Abstract
Rationale
Dual cell transplantation of cardiac progenitor cells (CPCs) and mesenchymal stem cells (MSCs) after infarction improves myocardial repair and performance in large animal models relative to delivery of either cell population.
Objective
To demonstrate that CardioChimeras (CCs) formed by fusion between CPCs and MSCs have enhanced reparative potential in a mouse model of myocardial infarction relative to individual stem cells or combined cell delivery.
Methods and Results
Two distinct and clonally derived CCs, CC1 and CC2 were utilized for this study. CCs improved left ventricular anterior wall thickness (AWT) at 4 weeks post injury, but only CC1 treatment preserved AWT at 18 weeks. Ejection fraction was enhanced at 6 weeks in CCs, and functional improvements were maintained in CCs and CPC + MSC groups at 18 weeks. Infarct size was decreased in CCs, whereas CPC + MSC and CPC parent groups remained unchanged at 12 weeks. CCs exhibited increased persistence, engraftment, and expression of early commitment markers within the border zone relative to combinatorial and individual cell population-injected groups. CCs increased capillary density and preserved cardiomyocyte size in the infarcted regions suggesting CCs role in protective paracrine secretion.
Conclusions
CCs merge the application of distinct cells into a single entity for cellular therapeutic intervention in the progression of heart failure. CCs are a novel cell therapy that improves upon combinatorial cell approaches to support myocardial regeneration.
Keywords: Cardiac progenitor cells, mesenchymal stem cells, cell fusion, myocardial infarction
INTRODUCTION
Cell therapy for regeneration of the myocardium after myocardial infarction (MI) involves two concurrent processes: 1) stimulation of endogenous repair, and 2) exogenous cellular commitment. Regenerative medicine would benefit tremendously from identification of optimal stem cell population(s) that exert both direct and indirect mechanisms to mediate survival of existing cardiac myocytes, support proliferation and differentiation of endogenous stem cells, reduce inflammation and prevent scar formation. Coupling intrinsic mechanisms of myocardial repair with the propensity of stem cells to undergo cardiomyogenesis should be carefully balanced and integrated with the existing heart scaffold. Delivery of single stem cell types promote relatively modest functional and structural recovery of the heart owing to limited reparative capacity of donated cell populations derived from cardiac and bone marrow origin. Increasing cell numbers can enhance beneficial cellular properties, but excess reactive oxidative species and inflammation after acute damage contributes to elimination of more than 90% of delivered cells after one-week1. While the genetic engineering of stem cells prior to delivery remains a promising alternative to enhance persistence and regeneration2, 3, potential additional benefits of combinatorial cell therapy remains largely unexplored.
Resident c-kit+ cardiac progenitor cells (CPCs) are a desirable cell choice due to enhanced proliferative capacity and ability to form cardiac myocytes, vascular smooth muscle and endothelial cells ex vivo4. Endogenous c-kit+ cells have limited capacity towards cardiomyogenic commitment during development and after myocardial injury5. Despite limited regenerative capability, clinical application of CPCs confers improvements in myocardial structure and function as highlighted in the Cardiac Stem Cell Infusion in Patients With Ischemic CardiOmyopathy (SCIPIO) patient trial6. Bone marrow is the most popular source of adult-derived stem cells because of proven safety and efficacy after transplantation7. In particular, mesenchymal stem cells (MSCs) are commonly used for autologous and allogeneic clinical therapies8. MSCs are valued for paracrine-mediated effects such as reducing inflammation and promoting pro-survival and growth cascades to surrounding cells9. MSC injection after infarction promotes robust recruitment of c-kit+ CPCs, induces cardiomyocyte cycling and facilitates the outgrowth of stem cells from myocardial biopsies ex vivo10. Recently, combining these two distinct stem cells types, CPCs and MSCs, was investigated in a porcine model of myocardial damage11. Functional recovery and detection of human derived cells in the myocardium was improved over injection of single cells alone, indicating synergism of combining two cell types11. However, cellular mechanisms of myocardial recovery were not addressed and ratios of cell numbers were skewed towards increased MSC numbers confer protective effects in vivo11.
Cell fusion and creation of syncytia is an endogenous and homeostatic process coupled with differentiation and organ development12. Although fusion is low at basal levels, fusion increases in acute and chronic settings of inflammation, DNA damage and apoptotic events after bone marrow cell (BMC) transplantation13, 14. Artificial cell fusion between the same or different cells types to produce heterokaryons can be accomplished with addition of polyethylene glycol, electric pulses or viral fusogens15. In rare events, mononucleated hybrids (synkaryons) from bi-nucleated cell states occur, which is largely dependent on the ability of one cell type to undergo DNA synthesis after fusion16. BMCs and MSCs have been observed to readily fuse to more mature cells, allowing successful transfer of mitochondria and phenotypic traits such as increased survival and proliferation17–19. Spontaneous in vivo cell fusion as a mechanism to support regenerative therapy have been underwhelming leading to the conclusion that cell fusion alone is not a major contributor to heart regeneration.
In this manuscript, we present the creation and characterization of CPC and MSC hybrids, referred to as CardioChimeras (CCs), generated by ex vivo viral cell fusion. CCs exhibit enhanced molecular and phenotypic traits relative to individual stem cells and these distinct hybrids were evaluated for in vivo therapeutic effects after myocardial damage in a mouse model. Recovery of anterior wall thickness (AWT) and ejection fraction (EF) were markedly improved, concomitant with increased engraftment and expression of early cardiomyogenic lineage markers in CC treated hearts. CardioChimeras represent a novel therapeutic that complements the paracrine effects of MSCs to orchestrate endogenous repair with direct cell contributions from CPCs in promotion of de novo cellular regeneration.
METHODS
Full materials and methods are available in the online data supplement.
Cell fusion and creation of CardioChimeras
Cell fusion was conducted using the GenomONE™ - CF EX Sendai virus (Hemagglutinating Virus of Japan or HVJ) Envelope Cell Fusion Kit (Cosmo Bio. USA). According to the manufacturer’s protocol, we subjected MSCs and CPCs to the plating method of cell fusion. Here, 100,000 MSCs expressing GFP in a 100mm dish were incubated in CPC media for 24 hours. Next day, 100,000 CPCs expressing mcherry were suspended in 20µL of cell fusion buffer and 10µL of Sendai virus and placed on ice for 5 minutes for absorption of the virus on the cell membrane. Media from the MSC plate was removed and washed once with cell fusion buffer, and CPCs plus Sendai virus was added. The plate was then centrifuged (10 minutes, 1200rpm at 4°C) to force cell-to-cell contact. Cells were placed at 37°C for a total of 15 minutes to induce cell fusion. Non-fused CPCs were removed and media was added back to the plate. The next day, media was changed, and within 48 hours cells were trypsinized and subjected to FACS to place one-cell per well of a 96-micro plate to allow for clonal expansion of double fluorescence cell populations.
