Abstract
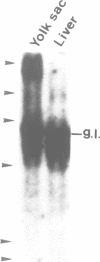
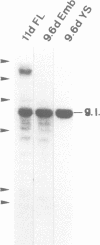
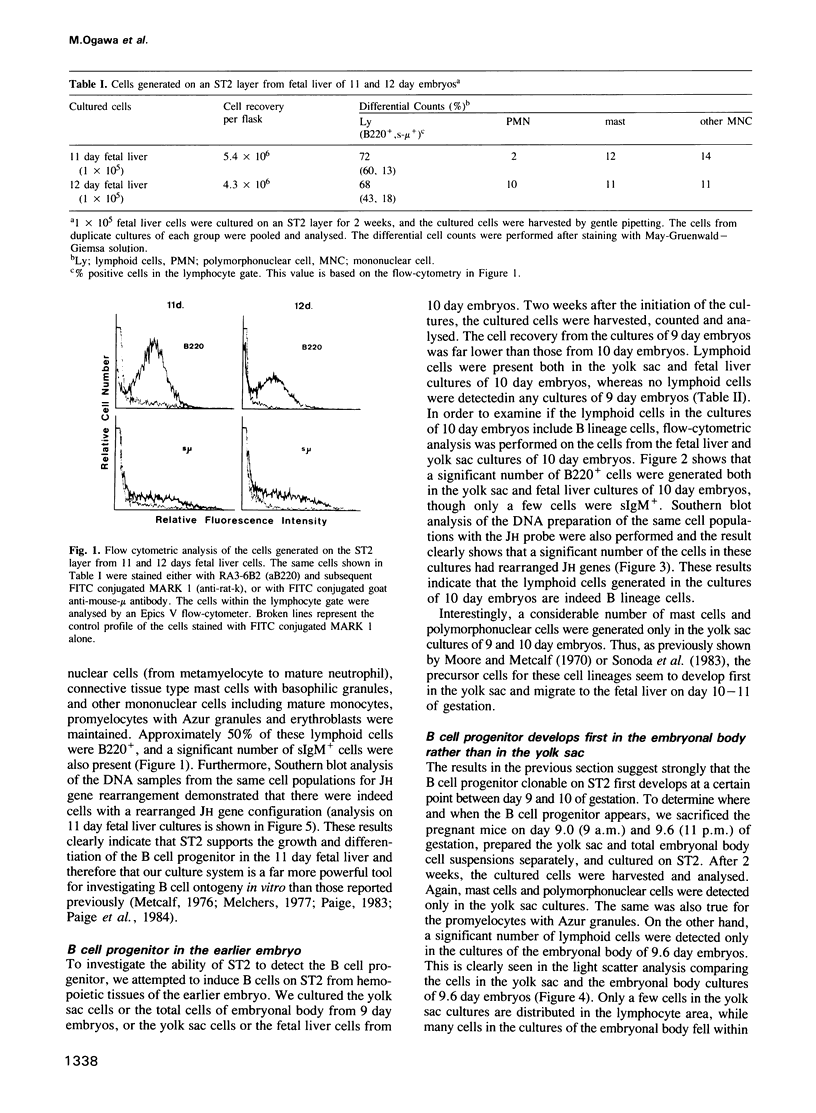
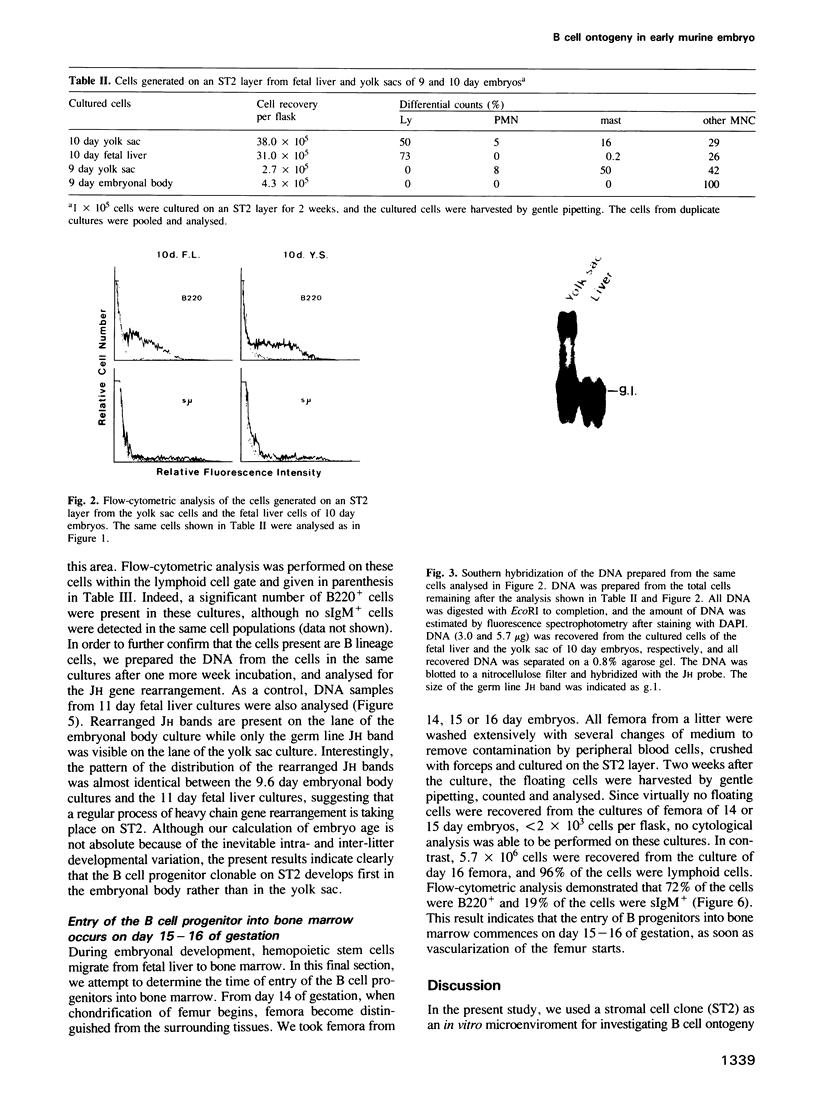
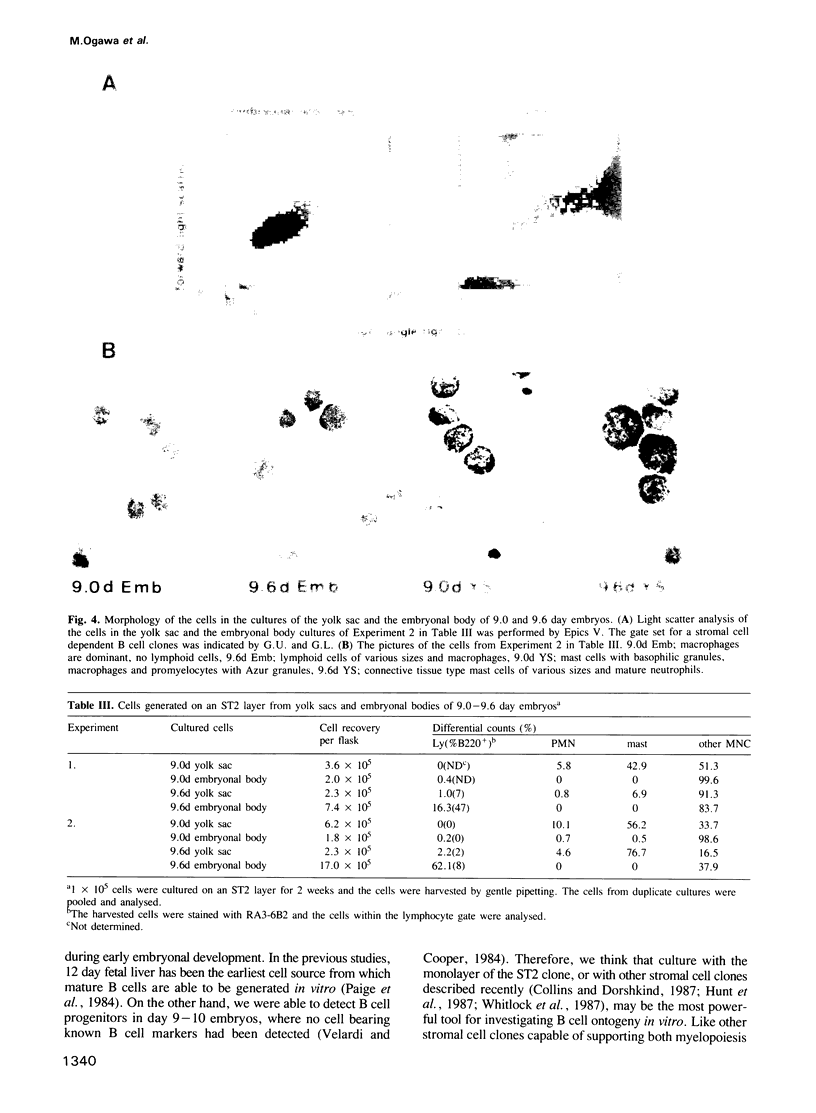
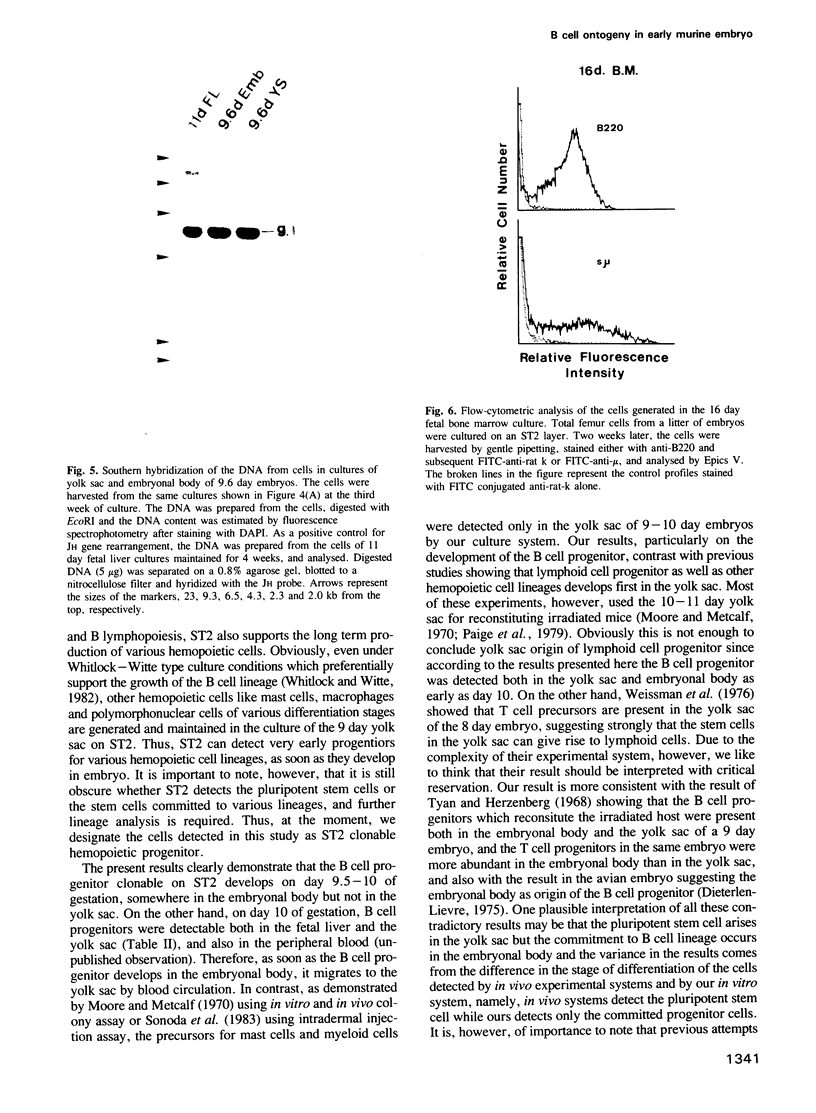
A stromal cell clone, ST2, which can support both myelopoiesis and B lymphopoiesis of adult bone marrow was used as an in vitro microenvironment for investigating the ontogeny of the B cell progenitor in murine embryos. The B cell progenitor clonable on an ST2 layer first become detectable in the embryonal body rather than in the yolk sac around day 9.5 of gestation. As soon as it develops in the embryo, it enters the blood circulation and becomes detectable both in the developing fetal liver and the yolk sac of the 10 day embryo. On the other hand, mast cell and polymorphonuclear cell progenitors, which are also generated on the ST2 layer, develop first in the yolk sac and migrate to the fetal liver around day 10-11 of gestation. At the late stage of embryonal development, day 15-16 of gestation, the B cell progenitor enters the femur as vascularization of the femur starts. These results suggest that the localization of the committed stem cells for various hemopoietic cell lineages differs in the early embryo, although the localization of the pluripotent stem cells is yet to be determined.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Collins L. S., Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987 Feb 15;138(4):1082–1087. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol. 1975 Jun;33(3):607–619. [PubMed] [Google Scholar]
