solvents were each dried in a vacuum centrifuge, and then re-dissolved in the same volume of either PBS or ethanol. Exchanging solvents produced changes in the intensity of various bands, but absorption maxima and band shapes were essentially independent of solvent, suggesting that distinct photochemical products with characteristic absorption spectra are formed in PBS and in ethanol.
UVB photoproducts of voriconazole N-oxide are toxic and sensitize cells to UVA
Because VNO exhibited the most notable UVB-induced changes in aqueous media, the parental drugs and their UVB-induced photoproducts were further studied in PBS. The UVB photoproducts of voriconazole and VNO will be subsequently denoted as vori’ and VNO’, respectively, to distinguish them from the parental compounds. Due to the clinical similarity to xeroderma pigmentosum that some patients on chronic voriconazole treatment exhibit, we assessed whether vori’ and VNO’ sensitized cells to UVB to screen for defects in nucleotide excision repair (Fig. 2). As we and others have previously reported, the thiazolyl blue assay, while not a direct measure of DNA repair, is quite sensitive to nucleotide excision repair defects 30,31. For example, as expected, the SV40-transformed xeroderma pigmentosum complementation group A (XPA) fibroblast cell line, XP12RO-SV40, was markedly sensitive to UVB, with virtually all cells killed by 88.5 J/m2 (8.85 mJ/cm2), while the repair-proficient SV40-transformed fibroblast cell line, GM637, had ~80% viability at the same dose (p<10−4, Fig. 2a). Keratinocytes were more resistant to UVB than fibroblasts, as has been previously observed 32. In the absence of UVB, voriconazole and vori’ were modestly toxic (Fig. 2b). In contrast, VNO exhibited minimal toxicity in the dark but VNO’ exhibited toxicity, resulting in a loss in viability of 50% of cells. Similar to our prior observations 16, neither voriconazole nor VNO sensitized keratinocytes to UVB doses up to 700 J/m2 (70 mJ/cm2) relative to diluent-treated controls, as measured by the thiazolyl blue assay 5 days following UVB (Fig. 2c). In addition, neither vori’ nor VNO’ further sensitized cells following UVB (Fig. 2d). Overall, these results suggest that voriconazole and VNO and their UVB photoproducts have no significant effect on the repair of direct UVB-generated DNA photoproducts. However, voriconazole, vori’ and VNO’ exhibit modest toxicity to keratinocytes that occurs through a non-UVB mechanism.
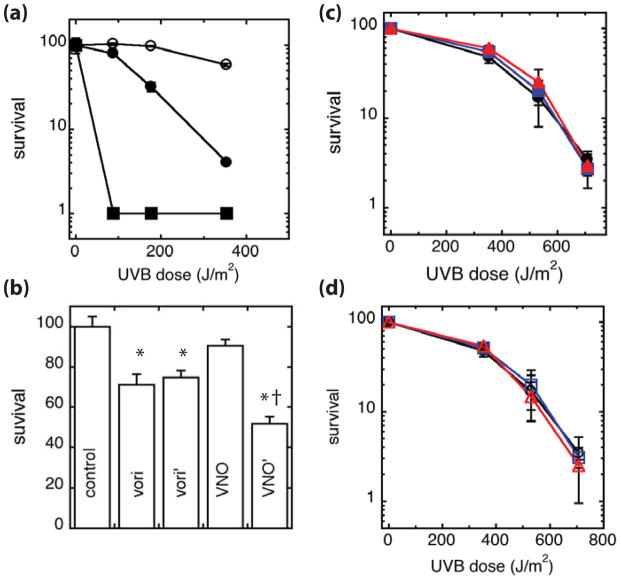
Figure 2.
UVB sensitivity of cells treated with voriconazole and voriconazole N-oxide photoproducts. (a) Normal human keratinocytes (○), GM637 (●), and XP12RO-SV40 cells (■) were irradiated with UVB and allowed to incubate in medium for 5 days before measuring survival. (b) Normal human keratinocytes were treated with PBS control, 30 μM voriconaozle (vori), vori’, VNO, or VNO’ for 6 days prior to measuring survival. Results are normalized to the PBS-treated control. (c) Normal human keratinocytes were treated with PBS control (●,○) or 30 μM (c) voriconazole (■) or VNO (▲), or (d) vori’ (□) or VNO’ (△) for 24 hours prior to and during UVB irradiation and then allowed to incubate in medium containing drug for five days before measuring survival. Results are normalized to unirradiated cells for each treatment group in panels (c, d). Results are the average at least 3 independent experiments. * denotes comparison to the PBS control, p ≤ 0.0001. † denotes comparison to the parental drug, p<0.0001.