RESULTS
Phenotypic characterization of CardioChimeras
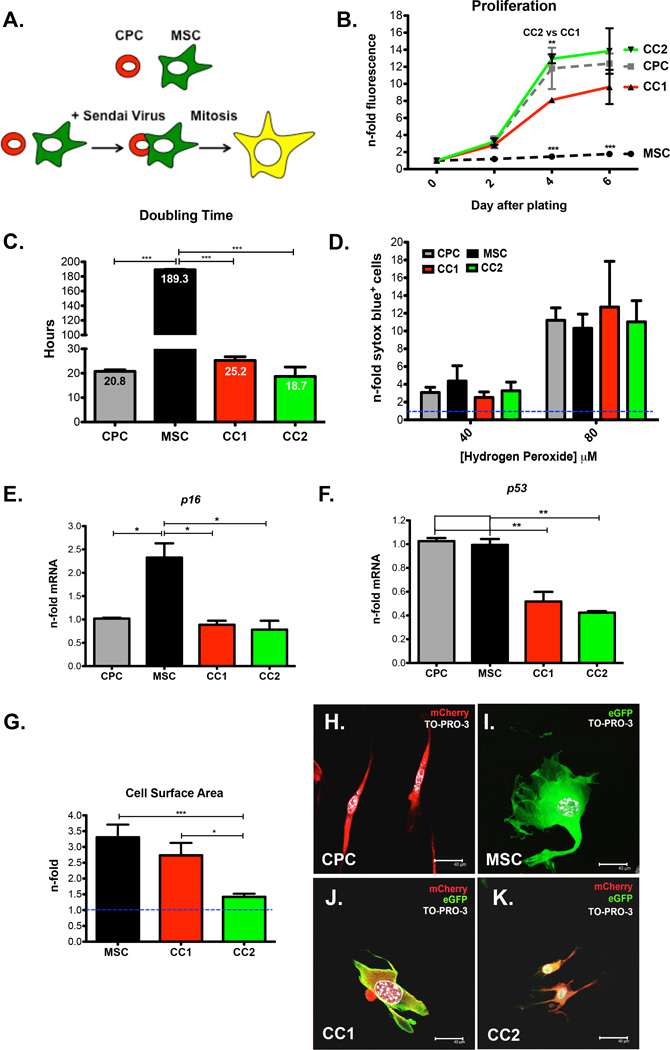
CardioChimeras (CCs) were created after fusion of fluorescently labeled CPCs (mcherry) and MSCs (eGFP) with an inactivated RNA Sendai virus (Figure 1A). After fusion, dual fluorescent hybrids were purified by fluorescent activated cell sorting and allowed to undergo clonal expansion (Figure 1A and Online Figure IIA). 18 mono-nucleated hybrids were successfully expanded one-month after initial sorting. Additional information concerning the analysis and selection criteria of the two CCs from the 18 clones is described in the online data supplement (Online Figure I and Online Table I). CC1 and CC2 were chosen from the 18 clones due to enhanced proliferation relative to the majority of clones, optimal cell survival, and the ability to provide pro-growth and survival factors when co-incubated with cardiac myocytes (Online Figure I, A–E and Online Table I). CC2 exhibits a proliferative rate similar to CPCs while CC1 shows modest proliferation, and all cells had increased proliferation over MSCs based on a fluorescent dependent cell proliferation assay and cell doubling time (Figure 1, B and C). CCs are not increasingly susceptible to cell death compared to parent cells (Figure 1D) and do not exhibit elevated expression of cell cycle arrest or senescence markers based on mRNA for p16 or p53 (Figure 1, E and F). CC1 has increased cell size and is morphologically similar to MSCs (Figure 1, G, I and J). CC2 displays a slight increase in cell size but is not significantly different from CPCs (Figure 1, G, H, and K).
Figure 1. Phenotypic Characterization of CardioChimeras.
(A) Schematic representation of the creation of CardioChimeras. (B) Proliferation of CCs, CPCs and MSCs represented as a fold change relative to day of plating. (C) Cell doubling time in hours. (D) Cell death assay of CCs and parents cells after treatment with 40µM or 80µM hydrogen peroxide represented as a fold change relative to cells not treated with hydrogen peroxide. (E) p16 and (F) p53 gene expression normalized to ribosomal 18s and represented as a fold change relative to CPCs. (G) Cell surface area represented as a fold change normalized to CPCs (blue dashed line, 1.0). Fluorescent images of (H) CPCs, (I) MSCs, (J) CC1 or (K) CC2. * p<0.05, ** p<0.01, *** p<0.001. Scale bar is 40µm.
Mononucleated CC1 and CC2 exhibit increased nuclear size and centromere intensity relative to parent cells after nuclear hybridization (Online Figure II, B–F). Collectively, CCs represent a novel stem cell population where increased DNA content does not negatively impact on survival or proliferation after induced cell fusion.
CardioChimeras exhibit increased basal level expression of cardiomyogenic commitment markers
MSCs and CC1 are low to negative for the stem/progenitor cells marker c-kit+, while CC2 and CPCs maintain 20% and 50% c-kit positivity respectively (Online Figure IIIA). Gap junction marker connexin43 and platelet endothelial cell adhesion molecule (pecam or cd31) mRNA are modestly upregulated in CC2 at basal levels (Online Figure III, B and C). MSCs express high levels of endothelial and smooth muscle markers as indicated by cd31 and smooth muscle 22 (sm22) gene expression (Online Figure III, C and D)20. Although sm22 was not upregulated in CCs, co-incubation of CPCs with MSCs at a 1:1 ratio increased mRNA expression of sm22 (Online Figure IIID). Interestingly, CC1 has increased mRNA for cardiac troponin T (cTNT or tnnt3) (Online Figure IIIE). Analysis of basal cardiomyogenic activity further confirmed the identification of CC1 and CC2 after cell fusion. CC1 has increased cardiogenic potential based on expression of cTNT, which corresponds to the lack of c-kit expression. CC2 retains low levels of c-kit expression but has increased expression of endothelial markers, a phenotype that has previously been reported to improve the regenerative capacity of CPCs2.
CardioChimeras promote cardiomyocyte growth after in vitro co-culture
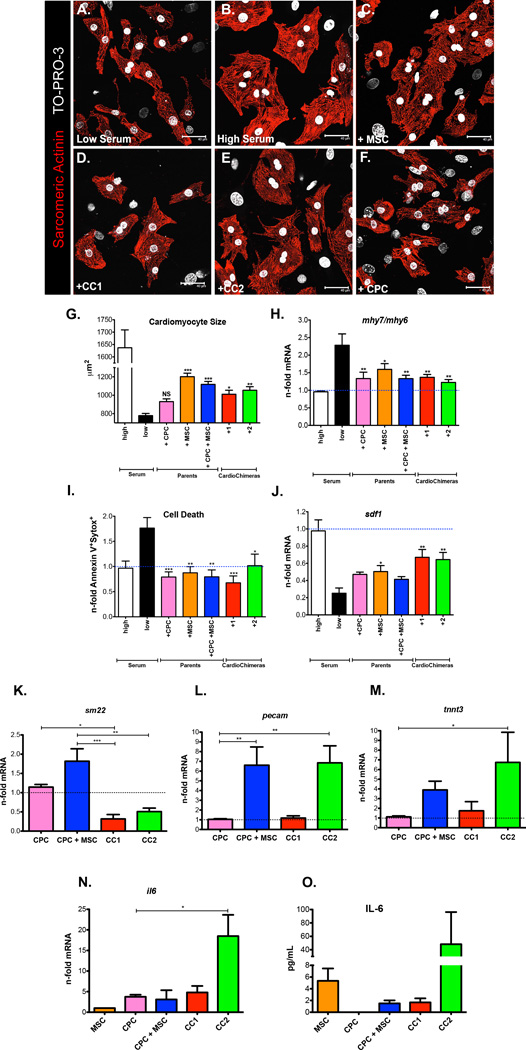
In order to test the beneficial effects mediated by CCs and parental cells before in vivo cell transfer, neonatal rat cardiac myocytes (NRCMs) were co-incubated with stem cell groups (CPC, MSCs, CPC + MSC, CC1 and CC2) at a ratio of 1:10 in serum depleted conditions. NRCMs maintained in low serum conditions (0.5%) resulted in smaller cardiac myocytes relative to NRCMs maintained in high serum conditions (10%) (Figure 2, A, B and G). Addition of MSCs, CPC + MSC, CC1 or CC2 to low serum treated NRCMs significantly increased cardiomyocyte size within 24 hours (Figure 2, C–E and G), but CPCs could not induce significant growth of NRCMs (Figure 2, F and G). Slow twitch β-myosin heavy chain (mhy7) over fast twitch α-myosin heavy chain (mhy6) gene expression was not significantly elevated in cardiac myocytes after 24 hours co-incubation with stem cell groups but is highly expressed in low serum conditions indicating that the addition of stem cells does not induce a maladaptive hypertrophic response in cardiac myocytes (Figure 2H). Regardless of the stem cell population added to cardiac myocytes, NRCMs were protected from cell death based on flow cytometric analysis of apoptotic and necrotic markers (Figure 2I). With the addition of CC1 and CC2, NRCMs had increased mRNA for stromal derived factor-1 (sdf-1) (Figure 2J) a cardioprotective cytokine and homing ligand for C-X-C chemokine receptor type 4 (CXCR-4) positive stem cells21.