- Fujita J., Nakayama H., Onoue H., Kanakura Y., Nakano T., Asai H., Takeda S., Honjo T., Kitamura Y. Fibroblast-dependent growth of mouse mast cells in vitro: duplication of mast cell depletion in mutant mice of W/Wv genotype. J Cell Physiol. 1988 Jan;134(1):78–84. doi: 10.1002/jcp.1041340109. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Ben-Shahar D., Ben-David E. Mast cell growth on fibroblast monolayers: two-cell entities. Immunology. 1982 Feb;45(2):371–380. [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Astle C. M., DeLaittre J. A. Processing by the thymus is not required for cells that cure and populate W/WV recipients. Blood. 1979 Nov;54(5):1152–1157. [PubMed] [Google Scholar]
- Hirayoshi K., Nishikawa S., Kina T., Hatanaka M., Habu S., Nomura T., Katsura Y. Immunoglobulin heavy chain gene diversification in the long-term bone marrow culture of normal mice and mice with severe combined immunodeficiency. Eur J Immunol. 1987 Jul;17(7):1051–1057. doi: 10.1002/eji.1830170723. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Katsura Y., Amagai T., Kina T., Sado T., Nishikawa S. Two subpopulations of stem cells for T cell lineage. J Immunol. 1985 Nov;135(5):3021–3027. [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Gravallese P. M., Stevens R. L. Coculture of interleukin 3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6485–6488. doi: 10.1073/pnas.83.17.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F. B lymphocyte development in fetal liver. I. Development of reactivities to B cell mitogens "in vivo" and "in vitro". Eur J Immunol. 1977 Jul;7(7):476–481. doi: 10.1002/eji.1830070714. [DOI] [PubMed] [Google Scholar]
- Melchers F., Von Boehmer H., Phillips R. A. B-lymphocyte subpopulations in the mouse. Organ distribution and ontogeny of immunoglobulin-synthesizing and of mitogen-sensitive cells. Transplant Rev. 1975;25:26–58. doi: 10.1111/j.1600-065x.1975.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Role of mercaptoethanol and endotoxin in stimulating B lymphocyte colony formation in vitro. J Immunol. 1976 Mar;116(3):635–638. [PubMed] [Google Scholar]
- Moore M. A., Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970 Mar;18(3):279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Gisler R. H., McKearn J. P., Iscove N. N. Differentiation of murine B cell precursors in agar culture. Frequency, surface marker analysis and requirements for growth of clonable pre-B cells. Eur J Immunol. 1984 Nov;14(11):979–987. doi: 10.1002/eji.1830141104. [DOI] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Moore M. A., Lee G. The fate of fetal and adult B-cell progenitors grafted into immunodeficient CBA/N mice. J Exp Med. 1979 Sep 19;150(3):548–563. doi: 10.1084/jem.150.