Because VNO and VNO’ both possess significant UVA absorption, we also examined their ability to sensitize cells to UVA radiation (Fig. 3). Keratinocytes treated with either voriconazole or vori’ did not sensitize keratinocytes to UVA relative to PBS-treated controls. In contrast, relative to the PBS-treated control, both VNO and VNO’ resulted in a greater than three-fold reduction in survival by 10 J/cm2 (104 mJ/cm2) UVA.
Figure 3.
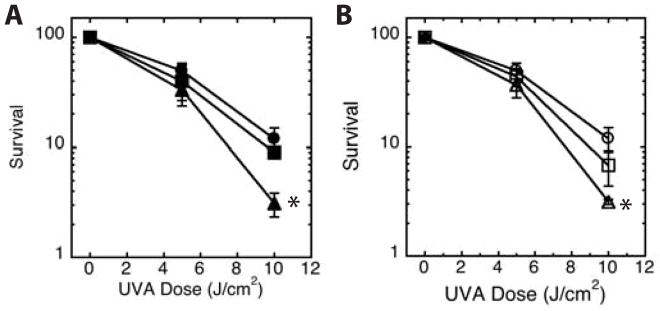
UVA sensitivity of cells treated with voriconazole and voriconazole N-oxide photoproducts. Normal human keratinocytes were treated with PBS control (●,○) or 30 μM (a) unirradiated voriconazole (■) or VNO (▲), or (b) vori’ (□) or VNO’ (△) for 24 hours prior to and during UVA irradiation and allowed to incubate in medium containing drug for five days before measuring survival. Results are the average of 3 independent experiments. * denotes comparison to matched PBS control, p<0.01.
Voriconazole N-oxide photoproducts generate ROS
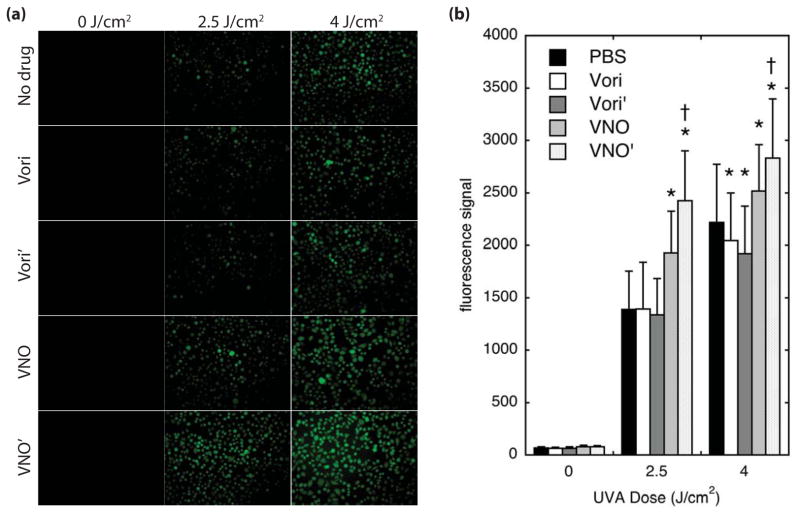
UVA can be associated with production of ROS when photodynamic sensitizers are present. To determine whether the UVA phototoxicity observed with VNO and VNO’ might be due to UVA-induced ROS generation, we monitored ROS levels using the fluorogenic reporter, CM-H2DCFDA (Fig. 4). CM-H2DCFDA is non-fluorescent and is added to cells as a diethyl ester derivative that is converted by intracellular esterases to the charged diacetate form, minimizing back-diffusion of intracellular dye to the extracellular space 23. The presence of intracellular ROS oxidizes CM-H2DCF to its fluorescent fluorescein derivative. Cells treated with either parental voriconazole or vori’ and with CM-H2DCFDA, then irradiated with UVA, exhibited background control intensities of fluorescent signal, but cells treated with VNO and VNO’ were more fluorescent than other cells at all UVA doses (Fig. 4a). Quantification of the fluorescent intensity showed that the signal from VNO and VNO’-treated cells was significantly higher than controls by 2.5 J/cm2 (2500 mJ/cm2), with VNO’ and VNO associated with ROS levels that were 75% and 40% higher than controls, respectively (Fig. 4b). Overall levels of ROS increased in a UVA dose-dependent manner under all treatment conditions, likely because CM-H2DCFDA is itself capable of some UVA-induced ROS generation 23. Over time this process as well as normal cellular metabolism served to produce significant fluorescent signal in all samples such that the differences in fluorescence become progressively smaller by 4 J/cm2 (4000 mJ/cm2), though even at this dose VNO and VNO’ generated significantly greater UVA-induced ROS than the PBS control, and ROS in VNO’-treated cells was greater than in VNO. For unclear reasons, cells treated with either voriconazole and vori’ exhibited significantly weaker fluorescence signals at 4 J/cm2 (4000 mJ/cm2).