Figure 2. CardioChimeras promote cell growth and have increased commitment and paracrine gene expression after in vitro co-culture with cardiac myocytes.
(A) NRCMs in low serum. (B) NRCMS in high serum. (C) NRCMs in low serum and after the addition of MSCs, (D) CC1, (E) CC2 or (F) CPCs for 24 hours. Cardiac myocytes were visualized by staining with sarcomeric actinin. TO-PRO-3 iodide was used to visualize nuclei. (G) Quantitation of cardiomyocyte size. (H) Gene expression of mhy7 over mhy6 represented as a fold change relative to high serum. (I) Cardiomyocyte cell death. Values are represented as fold change of Annexin V+ and Sytox Blue+ cells relative to high serum. (J) sdf-1 gene expression in cardiac myocytes alone after the addition of stem cells. (K–M) Gene expression in stem cells after a 7-day co-culture with NRCMs. (K) sm22 (L) pecam gene expression. (M and N) il6 gene expression analyzed in stem cells after a 24-hour co-culture with NRCMs. (O) IL-6 expression confirmed by ELISA.(G–J) Statistical values were determined by one-way ANOVA compared to low serum controls. * p<0.05, ** p<0.01, *** p<0.001. Scale bar is 40µm.
CardioChimeras have increased gene expression of commitment and paracrine markers after in vitro co-culture with cardiac myocytes
After co-culture with cardiac myocytes, sm22 was not significantly upregulated in CC groups (Figure 2K). However, CPC + MSC and CC2 displayed the largest induction of endothelial marker expression pecam, whereas CC2 induced cTNT gene expression after 7 days of co-culture with NRCMs (Figure 2, L and M). Paracrine factors are routinely touted as a mechanism for cardioprotection22, therefore we analyzed our stem cells for expression of growth and immunomodulatory factors. Gene and protein expression for Interleukin-6 (IL-6) is upregulated in CC2 after 24-hour incubation with serum starved NRCMs (Figure 2, N and O). Early release of immunomodulatory factors such as IL-6 after acute cardiac damage has been shown to have anti-apoptotic properties23. In summary, CC1 shows increased cellular size and expression of early cardiac commitment markers without impairment in cell proliferation. CC2 has similar morphological features to CPCs in addition to having a higher proliferative status relative to CC1. In fact, CC2 was most responsive to differentiation as evidenced by the up regulation of endothelial and cardiac markers in addition to increased expression of the immunomodulatory factor IL-6. This preliminary data further validates the in vivo application of these two distinct cell hybrids.
CardioChimeras improve left ventricular structure and cardiac function after myocardial injury
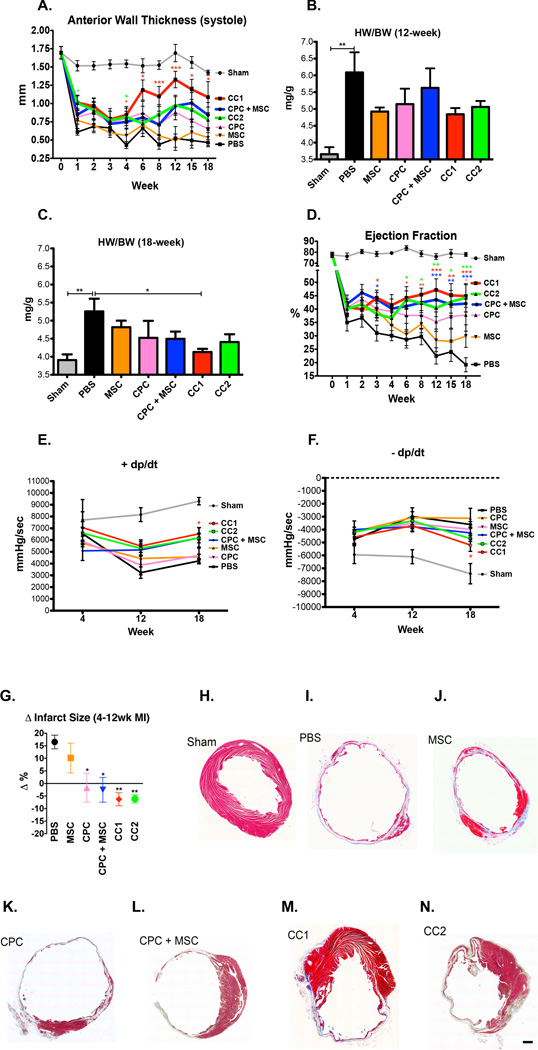
To establish the therapeutic efficacy of CCs relative to parent cells or parent cells combined, we injected a total of 100,000 cells in the border zone region of an acutely damaged mouse heart. At 1-week post injury (WPI), all groups had similar reductions in AWT and EF (Figure 3, A and D and Online Table II). CC1 and CC2 exhibited increased AWT at 4 WPI, but only CC1 treated hearts preserved AWT up to 18 WPI (Figure 3A). Heart weight to body weight ratios (HW/BW) at 12 and 18 weeks did not increase in CC treated hearts indicating that hypertrophy was not a contributing factor to increasing AWT (Figure 3, B and C). Rather, CC1 hearts had significantly reduced HW/BW relative to vehicle control (PBS) (Figure 3C). EF was increased in CC1 and CPC + MSC hearts starting at 3 WPI, and CC1 and CC2 had increased EF over PBS at 6 WPI (Figure 3D). CC and CPC + MSC-treated groups exhibited improved EF starting at 12 WPI, whereas the CPC treatment was beneficial for cardiac function only at 18 WPI (Figure 3D). Heart rates and structural/functional data are detailed in Online Table II. Correlating with improved EF, CC1 treatment significantly improved positive developed pressure over time (dP/dT) (Figure 3E) and negative dP/dT (Figure 3F). CC1, CC2, CPC + MSC and CPC hearts had smaller infarct sizes relative to PBS (Figure 3G). MSC groups exhibited increased infarct size when measuring scar between 4 and 12 WPI, CPC and CPC + MSC hearts remain unchanged, and CC1 and CC2 treatment reduced infarct size as represented by Masson’s Trichrome staining (Figure 3, G–N).