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J. Surface immunoglobulin-negative B-cell precursors detected by formation of antibody-secreting colonies in agar. Nature. 1983 Apr 21;302(5910):711–713. doi: 10.1038/302711a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Megson M., Owen J. J., Cooper M. D. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976 Jan 22;259(5540):224–226. doi: 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- Sonoda T., Hayashi C., Kitamura Y. Presence of mast cell precursors in the yolk sac of mice. Dev Biol. 1983 May;97(1):89–94. doi: 10.1016/0012-1606(83)90066-0. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Tyan M. L., Herzenberg L. A. Studies on the ontogeny of the mouse immune system. II. Immunoglobulin-producing cells. J Immunol. 1968 Sep;101(3):446–450. [PubMed] [Google Scholar]
- Tyan M. L. Studies on the ontogeny of the mouse immune system. I. Cell-bond immunity. J Immunol. 1968 Mar;100(3):535–542. [PubMed] [Google Scholar]
- Velardi A., Cooper M. D. An immunofluorescence analysis of the ontogeny of myeloid, T, and B lineage cells in mouse hemopoietic tissues. J Immunol. 1984 Aug;133(2):672–677. [PubMed] [Google Scholar]
- Whitlock C. A., Tidmarsh G. F., Muller-Sieburg C., Weissman I. L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]