Abstract
Objective
We investigated gay and bisexual men’s willingness to self-administer an anal cancer screening test at home.
Methods
We conducted two national, online cross-sectional surveys of self-identified gay and bisexual men: Study I in 2009 with men ages 20–59 (n=306) and Study II in 2013 with men ages 18–26 (n=428). We used multivariate logistic regression analyses to determine variables associated with willingness to self-administer the screening test.
Results
Most men were willing to self-administer an anal cancer screening test (78% Study I; 67% Study II). In Study I, willingness was higher among men who trusted anal Paps to find treatable cancer (adjusted odds ratio [aOR]=1.47, 95% CI 1.04–2.09) and who believed that men who have sex with men should be screened for anal cancer between one and three years vs. other intervals (aOR=2.19, 95% CI 1.17–4.10). In Study II, willingness was higher among men who perceived greater likelihood of anal cancer (aOR=1.57, 95% CI 1.12–2.20). Their most common concerns were not performing the test correctly and inaccuracy of results.
Conclusions
Many gay and bisexual men were willing to self-administer anal cancer screening tests at home. If routine screening is warranted, self-collected home testing could improve participation.
Keywords: human papillomavirus, anus neoplasms, anal cancer, screening, acceptability, men who have sex with men (MSM), homosexuality
Incidence of common cancers affecting men in the United States (prostate, lung, and colorectal cancer) have declined over the past decade.1 Anal cancer incidence, however, has increased over the past three decades, and currently 1.5 men per 100,000 are affected per year.1,2 This increase is attributable, at least in part, to high incidence rates among men who have sex with men (MSM; ~5 men per 100,000 per year), particularly those living with HIV/AIDS (65–131 men per 100,000 per year).2,3
The majority (>70%) of cases of anal cancer are associated with human papillomavirus (HPV) infection, specifically with high-risk type 16 or 18.4 Risk factors for new or persistent anal HPV infections include anal intercourse, having multiple sexual partners, and smoking.5 These risk factors may explain why high-risk HPV types are common in HIV-negative and HIV-positive MSM (12.5% and 35.4%, respectively, for type 16).3
Anal cytological screening and treatment of dysplastic lesions could represent an important strategy for preventing anal cancer.6 Routine cervical Papanicolaou (Pap) testing has resulted in a dramatic decrease in the incidence of invasive cervical cancer in women over the last 50 years.7 It is possible that anal cancer screening could have similar effects for MSM, given the similarities between cervical and anal cancers and studies showing anal cytology (Pap) tests have similar accuracy to cervical Pap tests.8 No studies have been conducted, though, to determine anal cancer screening’s effect on mortality or other patient-centered outcomes.9 Despite this uncertainty, some clinicians are now recommending high-risk men, including HIV-positive MSM, be screened for anal cancer because of the high incidence of anal cancer.10,11
Studies of anal cancer screening behavior among MSM have focused almost exclusively on men’s acceptance of physician-collected Pap tests.12 Self-collected anal Paps have been shown to have accuracy similar to clinician-collected tests.13 Studies of self-tests for HIV and other sexually transmitted diseases have found high rates of acceptability among MSM, and it is possible that allowing men to use a self-test for anal cancer would increase screening uptake.14,15 The purpose of the present study was to examine gay and bisexual men’s willingness to use a self-collected anal cancer screening test at home and to identify correlates of willingness to use a self-test. Findings could help inform future programs for promoting anal cancer screening among this higher-risk population.
Methods
We report data from two separate cross-sectional studies of gay and bisexual men. The studies are described in detail elsewhere and briefly here.16,17
Study I
Sample and procedures
The sample for Study I came from an existing, national panel of U.S. households maintained by Knowledge Networks (Palo Alto, CA) Panel members were recruited using list-assisted random-digit dialing. Panel members received free Internet access or small cash payments in exchange for completing surveys. Study I was limited to men aged 18–59 and oversampled for self-identified gay and bisexual men. Of the 874 eligible panel members invited to participate, 609 (70%) consented to and completed the survey in January 2009. Of those who completed the survey, 306 men self-identified as gay (n=236) or bisexual (n=70) and were included in the current analysis. The Institutional Review Board at the University of North Carolina approved this study.