Figure 4.
Reactive oxygen generation following UVA. Normal human keratinocytes at 90% confluence were treated with drugs as indicated for 30 minutes, followed by addition of CM-H2DCFDA for 30 minutes. (a) Cells were irradiated with UVA, and immediately visualized by epifluorescence microscopy. (b) Mean fluorescence intensity of each cell was measured and averaged over >200 cells. Error bars represent the standard deviation. * denotes comparison to matched PBS control, p<0.0001. † denotes comparison to the parental drug, p<0.001.
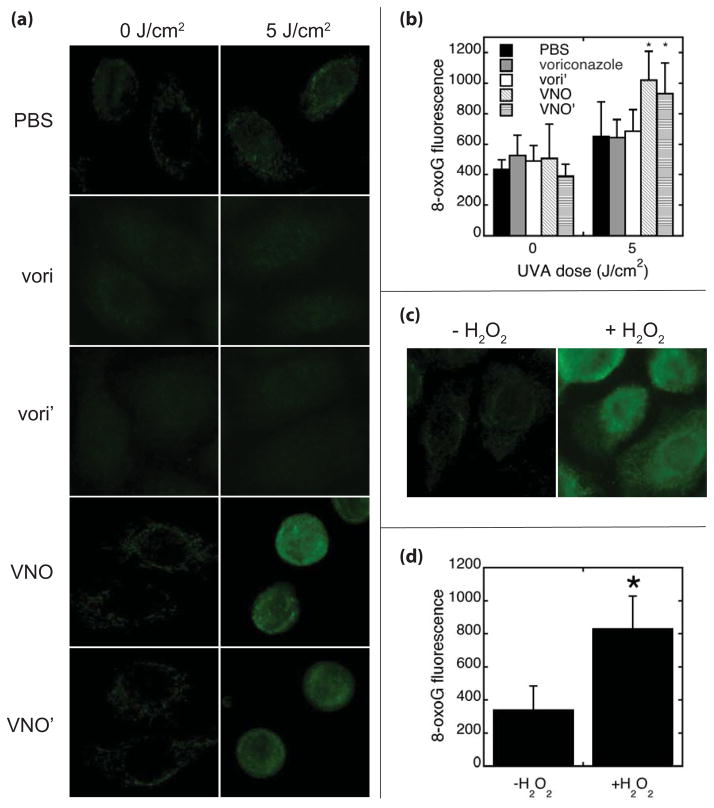
One anticipated consequence of ROS is the generation of oxidative DNA damage. To document whether such oxidative damage occurred, we assayed for the presence of 8-oxoguanine, a pre-mutagenic oxidation product of guanine in DNA 33. As shown in Fig. 5, UVA generated significantly increased cellular levels of 8-oxoguanine when cells were irradiated in the presence of VNO or VNO’ relative both to unirradiated cells and to identically treated voriconazole-, vori’- and PBS-treated controls (Fig. 5a, b). The 8-oxogaunine signal was comparable to that seen when cells were treated with hydrogen peroxide (Fig. 5c, d).
Figure 5.
8-oxoguanine formation following UVA. Normal human keratinocytes were treated with PBS, voriconazole (vori), vori’, VNO or VNO’ for 30 minutes before irradiation with UVA. (a) Cells were immediately fixed and stained with monoclonal antibody to 8-oxoguanine and fluorescently labeled secondary antibody before visualization. (b) Mean nuclear fluorescence intensity was measured from >200 cells from two independent samples. (c) As a positive control, normal human keratinocytes were treated with diluent or 1 mM H2O2 for 1 hour prior to fixation and staining with monoclonal antibody to 8-oxoguanine and fluorescently labeling with secondary antibody. (d) Mean nuclear fluorescence intensity was measured from >200 cells from samples treated with diluent or H2O2. Error bars represent the standard deviation. * denotes comparison to matched PBS control, p<0.001.