Figure 3. CardioChimeras improve left ventricular wall structure and cardiac function after myocardial injury.
(A) Longitudinal assessment of anterior wall thickness during systole (mm) over 18 weeks. (B) Heart weight to body weight ratio (mg/g) at 12 WPI (C) 18 WPI. Sample sizes of 3–5 mice per group. (D) Longitudinal assessment of ejection fraction (%). (E) Positive and (F) Negative developed pressure over time represented as mmHg/sec at 4, 12 and 18 WPI. (G) Change in infarct size between 4 and 12 weeks time points. P values were determined by one-way ANOVA compared to PBS treated controls. (H–N) Masson’s Trichrome staining and representative images of infarct size and fibrosis in (H) Sham, (I) PBS, (J) MSC, (K) CPC, (L) CPC + MSC, (M) CC1 and (N) CC2. Sample sizes are specified in the Online Table II. All statistical values were determined by two-way ANOVA compared to PBS treated hearts. * p<0.05, ** p<0.01, *** p<0.001. Colors of asterisk(s) correspond to heart group. Scale bar is 250µm.
Cellular engraftment of CardioChimeras 4 weeks after damage
Scar size measured at 4 WPI was not significantly different among infarcted heart groups (Online Figure IV, A and B–E). Next, we were interested in determining cell persistence at this time point and were able to detect CPCs labeled with mcherry in CPC alone and CPC + MSC treated hearts (Online Figure IV, F and G). Interestingly, mcherry+ CPCs were detected near small c-kit+/cTNT+ cardiac myocytes in the infarct area (Online Figure IVH). CC1 detected by both GFP and mcherry expression did not display evidence of commitment at this early time point (Online Figure IV, I–K).
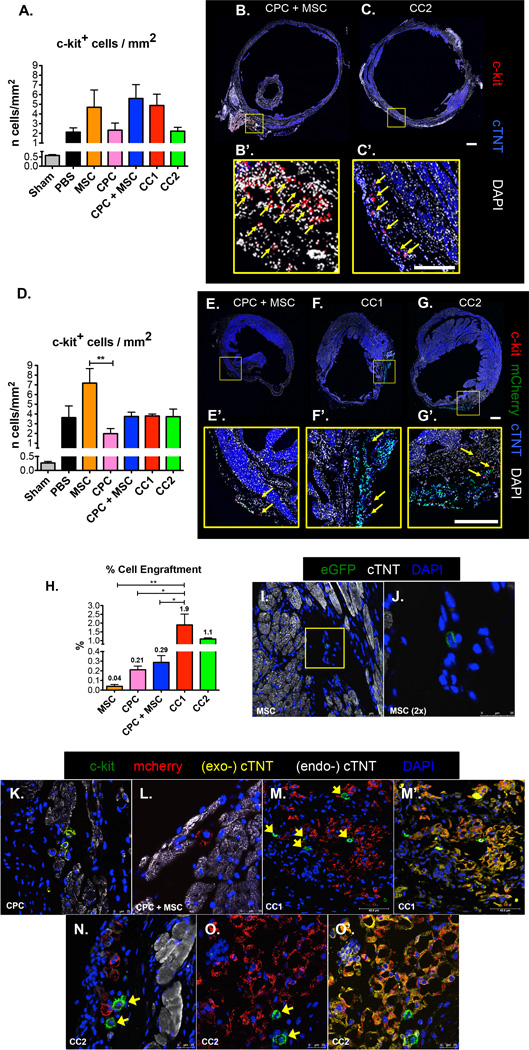
CardioChimeras have increased engraftment, expression of cardiomyogenic markers and support the increased presence of c-kit positive cells in the myocardium 12 weeks after damage
C-kit+ cell recruitment in damaged regions supports endogenous differentiation and myocardial repair22. Although infarction sizes were similar at the 4-week time point, induction of endogenous c-kit cells in the infarcted area was increased in MSC, CPC + MSC, and CC1 treated hearts (Figure 4, A–C). At 12 WPI, a high number of c-kit+ cells were observed in PBS and MSC treated hearts, yet c-kit+ cells remained visually present in CPC + MSC, CC1 and CC2 treated hearts surrounding mcherry+ cells in the border zone regions (Figure 4, D–G). The percentage of cell engraftment was increased in CC1 and CC2 hearts at 1.9% and 1.1% respectively relative to 0.21% and 0.29% in CPC and CPC + MSC hearts (Figure 4, H and K–O). MSCs were detected at a much lower level or 0.04% of the total left ventricular free wall (Figure 4, H, I and J). CPCs discovered in the border zone areas co-expressed c-kit and mcherry in CPC hearts and expressed mcherry alone in CPC + MSC hearts (Figure 4, K and L). CC1 and CC2 had increased levels of engraftment, expressed cTNT and were surrounded by endogenous c-kit+ cells (Figure 4, M–O).
Figure 4. CardioChimeras have increased engraftment, expression of cardiomyogenic markers and support the increased presence of c-kit+ cells in the myocardium 12 weeks after damage.
(A) Number of c-kit+ cells over the area of left ventricular free wall (mm2) in a 4-week damaged heart. Representative whole heart scans of (B) CPC + MSC and (C) CC2 treated hearts to visualize c-kit+ cells (red). Scale bar is 100µm. (B’) and (C’) C-kit+ cells are identified by yellow arrows. Scale bar is 50µm. (D) Number of c-kit+ cells in 12-week damaged heart.. Representative whole heart scans of (E) CPC + MSC, (F) CC1 and (G) CC2 treated hearts to visualize exogenous mcherry+ cells (green) and c-kit+ cells (red). Scale bar is 100µm. (E’), (F’) and (G’) C-kit+ cells are identified by yellow arrows. Scale bar is 100µm. (H) Cell engraftment efficiency (%). (I) MSC detected by GFP fluorescence at 12 weeks. (J) 2× zoom of a MSC in the border zone area. (K) C-kit+ /mcherry+ CPCs in the border zone area. (L) Mcherry+ CPC in CPC + MSC treated heart. (M) Mcherry+ CC1 visualized in the infarcted area surrounded by c-kit+ cells (green). (M’) Overlay of cTNT (exogenous-cTNT, yellow) in CC1 mcherry labeled cells. (N) Mcherry+ CC2 visualized in the infarcted area surrounded by c-kit+ cells (green). (O) Mcherry+ CC2 (red) visualized in the infarcted area surrounded by c-kit+ cells (green). (O) Overlay of cTNT (exogenous-cTNT, yellow) in CC2 mcherry labeled cells. Endogenous-cTNT (white) labels existing cardiac myocytes. Sample size of 3 mice per group. * p<0.05, ** p<0.01, *** p<0.001.