Measures
The Study I survey is available online at www.unc.edu/~ntbrewer/hpv.htm. We developed the survey items based on our previous work on HPV-related diseases.18,19
Our primary outcome was willingness to self-administer an anal cancer screening test at home. Introductory text to the section on anal cancer screening read, “Doctors can use an anal Pap test to identify anal cancer. An anal Pap test is when a doctor collects cells from the anus using a swab (like an extra-long Q-tip) and examines them for changes. An anal Pap test is not the same as a test for anal gonorrhea, a colonoscopy, or a digital rectal exam.” The survey assessed willingness to get a physician-collected anal Pap test under two conditions: (1) if it were free and (2) if it cost $150 out of pocket. Then the survey assessed willingness to self-administer an anal cancer screening test: “How willing would you be to use an anal swab on yourself to screen for anal cancer? You would do this test at home by yourself instead of going to a doctor’s office.” Response options for willingness items were definitely wouldn’t, probably wouldn’t, not sure, probably would, and definitely would. We combined responses into two categories: willing (probably or definitely would) and not willing (all other responses).
The survey assessed participants’ awareness of HPV prior to the study and knowledge of HPV using five factual items. We coded three to five correct responses as high HPV knowledge and the rest as low HPV knowledge, based on the median number of correct responses for the entire sample. The survey also measured respondents’ perceived knowledge of several HPV-related diseases (genital warts, oral cancer, and anal cancer), as well their worry about the disease, perceived likelihood of developing it, and belief that having the disease would change their lives. Because pilot testing showed men had low familiarity with HPV-related disease, we included brief informative statements about each disease prior to asking questions about them.
We measured several demographic and health-related characteristics as potential covariates. We defined “urban” as living in a metropolitan statistical area based on zip code. The survey also asked about history of sexually transmitted infections, genital warts, lifetime number of sexual partners, number of partners in the past year, and whether participants had disclosed sexual behavior with men to their physician.
Study II
Sample and procedures
The Study II sample was drawn from the Harris Interactive LGBT Panel, a subset of the Harris Poll Online Panel (Rochester, NY). The Harris Poll Online Panel is a voluntary research panel that includes members throughout the entire country; U.S. participants are similar to the general population for several demographic characteristics.20 Panel members who complete surveys receive points that can be exchanged for rewards. Inclusion criteria for Study II included being age 18–26 (because the primary focus of the study was on HPV vaccination among young adults), living in the US, and self-identifying as lesbian, gay, bisexual or transgender (LGBT). Of 2,014 eligible panel members, 1005 (50%) consented to and completed an online survey in October or November 2013. Of those who completed the survey, the 428 men who self-identified as gay (n=309) or bisexual (n =119) were included in the current analysis. The Institutional Review Board at The Ohio State University approved Study II.
Measures
We based the Study II survey on the survey used for Study I, as well as subsequent surveys on HPV vaccination.21,22 Our primary outcome was willingness to self-administer an anal cancer screening test at home. The survey item read, “There is also a home test that may help screen for anal cancer. This test would involve using a swab (like a Q-tip) to get an anal specimen. You would do this test at home by yourself instead of going to a doctor’s office. How willing would you be to use an anal swab on yourself to screen for anal cancer?” As with Study I, the five response options were combined into willing to self-test (probably or definitely willing) and not willing (note sure, probably or definitely not willing). An additional item asked participants to select potential concerns they had about using a home test for anal cancer from a list of predefined response options.
The survey assessed participants’ awareness of HPV prior to the study and knowledge of HPV using five factual items. We coded four or five correct responses as high HPV knowledge and less than four correct responses as low HPV knowledge, based on the median number correct for the entire sample. The survey also measured respondents’ worry about and perceived seriousness of HPV-related disease, as well as their perceived likelihood of developing specific diseases (genital warts, oral cancer, and anal cancer).
We measured several demographic and health-related characteristics as potential covariates. We defined “urban” as self-report of living in an urban or suburban area. The survey also asked about history of sexually transmitted infections, genital warts, lifetime number of sexual partners, number of partners in the past year, and whether participants had disclosed their sexual orientation to their physician.
Analyses
We analyzed data from the two studies separately, given their different samples. We used logistic regression to identify bivariate correlates of willingness to self-administer an anal cancer screening test. We entered statistically significant (p<0.05) bivariate correlates into multivariate logistic regression models for each study.
We used McNemar’s test to compare Study I participants’ willingness to self-administer an anal cancer screening test to their willingness to get a physician collected anal cancer screening test that cost $150 or that was free. We used Pearson’s chi-squared test to compare the two study samples’ willingness to self-administer the test.