DISCUSSION
Drug phototoxicity due to generation of ROS, is well-known 25,34. Less well-described is photosensitivity due to drug metabolites and photoconversion of drugs to other photosensitizers. Our results demonstrate that VNO is particularly labile following UVB irradiation, such that UVB converts VNO in aqueous solution to a spectroscopically distinct photoproduct, denoted here as VNO’, with enhanced UVA absorption. The photoconversion occurs at even relatively low UVB doses, and is essentially complete by 800 J/m2 (80 mJ/cm2). The identity of VNO’ is currently being investigated, but the current results indicate that VNO’ may also need to be considered when fully accounting for both the phototoxicity as well as potential photocarcinogenicity associated with voriconazole. Interestingly, the UVB-induced photoproducts of VNO appear to be solvent-dependent, suggesting that different photobiology could occur depending on the partitioning of VNO in aqueous and hydrophobic environments within cells.
We find that, while voriconazole and VNO and their UVB-generated photoproducts all exhibit some toxicity to cells in the dark, none further sensitizes cells to UVB exposure. This result is consistent with our prior report that voriconazole and VNO alone do not sensitize cells to UVB exposure 16, and further suggests that neither these drugs nor their UVB photoproducts impair nucleotide excision repair of UVB-induced DNA lesions. Despite some clinical similarities in skin appearance of patients receiving chronic voriconazole to xeroderma pigmentosum patients in which deficiencies in nucleotide excision repair lead to significant UVB sensitization5 (Fig. 2a), no such sensitization was observed following treatment of cells with voriconazole, VNO or their UVB photoproducts.
Our results instead indicate that VNO and VNO’, but not voriconazole or its UVB photoproduct, can sensitize cells to UVA radiation. This is consistent with a recent report that found clinical photosensitivity to UVA but not UVB wavelengths 22. Both VNO and VNO’ absorption spectra possess bands in the UVA range, though VNO’s long-wavelength absorption band is only partially in the UVA region while VNO’ has an absorption band that is predominantly in the UVA region. Interestingly, these differences in UVA absorption as well as in UVA-induced ROS generation in VNO and VNO’ were not reflected in cellular survival or in 8-oxoguanine levels. It is possible that VNO and VNO’ differentially elicit other biological responses that negate their differences in ROS generation when acute survival or oxidative DNA damage following UVA is measured.
The precise photochemistry leading to VNO’ formation as well as to ROS generation by VNO and VNO’ is beyond the scope of this study. However, the notable solvent dependence of both of VNO’s major absorption bands suggests that these transitions to the excited state by UVB photon absorption are accompanied by significant changes in VNO’s electronic charge distribution, potentially creating charge-separated excited state capable of ROS generation or other redox photochemistry 35. Along these lines, both VNO and VNO’ have extremely weak fluorescence emission relative to voriconazole, suggesting that non-radiative mechanisms such as electron transfer or other redox reactions may be important pathways for releasing absorbed UVB energy in these compounds (K. Ona-Vu and D.H. Oh, unpublished results).
The ability of ROS to generate oxidative DNA lesions such as 8-oxoguanine is expected to affect genomic stability and be carcinogenic. 8-oxoguanine itself is highly mutagenic 33. These results are also consistent with the clinical behavior of voriconazole-associated photosensitivity and cancers. Unlike other drugs in which photosensitivity occurs relatively acutely, voriconazole’s phototoxicity occurs much later 1. Our results are consistent with a model in which voriconazole’s adverse biological side effects may occur only after sufficient VNO has accumulated in the skin, either following hepatic metabolism or through local epidermal generation, and additionally has had opportunity to be exposed to sufficient UVA doses to generate enough ROS to produce cellular damage. Generation of VNO’ though UVB exposure is an alternative pathway for sensitizing epidermal cells to UVA. Thus, a multistep mechanism that involves generation of the VNO metabolite and subsequent conversion to UVA photosensitizer may explain the relatively long latency in both photosensitivity as well as skin cancer development in patients receiving long-term voriconazole therapy. In addition to reinforcing the need for UVA photoprotection in patients receiving voriconazole, our results also suggest that preventing formation of the N-oxide derivative may be an approach to minimize voriconazole’s photo-induced adverse effects.