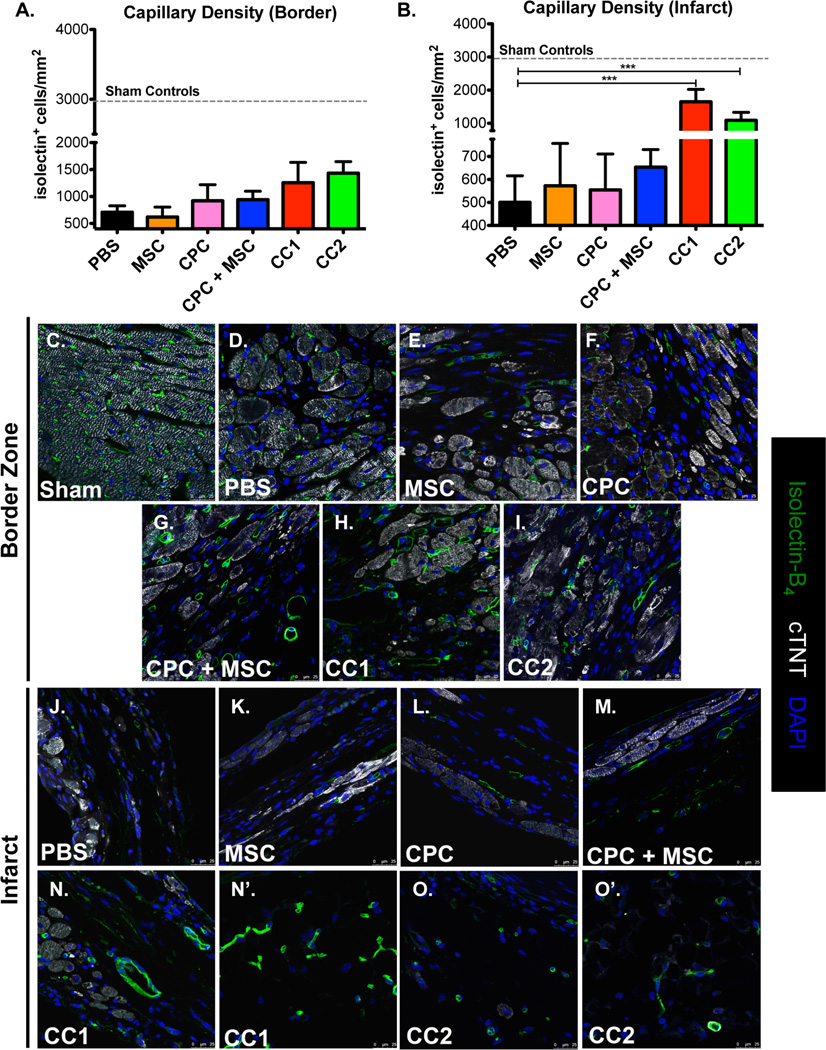
CardioChimeras increase capillary density in the infarct area
Capillary density was measured in the border zone and infarcted areas at 12 WPI. Shams, non-injured controls, are included as a standard for capillary density compared to injured hearts (Figure 5, A, B and C). Parent cells, individual or combined, or CC treatment did not significantly increase capillary density in the border zone regions relative to PBS (Figure 5, A and C–I). MSC, CPC or CPC + MSC treated hearts similarly did not affect the number of capillaries discovered in the infarct zone (Figure 5, B and J–M). Notably, CC1 and CC2 treated hearts had significant increases in isolectin+ structures in the infarct regions at 12 WPI (Figure 5, B and N–O).
Figure 5. CardioChimeras increase capillary density in the infarct area.
(A) Capillary density in the border zone and (B) Infarcted heart regions. Sample sizes are 3–4 mice per group. Sham controls (dashed line) are represented as control for baseline density of isolectin+ structures per mm2. (C–I) Representative border zone images to visualize isolectin+ structures. (J–O) Representative infarct zone images to visualize and quantitate isolectin+ structures. Green= Isolectin B4, White=cardiac troponin T and Blue= DAPI to stain for nuclei. Scale bar is 25µm.* p<0.05, ** p<0.01, *** p<0.001.
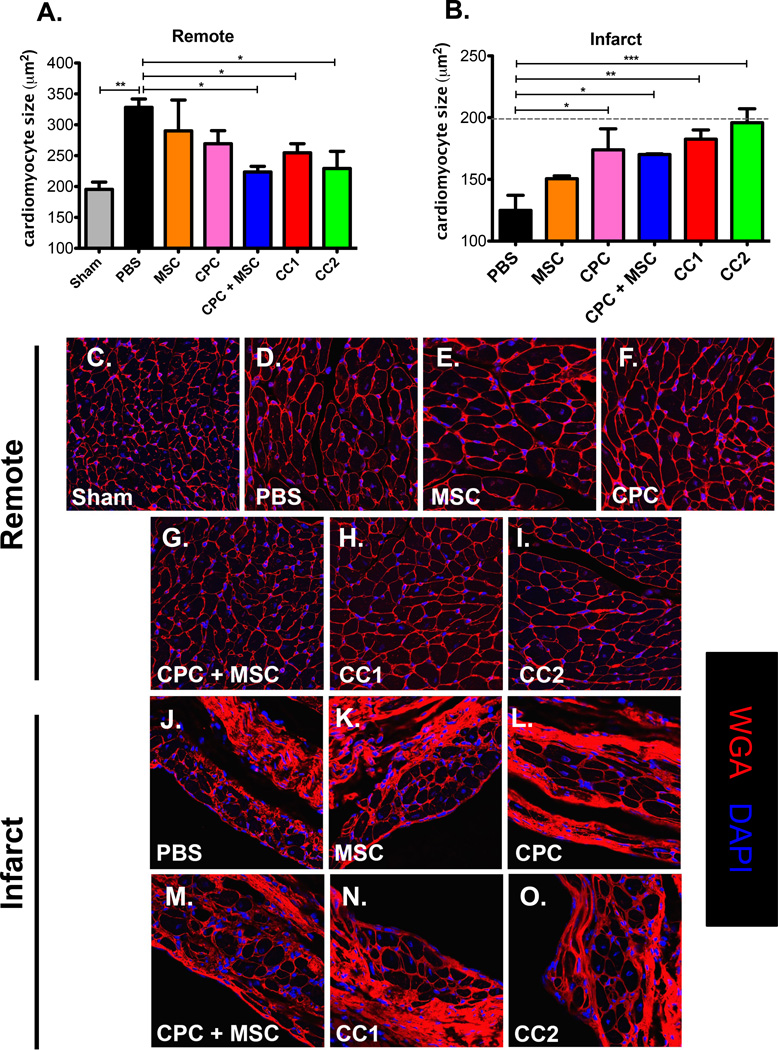
CPC, MSC and CardioChimera treatment antagonizes cardiomyocyte hypertrophy in the remote region and preserves cardiomyocyte size in the infarcted regions
Cellular treatment and long term engraftment of cells is reported to induce compensatory hypertrophy in areas of damage preventing progression of heart failure after MI24. MSC and vehicle treated hearts showed increased cardiomyocyte size in the remote area relative to sham (Figure 6, A and C–E). CPC + MSC, CC1 and CC2 treated hearts maintained cardiomyocyte size in the remote region similar to non-injured controls (Fig 6, A, C and G–I). Although stem cell treatments could not modify border zone cardiomyocyte size (Online Figure V, A–G), injection of CPC, CPC + MSC, and both CCs increased cardiomyocyte size in the infarcted regions relative to PBS and MSC treated hearts up to 12 WPI (Figure 6, B and J–O). This data indicates that improved engraftment of stem cells correlates with the presence of microvascular structures and preservation of cardiomyocyte size in the remote and infarct regions relative to failing and severely damaged hearts.
Figure 6. CPC, MSC and CardioChimera treatment antagonizes cardiomyocyte hypertrophy in the remote region and preserves cardiomyocyte size in the infarcted regions.
(A) Mean cardiomyocyte size in the remote and (B) Infarct regions. Sample size is 3–4 mice per group. (C–I) Representative images of remote area cardiomyocyte size. (J–O) Representative images of infarct area cardiac myocytes. Red=Wheat germ agglutinin, White=cardiac troponin T and Blue=DAPI to stain for nuclei. Scale bar is 25µm.* p<0.05, ** p<0.01, *** p<0.001.