We analyzed data with Stata release 13 (StataCorp LP, 2013, College Station, TX, USA). All statistical tests were 2-tailed, using a critical α=0.05. Missing values were replaced using mean substitution.
The reporting of this paper conforms to the STROBE statement, and a completed STROBE checklist is available online (Supplementary Digital Content: Table 1).23
Role of the Funding Source
Study I was supported by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. Merck Sharp & Dohme Corp. played no role in the study design, planning, implementation, analysis, or reporting of the findings. Additional support for these studies was provided by the American Cancer Society and the National Cancer Institute at the National Institutes of Health.
Results
Participant Characteristics
Most men in Study I identified as gay (77%) and were HIV-negative (83%) (Table 1). Their median age was 47 years (range 20–59), and 96% were older than 26. Most were non-Hispanic White (81%), had a college degree (56%), were insured (86%), or lived in an urban area (93%). Nearly half were married or living with a partner (48%) and most had at least five lifetime sexual partners (89%). Only 23% had ever heard of an anal Pap before the survey, and 14% reported having an anal Pap in the past.
Table 1.
Participant Characteristics
| Study I (n=306) No. (%) | Study II (n=428) No. (%) | |
|---|---|---|
| Sexual orientation | ||
| Gay | 236 (77) | 309 (72) |
| Bisexual | 70 (23) | 119 (28) |
| Race/ethnicity | ||
| Non-Hispanic White | 247 (81) | 273 (64) |
| Non-Hispanic Black | 14 (5) | 28 (7) |
| Hispanic | 29 (9) | 79 (18) |
| Other | 16 (5) | 48 (11) |
| Age, mean (range, median) | 46 (20–59,47) | 23 (18–26, 23) |
| Annual household income | ||
| ≥$50,000 | 204 (67) | 151 (35) |
| <$50,000 | 102 (33) | 231 (54) |
| Prefer not to answer | -- | 46 (11) |
| Education | ||
| Less than college degree | 136 (44) | 228 (53) |
| College degree or higher | 170 (56) | 200 (47) |
| Health insurance | ||
| No | 44 (14) | 86 (20) |
| Yes | 262 (86) | 342 (80) |
| Reside in urban area | ||
| No | 20 (7) | 67 (16) |
| Yes | 286 (93) | 361 (84) |
| Relationship status | ||
| Unmarried or not living with partner | 160 (52) | 357 (83) |
| Married or living with partner | 146 (48) | 71 (17) |
| HIV status | ||
| Negative | 255 (83) | 410 (96) |
| Positive | 51 (17) | 18 (4) |
| Number of lifetime sexual partners | ||
| 0 – 4 | 35 (11) | 202 (47) |
| 5 or more | 271 (89) | 226 (53) |
A similar proportion of men in Study II identified as gay (72%). Almost all men in Study II were HIV-negative (96%). Men in Study II were younger than in Study I (23 years, range 18–26). Fewer men in Study II identified as non-Hispanic White (64%), had a college degree (47%), were insured (80%), or lived in an urban area (84%). Fewer were married or partnered (53%), and only 53% had five or more lifetime sexual partners.
Willingness to Self-Administer an Anal Cancer Screening Test
Most men in Study I (78%, 95% confidence interval [CI] 73%–82%) were willing to self-administer an anal cancer screening test at home (Figure 1). Men were somewhat less willing to self-administer the test than to get a free physician-collected test (78% vs. 83%; χ2=4.41; p=0.04). They were much more willing to self-administer the test than to get a physician-collected test that cost $150 (78% vs. 31%; χ2=129.60, p<0.001).
Figure 1. Men’s willingness to screen for anal cancer by self-collected home test and physician-collected anal Pap test.
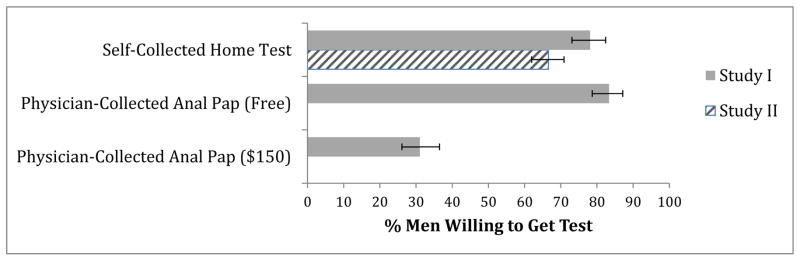
Error bars show 95% confidence intervals. Data on physician-collected anal Pap testing were not available for Study 2.