What’s already known about this topic?
Voriconazole is associated with photosensitivity and aggressive skin cancers
Voriconazole does not confer sensitivity to UVB but UVA sensitivity has been reported in patients
What does this study add?
The hepatically generated N-oxide derivative of voriconazole and its UVB photoproduct sensitize keratinocytes to UVA but not to UVB
In keratinocytes treated with voriconazole N-oxide or its UVB photoproduct, UVA induces reactive oxygen species and oxidative DNA damage, suggesting a mechanism for the phototoxicity and photocarcinogenesis associated with voriconazole in skin.
Acknowledgments
We thank A. Scandurra for technical assistance, and J.G.C. Angeles, S.T. Arron, and J.E. Cleaver for helpful discussions and review of the manuscript. This work was supported by a VA Merit Award and NIH Grant AR064958 (D.H.O.).
Footnotes
Disclosures: None declared
References
- 1.Epaulard O, Leccia M-T, Blanche S, et al. Phototoxicity and photocarcinogenesis associated with voriconazole. Med Mal Infect. 2011;41:639–45. doi: 10.1016/j.medmal.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Bernhard S, Kernland Lang K, Ammann RA, et al. Voriconazole-induced phototoxicity in children. Pediatr Infect Dis J. 2012;31:769–71. doi: 10.1097/INF.0b013e3182566311. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy KL, Playford EG, Looke DFM, et al. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis. 2007;44:e55–e6. doi: 10.1086/511685. [DOI] [PubMed] [Google Scholar]
- 4.Vanacker A, Fabré G, Van Dorpe J, et al. Aggressive cutaneous squamous cell carcinoma associated with prolonged voriconazole therapy in a renal transplant patient. Am J Transplant. 2008;8:877–80. doi: 10.1111/j.1600-6143.2007.02140.x. [DOI] [PubMed] [Google Scholar]
- 5.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in chilren and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62:31–7. doi: 10.1016/j.jaad.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller DD, Cowen EW, Nguyen JC, et al. Melanoma associated with long-term voriconazole therapy: A new manifestation of chronic photosensitivity. Arch Dermatol. 2010;146:300–4. doi: 10.1001/archdermatol.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim SF, Singer JP, Arron ST. Catastrophic squamous cell carcinoima in lung transplant patients treated with voriconazole. Dermatol Surg. 2010;36:1752–5. doi: 10.1111/j.1524-4725.2010.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vadnerkar A, Nguyen MH, Mitasani D, et al. Voriconazole exposure and geographic location are independent risk factors for squamous cell carcinoma of the skin among lung transplant recipients. J Heart Lung Transplant. 2010;29:1240–4. doi: 10.1016/j.healun.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Singer JP, Boker A, Metchnikoff C, et al. High cumulative dose exposre to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J Heart Lung Transplant. 2012;31:694–9. doi: 10.1016/j.healun.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwald FO, Spratt M, Lemos BD, et al. Duration of voriconazole exposure: An independent risk factor for skin cancer after lung transplantation. Dermatol Surg. 2012;38:1369–3974. doi: 10.1111/j.1524-4725.2012.02418.x. [DOI] [PubMed] [Google Scholar]
- 11.Feist A, Lee R, Osborne S, et al. Increased incidence of cutaneous squamous cell carcinoma in lung transplant recipients taking long-term voriconazole. J Heart Lung Transplant. 2012;31:1177–81. doi: 10.1016/j.healun.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Williams K, Mansh M, Chin-Hong P, et al. Voriconazole-associated cutaneous malignancy: A literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014;58:997–1002. doi: 10.1093/cid/cit940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin JM, Equils O, Somerville KT, et al. Risk-adjusted relationship between voriconazole utilization and non-melanoma skin cancer among lung and heart/lung transplant patients. Transpl Infect Dis. 2013;15:329–43. doi: 10.1111/tid.12063. [DOI] [PubMed] [Google Scholar]
- 14.Epaulard O, Villier C, Ravaud P, et al. A multistep voriconazole-related phototoxic pathway may lead to skin carcinoma: Results from a French nationwide study. Clin Infect Dis. 2013;57:e182–8. doi: 10.1093/cid/cit600. [DOI] [PubMed] [Google Scholar]