DISCUSSION
The restorative impact of cell therapy to advance regenerative medicine remains to be fully realized and continues to be the focus of intense investigation. Increased knowledge of stem cell biology emerges from the use and application of a variety of adult stem cells. Unfortunately, ideal cellular properties are compromised by massive cellular death upon introduction into damaged myocardium1. In this report, we demonstrate a novel approach by using cell fusion to enhance delivery of novel and unique stem cell properties created within a single cell. Cardiac-derived CPCs and bone marrow derived MSCs were chosen for this study as both of these cell types have established roles in the heart: CPCs contribute to direct cardiomyogenic differentiation whereas MSCs provide for protective immunomodulatory and growth factor paracrine secretion4, 9. CCs injected into the acutely damaged heart improved structural integrity and reduced infarct size (Figure 3). Furthermore, functional improvements were observed in CC treated hearts, and increased engraftment was apparent in the border zones after 12 WPI (Figure 3 and 4). Specifically, CC1 significantly improved myocardial wall structure compared to control groups, and both CC1 and CC2 showed increased cellular engraftment in the border zone regions corresponding to a reduced infarct size and preservation of vascular structures in the neighboring infarct (Figure 3, 4 and 5). Notably, CCs improved EF earlier in the assessment (6 WPI) relative to combined and single cell injections (Figure 3). At 12 WPI, CC therapy promoted increases in cardiac function, induction of endogenous c-kit cells and maintenance of cardiomyocyte size that were comparable to mixed cell injections (Figure 3, 4 and 6). Initial improvements in cardiac function are most likely mediated by the combination of increased cell persistence and growth factor secretions conferred by CCs supporting long-term vascular stability and mitigation of adverse scar remodeling that is improved over combined therapy of CPCs and MSCs (Figure 3 and 5).
BMCs, the most common stem cell for cardiac therapy, apparently undergo engraftment through a combination of cell fusion and to a lesser degree by direct transdifferentiation events25. Membrane fusion is dependent upon signaling mechanisms involving paxillin induced focal adhesions and recycling of integrins as demonstrated between macrophages and myoblasts26. In the heart, cell fusion is increased between exogenous stem cells and apoptotic cardiac myocytes similar to enhanced myoblast fusion in the presence of phosphatidylserine presenting cells14, 27. Altered DNA content has been raised as an issue following fusion events as genomic instability leads to cellular aging28. Somatic cells exhibiting chromosomal mosaicism such as through the loss or deletions of chromosomes do not significantly affect stem cell properties or cell fate29. As a result, CCs do not appear transformed but rather retain properties of CPCs and MSCs to support enhanced myocardial repair. To this effect, we were interested in correlating the in vitro properties of CC1 and CC2 to the observed effects in the myocardium. Although, both CC1 and CC2 were responsive to co-culture with cardiac myocytes (Figure 2), CC treated hearts showed only a modest up regulation of cTNT in vivo (Figure 4). CC1 in culture did not undergo significant cardiomyogenic commitment or secrete IL-6, yet CC1 hearts had stabilized AWT (Figure 3). We hypothesize that the larger cell body of CC1 contributed to higher rates of engraftment contributing to the improvement in myocardial structure without significant evidence of cardiogenic commitment (Figure 1 and 4). Prior to injection, CC2 exhibited a predominately CPC phenotype, and supported in vivo effects such as enhanced persistence and increased cardiac function similar to CC1 and CPC + MSC treated hearts. We propose that the high proliferative capacity of CC2 and expression of immunomodulatory factor IL-6 contributed to structural and functional benefits but through the contribution of distinct phenotypic characteristics from CC1 and CC2 respectively.
Increased basal expression of cardiomyogenic factors was observed in CCs (Online Figure III). Pre-committed cells, but not fully mature stem cell derived cardiac myocytes improve exogenous cell coupling and formation of gap junction proteins30. CCs display coordinated phenotypic properties of commitment and increased paracrine abilities to promote cardiomyocyte health much like the MSC parent and CPC + MSC parents combined (Figure 2). Factor(s) that promote growth of cardiac myocytes and stabilization or the creation of microvasculature (Figure 5 and 6) remain to be established in our model of mouse CCs, but is certainly a subject of future investigations. Gene dosage effects as well as modifying the ratio of cell numbers before cell fusion leads to unique phenotypic properties such as proliferation and inhibition of senescence31–33. Embryonic stem cell (ESC) fusion with somatic cells facilitates reprogramming using equal cellular ratios indicating that ESCs are the more dominant cell type34. In this report, the CPC parent phenotype dominates in the fused progeny and most likely mediates early cardiomyogenic factors in CCs, whereas paracrine mediated effects from the MSC parent is secondary. For future studies, selecting the optimal cells and gene dosage for fusion will allow us to more effectively design hybrids for stronger traits towards commitment or paracrine effects.
Therapeutic delivery of MSCs improves cardiac function and structure mainly through paracrine mediated effects. Secretion of factors such as SDF-1 and IGF-1 support endogenous recruitment of c-kit+ progenitor cells and further facilitates cardiomyocyte cell cycle entry and survival35–37. Immunomodulatory functions of MSCs to inhibit excess scar formation is an attractive therapy for several disease states38. In this study, MSC treatment was unable to prevent increases in scar size or decreases in cardiac function up to 18 weeks similar to the deteriorating PBS treated hearts. Although, MSC addition did maintain size and survival of the responding cardiac myocytes, these beneficial effects were not recapitulated in vivo after MSC transfer (Figure 2 and 3). Apoptosis and slow proliferation rate are likely contributing factors to the disappearance of MSCs at later time points (Figure 1 and 4). Instead, MSC and PBS treated hearts sustained increases in c-kit+ cells, which are most likely increased through chronic inflammation and recruitment of hematopoietic derived c-kit+ mast cells (Figure 4).
The optimal cell number chosen for therapy is a critical aspect to promote structural and functional recovery after MI. Delivery of human CPC + MSC in a pig model of ischemia resulted in positive remodeling and engraftment using 200-fold more MSCs relative to CPCs11. For our study, we placed CPCs to MSCs at a one-to-one ratio as the appropriate control compared to our CCs. The engraftment efficiency of MSCs could have been greatly limited from the beginning of the experiment due to reduced MSC cell numbers (Figure 4). Benefits of co-culture of CPCs with MSCs are consistent with previous findings as MSC co-incubation with CPCs at equal ratios increased basal differentiation markers such as sm22, which was not observed in CCs (Online Figure III). Furthermore, during co-culture with NRCMs, CPC + MSC groups exhibited increased cardiomyogenic markers sm22, pecam and cTNT (Figure 2). It remains unclear if differentiation resulted from CPCs alone in culture with MSCs, although significant cell death of MSCs alone was observed when co-cultured with NRCMs for seven days. The comparatively modest therapeutic benefit of unmodified CPCs has been previously shown from our laboratory2, 3. Clearly, pinpointing the mechanistic contribution of MSCs to support CPCs in our CPC + MSC treated hearts is an important unanswered question to be resolved in future investigations.
Although engraftment efficiency of CPCs co-injected with MSCs was not significantly improved relative to CPC hearts alone, function was improved in CPC + MSC hearts at a much earlier time point. We can hypothesize that MSCs in the acute stages of damage (<4weeks) facilitated protective endogenous cell reprogramming without long-term persistence, which was not sufficient to impact on exogenous CPC proliferation and/or engraftment, consistent with reports from other groups10.