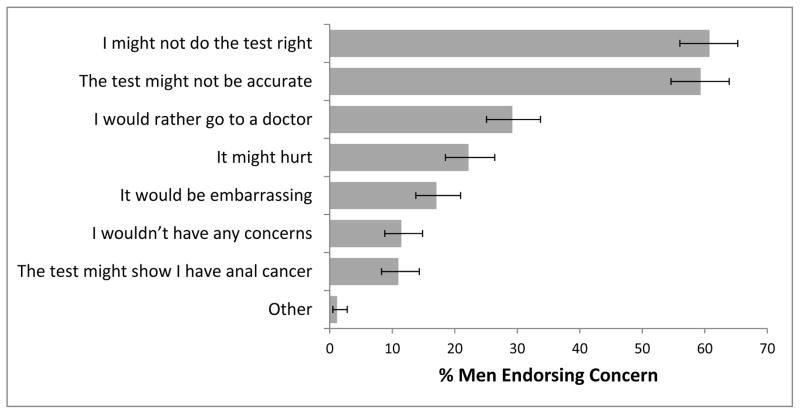
Two-thirds of Study II men (67%, 95% CI 62%–71%) were willing to self-administer an anal cancer screening test at home, a lower percentage than among Study I participants (p<0.001). The most common concerns about self-administrating the test that participants cited were “I might not do the test right” (61%) and “the test might not be accurate” (59%) (Figure 2). About 29% of the men said they would rather go to a doctor to get screened for anal cancer, 22% were concerned the test would hurt, and 17% thought it would be embarrassing.
Figure 2. Study II Concerns About Self-Administering an Anal Cancer Screening Test at Home.
Error bars show 95% confidence intervals.
Correlates of Willingness
In Study I, men’s willingness to self-administer an anal cancer test was higher if they believed MSM should be screened between one and three years (87%) rather than screening at other intervals (adjusted odds ratio [aOR]=2.19, 95% CI 1.17–4.10) in multivariate analysis (Table 2). Willingness was also higher among men who agreed more with the statement: “If I got regular anal pap tests, I would trust them to find anal cancer when it is still treatable.” (aOR=1.47, 95% CI 1.04–2.09). Additional correlates of willingness in bivariate analysis were higher worry about anal cancer, higher knowledge about HPV, belief that HIV affects a man’s chances of getting anal cancer, being HIV-positive, and being non-Hispanic white when compared to “other” race/ethnicity (all p<0.05).
Table 2.
Study I Correlates of Willingness to Self-Administer an Anal Cancer Screening Test
| No. Willing/Total No. (%) | Bivariate OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|
| HPV, anal Cancer, and screening | |||
| Perceived knowledge of anal cancer A | -- | 1.24 (0.84–1.83) | -- |
| Worry about anal cancer B | -- | 1.68 (1.07–2.66)* | 1.54 (0.94–2.52) |
| Perceived severity of anal cancer B | -- | 1.25 (0.89–1.75) | -- |
| Perceived likelihood of anal cancer C | -- | 1.32 (0.86–2.02) | -- |
| Belief that HIV status affects chances of getting anal cancer D | -- | 2.50 (1.23–5.07)* | 1.95 (0.87–4.40) |
| Trust in anal Pap to find treatable cancer E | -- | 1.74 (1.27–2.40)** | 1.47 (1.04–2.09)* |
| Belief that only people who have anal sex need anal Pap tests E | -- | 1.18 (0.93–1.50) | -- |
| Awareness of HPV F | |||
| Unaware | 44/63 (70) | 1.00 | 1.00 |
| Aware, low knowledge | 98/127 (77) | 1.46 (0.74–2.88) | 1.25 (0.59–2.67) |
| Aware, high knowledge | 97/116 (84) | 2.20 (1.06–4.57)* | 1.46 (0.65–3.26) |
| Aware of anal pap test | |||
| No | 178/235 (76) | 1.00 | -- |
| Yes | 61/71 (86) | 1.95 (0.94–4.06) | |
| Ever had an anal Pap test | |||
| No | 203/262 (77) | 1.00 | -- |
| Yes | 36/44 (82) | 1.31 (0.58–2.97) | |
| Belief that doctors recommend anal Pap tests for MSM | |||
| No | 48/60 (80) | 1.00 | -- |
| Yes | 95/112 (85) | 1.40 (0.62–3.16) | |
| Don’t know | 96/134 (72) | 0.63 (0.30–1.32) | |
| Believed anal cancer screening should happen every 1–3 y among MSM | |||
| No | 115/163 (71) | 1.00 | 1.00 |
| Yes | 124/143 (87) | 2.72 (1.51–4.91)** | 2.19 (1.17–4.10)* |
| History of digital rectal exam | |||
| No or don’t know | 57/79 (72) | 1.00 | -- |
| Yes | 182/227 (80) | 1.56 (0.87–2.82) | |