- 15.Pfizer. Vfend prescribing information. 2014. [Google Scholar]
- 16.Angeles JGC, Cleaver JE, Feeney L, et al. Voriconazole does not potentiate photo damage from UVB exposure. Exp Dermatol Res. 2013;4:173. [Google Scholar]
- 17.Murayama N, Imai N, Nakane T, et al. Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol. 2007;78:2020–6. doi: 10.1016/j.bcp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos. 2003;31:540–7. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 19.Ratushny V, Gober MD, Hick R, et al. From keratinocyte to cancer: The pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. 2012;122:464–72. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunte DM, Simmel F, Frimodt-Moller N, et al. In vivo efficacy and pharmacokinetics of voriconazole in an animal model of dermatophytosis. Antimicrob Agents Chemother. 2007;51:3317–21. doi: 10.1128/AAC.01185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron JM, Holler D, Schiffer R, et al. Expression of multiple cytochrome P450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J Invest Dermatol. 2001;116:541–8. doi: 10.1046/j.1523-1747.2001.01298.x. [DOI] [PubMed] [Google Scholar]
- 22.Haylett AK, Felton S, Denning DW, et al. Voriconazole-induced photosensitivity: photobiological assessment of a case series of 12 patients. Br J Dermatol. 2013;168:179–85. doi: 10.1111/j.1365-2133.2012.11196.x. [DOI] [PubMed] [Google Scholar]
- 23.King BA, Oh DH. Spatial Control of Reactive Oxygen Species Formation in Fibroblasts Using Two-Photon Excitation. Photochem Photobiol. 2004;80:1–6. doi: 10.1562/2004-03-01-RA-093.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan S, Hottiger MO. Histone acetyl transferases: a role in DNA repair and DNA replication. J Mol Med. 2002;80:463–74. doi: 10.1007/s00109-002-0341-7. [DOI] [PubMed] [Google Scholar]
- 25.Kochevar IE. Phototoxicity mechanisms: Chlorpromazine photosensitized damage to DNA and cell membranes. J Invest Dermatol. 1981;77:59–64. doi: 10.1111/1523-1747.ep12479244. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson BE, Oh DH. Proficient global nucleotide excision repair in human keratinocytes but not fibroblasts deficient in p53. Cancer Res. 2005;65:8723–9. doi: 10.1158/0008-5472.CAN-05-1457. [DOI] [PubMed] [Google Scholar]
- 27.Laurion I, Lean DRS, Vincent WF. UVB effects on a plankton community: results from a large-scale enclosure assay. Acquatic Microbioal Ecology. 1998;16:189–98. [Google Scholar]
- 28.Gasparro FP, Brown DB. Photobiology 102: UV sources and dosimetry - the proper use and measurement of “photons as a reagent”. J Invest Dermatol. 2000;114:613–5. doi: 10.1046/j.1523-1747.2000.00940.x. [DOI] [PubMed] [Google Scholar]
- 29.Oh DH, Hanawalt PC. Binding and Reactivity of Psoralen Linked to Triple Helix-Forming Oligonucleotides. Photochem Photobiol. 2000;72:298–307. [PubMed] [Google Scholar]
- 30.Revet I, Feeney L, Bruguera S, et al. Functional relevance of the histone γH2Ax in the response to DNA damaging agents. Proc Natl Acad Sci. 2011;108:8663–7. doi: 10.1073/pnas.1105866108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuschal C, Thoms KM, Boeckmann L, et al. Cyclosporin A inhibits nucleotide excision repair via downregulation of the xeroderma pigmentosum group A and G proteins, which is mediated by calcineurin inhibition. Exp Dermatol. 2011;20:795–9. doi: 10.1111/j.1600-0625.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 32.D’Errico M, Teson M, Calcagnile A, et al. Apoptosis and efficient repair of DNA damage protect human keratinocytes against UVB. Cell Death Differ. 2003;10:754–6. doi: 10.1038/sj.cdd.4401224. [DOI] [PubMed] [Google Scholar]
- 33.Bjelland S, Seeberg E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Hasan T, Khan AU. Phototoxicity of the tetracyclines: Photosensitized emission of singlet delta dioxygen. Proc Nat Acad Sci USA. 1986;83:4604–6. doi: 10.1073/pnas.83.13.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh DH, Sano M, Boxer SG. Electro-absorption (Stark Effect) spectroscopy of mono- and bi-ruthenium valence complexes. J Am Chem Soc. 1991;113:6880–90. [Google Scholar]