From the numerous cell types touted to be efficacious for cardiac clinical therapy, CPCs and MSCs are particularly promising because of established protocols for cell isolation and expansion in clinical settings6, 8. Although MSCs show much lower rates of persistence in the damaged heart than CPCs, cell therapeutic practices could benefit from investigation of how to enhance immunomodulatory effects of MSCs9. Currently, “Off-the-shelf” allogeneic cellular options include cardiosphere derived cells and MSCs that may exert beneficial effects after MI but suffer from poor persistence following delivery8, 39. In comparison, ESCs and induced pluripotent stem cells exhibit extended proliferation and are less prone to immuno rejection and/or cell senescence after transplantation40. However, ESCs have reduced capacity for integrative cardiomyogenesis as demonstrated by arrhythmogenic events in large animal models41. Our cell fusion approach aims to capitalize on adult stem cells that have validated cardiac therapeutic effects in order to create an exceptional composite hybrid with anti-inflammatory functions arising from the inclusion of allogeneic MSCs. Transplanted MSCs have suggested immunomodulatory functions by regulation of immune cells in the damaged setting. Mechanistically, MSCs have the potential to balance the inhibition of T cell proliferation by secretion of indoleamine and promotion of dendritic cell differentiation into T regulatory cells by secretion of IL-6 and interleukin-10 making this cellular source an essential component of future cardiac stem cell hybrids42. Additionally, fusion of aged stem cells with more youthful cells could confer cell rejuvenation and reverse signs of cellular aging33, 43. In the era of human cord blood banking, the isolation of immunoprivileged stromal cells from the same patient can be easily fused with stem cells harboring tissue specific regenerative capacity, resulting in a novel cell type that is resistant to rejection in addition to having desired cellular effects such as proliferation and direct tissue commitment. From a translational perspective, cell fusion is an adaptable genetic engineering strategy that qualitatively enhances adult stem cell properties such as persistence, anti-inflammatory and growth factor secretion and direct cardiomyogenesis to sustain long-term cardiac repair.
Supplementary Material
Novelty and Significance.
What Is Known?
Adult stem cell therapy leads to modest reparative effects due to low proliferation and survival of delivered cells in the damaged myocardium.
Combined delivery of cardiac progenitor cells (CPCs) and bone marrow derived mesenchymal stem cells (MSCs) support enhanced cellular engraftment and reduction in scar size after acute myocardial infarction (MI).
What New Information Does this Article Contribute?
Cell fusion to create cardiac stem cell hybrids or CardioChimeras between CPCs and MSCs combines optimal traits such as proliferation, survival, paracrine secretion and cardiomyogenic differentiation ability in a single cell type.
Adoptive transfer of CardioChimeras after acute MI promotes long-term improvements in anterior wall thickness and cardiac function.
CardioChimeras exhibit sustained engraftment concomitant with increased vascular stability and prevention of maladaptive cardiomyocyte hypertrophy.
Cardiac stem cell based therapy for the treatment of ischemic damage is popularized by the application of diverse cell types that exhibit distinct phenotypic traits. Specifically, delivery of CPCs or MSCs individually reveals contrasting but complementary in vivo functions such as through direct cardiomyogenic differentiation or paracrine growth factor secretion respectively. Dual cell delivery has emerged as a unique strategy to combine desirable functions of distinct cells to mitigate cardiac damage. In this study we are the first to utilize novel cardiac stem cell hybrids created by cell fusion between CPCs and MSCs, also known as CardioChimeras, to support and enhance combinatorial cell delivery approaches. By inheriting properties of CPCs and MSCs, CardioChimeras exhibit optimal properties such as cardiac commitment and enhanced paracrine secretion. CardioChimeras transplanted after MI improves myocardial structure and reduces infarct size. Importantly, CardioChimeras have increased engraftment in the left ventricle compared to groups treated with CPCs or MSCs individually and/or combined. Mechanistically, CardioChimeras promote an increase in capillary density and preserve cardiomyocyte size in the infarct area 12 weeks after damage. CardioChimeras represent an efficient fused product with beneficial and cardioprotective properties for effective cardiac repair.
ACKNOWLEDGMENTS
We thank all the members of the Sussman laboratory for their critical feedback.
SOURCES OF FUNDING
P. Quijada is supported by NIH grant F31HL117623, Rees Stealy Research Foundation Fellowship and SDSU Heart Institute Fellowship and Achievement Rewards for College Scientists Scholarship. N. Hariharan is supported by the American Heart Association 15BGIA23010047. M. A. Sussman is supported by NIH grants R01HL067245, R37HL091102, R01HL105759, R01HL113656, R01HL113647, R01HL122525 as well as an award from the Fondation Leducq.
M. A. Sussman is a founder and co-owner of CardioCreate Inc.
Nonstandard Abbreviations and Acronyms
- 2N
diploid, euploid DNA content
- 4N
tetraploid, aneuploid DNA content
- ANOVA
analysis of variance
- AWT
anterior wall thickness
- BMC
bone marrow cell
- CC
CardioChimera
- CC1
CardioChimera 1
- CC2
CardioChimera 2
- CPC
cardiac progenitor cell
- CPC + MSC
cardiac progenitor cells and mesenchymal stem cells
- CSC
cardiac stem cell
- cTNT
cardiac troponin T
- DAPI
4',6-diamidino-2-phenylindole
- dP/dT
developed pressure over time
- EF
ejection fraction
- eGFP
enhanced green fluorescent protein
- FACS
fluorescence activated cell sorting
- HW/BW
heart weight to body weight ratio
- IL-6
interleukin-6
- iPSC
induced pluripotent stem cell
- LAD
left anterior descending artery
- MI
myocardial infarction
- MSC
mesenchymal stem cell
- Myh6
myosin heavy chain α
- Myh7
myosin heavy chain β
- NRCM
neonatal rat cardiomyocyte
- PBS
phosphate buffered saline, vehicle control group
- PECAM (CD31)
platelet endothelial cell adhesion molecule
- PGK
phosphoglycerate kinase
- SDF-1
stromal derived factor-1
- SM22
smooth muscle 22
- TOPRO
TO-PRO-3 iodide
Footnotes
DISCLOSURES
All other authors have no disclosures.