| Sexual history | |||
| Disclosed sex with men to PCP | |||
| Yes | 151/186 (81) | 1.00 | 1.00 |
| n/a or missing | 26/39 (67) | 0.46 (0.22–0.99)* | 0.73 (0.31–1.69) |
| No | 62/81 (77) | 0.76 (0.40–1.42) | 1.05 (0.52–2.10) |
| Age at first sexual intercourse (oral, anal, or vaginal) | |||
| <16 | 88/108 (81) | 1.00 | -- |
| ≥16 | 151/198 (76) | 0.73 (0.41–1.31) | |
| Number of lifetime sexual partners | |||
| ≤4 | 24/35 (69) | 1.00 | -- |
| ≥5 | 215/271 (79) | 1.76 (0.81–3.81) | |
| History of cancer (oral, anal, penile) or lesions (anal, penile) | |||
| No | 234/301 (78) | 1.00 | -- |
| Yes | 5/5 (100) | -- | |
| History of genital warts | |||
| No | 204/263 (78) | 1.00 | -- |
| Yes | 35/43 (81) | 1.27 (0.56–2.88) | |
| HIV status | |||
| Negative | 193/255 (76) | 1.00 | 1.00 |
| Positive | 46/51 (90) | 2.96 (1.12–7.77)* | 2.25 (0.80–6.34) |
| History of sexually transmitted disease other than HIV | |||
| No | 169/221 (76) | 1.00 | -- |
| Yes | 70/85 (82) | 1.44 (0.76–2.72) | |
| Demographic characteristics | |||
| Sexual orientation | |||
| Gay | 190/236 (81) | 1.00 | -- |
| Bisexual | 49/70 (70) | 0.56 (0.31–1.03) | |
| Health insurance | |||
| No | 32/44 (73) | 1.00 | -- |
| Yes | 207/262 (79) | 1.41 (0.68–2.92) | |
| Current smoker | |||
| No | 169/219 (77) | 1.00 | -- |
| Yes | 70/87 (80) | 1.22 (0.66–2.26) | |
| Race/ethnicity | |||
| Non-Hispanic White | 197/247 (80) | 1.00 | 1.00 |
| Non-Hispanic Black | 11/14 (79) | 0.93 (0.25–3.46) | 0.59 (0.13–2.61) |
| Hispanic | 22/29 (76) | 0.80 (0.32–1.97) | 0.94 (0.35–2.48) |
| Other | 9/16 (56) | 0.33 (0.12–0.92)* | 0.42 (0.14–1.31) |
| Age, mean y (S.D.) | 46.60 (8.44) | 1.01 (0.98–1.04) | -- |
| Annual income | |||
| <$50,000 | 73/102 (72) | 1.00 | -- |
| ≥$50,000 | 166/204 (81) | 1.74 (1.00–3.03) | |
| Education | |||
| No college degree | 102/136 (75) | 1.00 | -- |
| College degree | 137/170 (81) | 1.38 (0.80–2.38) | |
| Reside in urban area | |||
| No | 17/20 (85) | 1.00 | -- |
| Yes | 222/286 (78) | 0.61 (0.17–2.15) | |
| Relationship status | |||
| Unmarried or not living with partner | 122/160 (76) | 1.00 | -- |
| Married or living with partner | 117/146 (80) | 1.26 (0.73–2.17) | |
p<0.05
p≤0.001
HPV = human papillomavirus MSM = men who have sex with men PCP = primary care provider
4-point response scale, from nothing at all (coded as 1) to quite a lot (coded as 4)
4-point scale, from not at all (coded as 1) to quite a lot (coded as 4)
5-point scale, from no chance (coded as 1) to certain (coded as 5)
3-point scale, decreases chances (coded as 1), has no effect (coded as 2), or increases chances (coded as 3)
5-point scale, from strongly disagree (coded as 1) to strongly agree (coded as 5)
Cutoff for high-knowledge was ≥3/5 HPV knowledge items correct, based on median # correct for entire study
In Study II, willingness to self-administer an anal cancer screening test was higher among men who had higher perceived likelihood of developing anal cancer (aOR=1.57, 95% CI 1.12–2.20) in multivariate analysis (Table 3). In bivariate analyses, correlates of willingness to use a self-test were higher worry about diseases caused by HPV, higher knowledge of HPV, having five or more lifetime sexual partners, having a college degree, and being older (all p<0.05).