REFERENCES
- 1.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. C-kit+ cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human cardiac progenitor cells engineered with pim-i kinase enhance myocardial repair. Journal of the American College of Cardiology. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiological reviews. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 5.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. C-kit+ cells minimally contribute cardiac myocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. Jama. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nature immunology. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 10.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with purkinje neurons, cardiac myocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 13.Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with purkinje neurons in response to chronic inflammation. Nature cell biology. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang WJ, Li SH, Weisel RD, Liu SM, Li RK. Cell fusion contributes to the rescue of apoptotic cardiac myocytes by bone marrow cells. Journal of cellular and molecular medicine. 2012;16:3085–3095. doi: 10.1111/j.1582-4934.2012.01600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soza-Ried J, Fisher AG. Reprogramming somatic cells towards pluripotency by cellular fusion. Curr Opin Genet Dev. 2012;22:459–465. doi: 10.1016/j.gde.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Tsubouchi T, Soza-Ried J, Brown K, Piccolo FM, Cantone I, Landeira D, Bagci H, Hochegger H, Merkenschlager M, Fisher AG. DNA synthesis is required for reprogramming mediated by stem cell fusion. Cell. 2013;152:873–883. doi: 10.1016/j.cell.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, Christov C, Baudin X, Auber F, Yiou R, Dubois-Rande JL, Rodriguez AM. Human mesenchymal stem cells reprogram adult cardiac myocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–824. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takei S, Yamamoto M, Cui L, Yue F, Johkura K, Ogiwara N, Iinuma H, Okinaga K, Sasaki K. Phenotype-specific cells with proliferative potential are produced by polyethylene glycol-induced fusion of mouse embryonic stem cells with fetal cardiac myocytes. Cell Transplant. 2005;14:701–708. doi: 10.3727/000000005783982693. [DOI] [PubMed] [Google Scholar]
- 19.Islam MQ, Meirelles Lda S, Nardi NB, Magnusson P, Islam K. Polyethylene glycol-mediated fusion between primary mouse mesenchymal stem cells and mouse fibroblasts generates hybrid cells with increased proliferation and altered differentiation. Stem Cells Dev. 2006;15:905–919. doi: 10.1089/scd.2006.15.905. [DOI] [PubMed] [Google Scholar]
- 20.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108(Suppl 1):II253–II258. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 21.Zaruba MM, Franz WM. Role of the sdf-1-cxcr4 axis in stem cell-based therapies for ischemic cardiomyopathy. Expert opinion on biological therapy. 2010;10:321–335. doi: 10.1517/14712590903460286. [DOI] [PubMed] [Google Scholar]
- 22.Zacchigna S, Giacca M. Extra- and intracellular factors regulating cardiomyocyte proliferation in postnatal life. Cardiovascular research. 2014;102:312–320. doi: 10.1093/cvr/cvu057. [DOI] [PubMed] [Google Scholar]
- 23.Fontes JA, Rose NR, Cihakova D. The varying faces of il-6: From cardiac protection to cardiac failure. Cytokine. 2015 doi: 10.1016/j.cyto.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo J, Sano M, Fujita J, Hayashida K, Yuasa S, Aoyama N, Takehara Y, Kato O, Makino S, Ogawa S, Fukuda K. Bone marrow derived cells are involved in the pathogenesis of cardiac hypertrophy in response to pressure overload. Circulation. 2007;116:1176–1184. doi: 10.1161/CIRCULATIONAHA.106.650903. [DOI] [PubMed] [Google Scholar]
- 25.Wu JM, Hsueh YC, Ch'ang HJ, Luo CY, Wu LW, Nakauchi H, Hsieh PC. Circulating cells contribute to cardiomyocyte regeneration after injury. Circ Res. 2015;116:633–641. doi: 10.1161/CIRCRESAHA.116.304564. [DOI] [PubMed] [Google Scholar]
- 26.Pajcini KV, Pomerantz JH, Alkan O, Doyonnas R, Blau HM. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J Cell Biol. 2008;180:1005–1019. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA, Klibanov AL, Yan Z, Mandell JW, Ravichandran KS. Phosphatidylserine receptor bai1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263–267. doi: 10.1038/nature12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estrada JC, Torres Y, Benguria A, Dopazo A, Roche E, Carrera-Quintanar L, Perez RA, Enriquez JA, Torres R, Ramirez JC, Samper E, Bernad A. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell death & disease. 2013;4:e691. doi: 10.1038/cddis.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson SE, Westra JW, Rehen SK, Young H, Bushman DM, Paczkowski CM, Yung YC, Lynch CL, Tran HT, Nickey KS, Wang YC, Laurent LC, Loring JF, Carpenter MK, Chun J. Normal human pluripotent stem cell lines exhibit pervasive mosaic aneuploidy. PLoS One. 2011;6:e23018. doi: 10.1371/journal.pone.0023018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boheler KR, Joodi RN, Qiao H, Juhasz O, Urick AL, Chuppa SL, Gundry RL, Wersto RP, Zhou R. Embryonic stem cell-derived cardiomyocyte heterogeneity and the isolation of immature and committed cells for cardiac remodeling and regeneration. Stem Cells Int. 2011;2011:214203. doi: 10.4061/2011/214203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlath GK, Blau HM. Expression of muscle genes in heterokaryons depends on gene dosage. J Cell Biol. 1986;102:124–130. doi: 10.1083/jcb.102.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam MQ, Ringe J, Reichmann E, Migotti R, Sittinger M, da SML, Nardi NB, Magnusson P, Islam K. Functional characterization of cell hybrids generated by induced fusion of primary porcine mesenchymal stem cells with an immortal murine cell line. Cell Tissue Res. 2006;326:123–137. doi: 10.1007/s00441-006-0224-2. [DOI] [PubMed] [Google Scholar]
- 33.Islam MQ, Panduri V, Islam K. Generation of somatic cell hybrids for the production of biologically active factors that stimulate proliferation of other cells. Cell Prolif. 2007;40:91–105. doi: 10.1111/j.1365-2184.2007.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foshay KM, Looney TJ, Chari S, Mao FF, Lee JH, Zhang L, Fernandes CJ, Baker SW, Clift KL, Gaetz J, Di CG, Xiang AP, Lahn BT. Embryonic stem cells induce pluripotency in somatic cell fusion through biphasic reprogramming. Mol Cell. 2012;46:159–170. doi: 10.1016/j.molcel.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong F, Harvey J, Finan A, Weber K, Agarwal U, Penn MS. Myocardial cxcr4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation. 2012;126:314–324. doi: 10.1161/CIRCULATIONAHA.111.082453. [DOI] [PubMed] [Google Scholar]
- 36.Taghavi S, Sharp TE, 3rd, Duran JM, Makarewich CA, Berretta RM, Starosta T, Kubo H, Barbe M, Houser SR. Autologous c-kit+ mesenchymal stem cell injections provide superior therapeutic benefit as compared to c-kit+ cardiac-derived stem cells in a feline model of isoproterenol-induced cardiomyopathy. Clinical and translational science. 2015 doi: 10.1111/cts.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poynter JA, Herrmann JL, Manukyan MC, Wang Y, Abarbanell AM, Weil BR, Brewster BD, Meldrum DR. Intracoronary mesenchymal stem cells promote postischemic myocardial functional recovery, decrease inflammation, and reduce apoptosis via a signal transducer and activator of transcription 3 mechanism. Journal of the American College of Surgeons. 2011;213:253–260. doi: 10.1016/j.jamcollsurg.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: The mesenchymal stromal cells breakthrough. Stem Cells Int. 2014;2014:340257. doi: 10.1155/2014/340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yee K, Malliaras K, Kanazawa H, Tseliou E, Cheng K, Luthringer DJ, Ho CS, Takayama K, Minamino N, Dawkins JF, Chowdhury S, Duong DT, Seinfeld J, Middleton RC, Dharmakumar R, Li D, Marban L, Makkar RR, Marban E. Allogeneic cardiospheres delivered via percutaneous transendocardial injection increase viable myocardium, decrease scar size, and attenuate cardiac dilatation in porcine ischemic cardiomyopathy. PLoS One. 2014;9:e113805. doi: 10.1371/journal.pone.0113805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quijada P, Sussman MA. Making it stick: Chasing the optimal stem cells for cardiac regeneration. Expert review of cardiovascular therapy. 2014;12:1275–1288. doi: 10.1586/14779072.2014.972941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiac myocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 43.Tat PA, Sumer H, Pralong D, Verma PJ. The efficiency of cell fusion-based reprogramming is affected by the somatic cell type and the in vitro age of somatic cells. Cell Reprogram. 2011;13:331–344. doi: 10.1089/cell.2011.0002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.