Table 3.
Study II Correlates of Willingness to Self-Administer an Anal Cancer Screening Test
| No. Willing/Total No. (%) | Bivariate OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|
| HPV, Anal Cancer, and Screening | |||
| Worry about diseases caused by HPV A | 1.36 (1.06–1.74)* | 1.03 (0.78–1.35) | |
| Perceived severity of disease caused by HPV B | 1.09 (0.82–1.45) | -- | |
| Perceived likelihood of anal cancer C | 1.69 (1.22–2.33)** | 1.57 (1.12–2.20)* | |
| Awareness of HPV D | |||
| Unaware | 33/59 (56) | 1.00 | 1.00 |
| Aware, low knowledge | 138/221 (62) | 1.31 (0.73–2.34) | 1.09 (0.59–1.99) |
| Aware, high knowledge | 114/148 (77) | 2.64 (1.39–5.01)* | 1.94 (0.98–3.85) |
| Vaccinated for HPV | |||
| No | 245/372 (66) | 1.00 | -- |
| Yes | 40/56 (71) | 1.30 (0.70–2.40) | |
| Sexual History | |||
| Disclosed sexual orientation to PCP | |||
| Yes | 98/136 (72) | 1.00 | -- |
| Somewhat | 17/27 (63) | 0.66 (0.28–1.57) | |
| No | 170/265 (64) | 0.69 (0.44–1.09) | |
| Age at first sexual intercourse (oral, anal, or vaginal) | |||
| <16 | 68/102 (67) | 1.00 | -- |
| ≥16 | 217/326 (67) | 0.99 (0.62–1.60) | |
| Number of lifetime sexual partners | |||
| 0 – 4 | 120/202 (59) | 1.00 | 1.00 |
| 5 or more | 165/226 (73) | 1.85 (1.23–2.77)* | 1.42 (0.90–2.23) |
| History of genital warts | |||
| No | 268/408 (66) | 1.00 | -- |
| Yes | 17/20 (85) | 2.96 (0.85–10.27) | |
| HIV status | |||
| Negative | 272/410 (66) | 1.00 | -- |
| Positive | 13/18 (72) | 1.32 (0.46–3.78) | |
| History of sexually transmitted disease other than HIV | |||
| No | 256/386 (66) | 1.00 | -- |
| Yes | 29/42 (69) | 1.13 (0.57–2.25) | |
| History of HPV infection | |||
| No | 267/406 (66) | 1.00 | -- |
| Yes | 18/22 (82) | 2.34 (0.78–7.06) | |
| Demographic Characteristics | |||
| Sexual orientation | |||
| Gay | 217/309 (70) | 1.00 | 1.00 |
| Bisexual | 68/119 (57) | 0.57 (0.37–0.88)* | 0.69 (0.43–1.10) |
| Health insurance | |||
| No | 53/86 (62) | 1.00 | -- |
| Yes | 232/342 (68) | 1.31 (0.80–2.14) | |
| Race/ethnicity | |||
| Non-Hispanic White | 182/273 (67) | 1.00 | -- |
| Non-Hispanic Black | 20/28 (71) | 1.25 (0.53–2.95) | |
| Hispanic | 47/79 (59) | 0.73 (0.44–1.23) | |
| Other | 36/48 (75) | 1.50 (0.74–3.02) | |
| Age, mean yrs (S.D.) | 23.07 (2.40) | 1.11 (1.02–1.20)* | 1.04 (0.94–1.15) |
| Annual income | |||
| <$50,000 | 151/231 (65) | 1.00 | -- |
| ≥$50,000 | 107/151 (71) | 1.29 (0.83–2.01) | |
| Not reported | 27/46 (59) | 0.75 (0.39–1.44) | |
| Education | |||
| No college degree | 139/228 (61) | 1.00 | 1.00 |
| College degree | 146/200 (73) | 1.73 (1.15–2.61)* | 1.36 (0.83–2.24) |
| Reside in urban area | |||
| No | 45/67 (67) | 1.00 | -- |
| Yes | 240/361 (66) | 0.97 (0.56–1.69) | |
| Relationship status | |||
| Unmarried or not living with partner | 231/357 (65) | 1.00 | -- |
| Married or living with partner | 54/71 (76) | 1.73 (0.96–3.12) | |
p<0.05
p≤0.001
HPV = human papillomavirus PCP = primary care provider
4-point scale, from not at all (coded as 1) to quite a lot (coded as 4)
4-point scale, from not at all (coded as 1) to very (coded as 4)
4-point scale, from no chance (coded as 1) to high chance (coded as 4)
Cutoff for high-knowledge was ≥4/5 HPV knowledge items correct, based on median # correct for entire study
Discussion
In the two national samples, most gay and bisexual were willing to self-administer an anal cancer screening test at home. The proportion of men willing to use a self-collected test was similar to or higher than those previously reported for physician-collected Pap tests.16,24 The possibility that men may prefer a self-collected home test is supported by our findings that Study I men were more willing to use the self-collected test than to get a physician-collected Pap that cost $150 and that fewer than a third of Study II participants indicated they would rather go to a doctor to get screened for anal cancer as a concern about self-testing. This is consistent with the literature on cervical cancer screening, which has shown that allowing women to self-collect HPV samples improves participation in screening programs.25 Men in Study I were slightly less willing to use a self-collected test than to undergo a physician-collected Pap if offered at no cost. Offering subsidized physician-based testing and promoting home-testing may therefore both be reasonable options to improve screening in the future.
We found much greater willingness to use a self-collected anal cancer screening test than a previous study of acceptability among MSM; Gilbert et al. reported only 35% of men were willing to use a self-collected test, but their sample was limited to men attending MSM-frequented venues and the test was collected in venue bathrooms.26 The increased privacy, comfort, and safety of collecting the test at home may be important components of men’s willingness to self-administer an anal cancer screening test.27
We identified three potentially modifiable correlates of willingness to use self-collected testing in multivariate analyses. Men in Study II who perceived they had a higher likelihood of anal cancer were more willing to self-administer an anal cancer screening test. Reed et al. previously identified this association in their analysis of willingness to get a physician-collected anal Pap test.16 Multiple health behavior studies have established that risk perceptions, including perceived likelihood, are important predictors of cancer screening participation and other health protective actions.28 This study extends that link to the use of self-administered cancer screening tests.
Men’s willingness to use a self-collected test in Study I was associated with their trust in anal Paps to find cancer when it is still treatable. Belief in the efficacy or benefits of screening is another construct that has been correlated with participation in cancer screening programs.29 However, Reed et al. did not find a statistically significant association between perceived test efficacy and willingness to get a physician-collected Pap.17 Similarly, D’Souza et al. found that belief in the utility of anal Pap tests was associated with acceptance of a free test among MSM in bivariate analyses, but the association was not significant in their multivariate model.24 It is possible that test effectiveness is more important to men when considering a home-based test, as a test performed by a physician may be assumed to have some minimal level of effectiveness. This idea is supported by qualitative work showing some people worry that home-based STI tests are less accurate and our own findings that the majority of men in Study II had concerns about the self-collected test’s accuracy and their ability to perform it correctly.30
The association between believing that MSM should get an anal Pap test to screen for anal cancer and men’s willingness to use a self-collected test may simply show that men who thought MSM need to be screened were themselves more willing to be screened. Perceived norms about engaging in health-promoting behavior have been linked to adherence to colorectal cancer screening recommendations.31 Our study shows that this relationship could also be true for anal cancer screening.
The strengths of this study were its use of two large, national samples with high participation rates. To our knowledge, this was the first study to explore gay and bisexual men’s willingness to self-administer an anal cancer screening test using a national sample.
Limitations include the cross-sectional designs and reliance on self-report for the measures, including HIV status and screening history. Study sampling was based on sexual identity rather than behavior, and thus may not be representative of all MSM. Furthermore, as most men lived in urban areas, had health insurance, and were non-Hispanic White, the generalizability of our findings to other MSM remains to be established. Currently, no self-administered anal cancer screening test is licensed for home use outside of research settings. Our findings for willingness may overestimate men’s actual use should such a test become available. Future studies should therefore examine use if a self-administered home test becomes available. We did not establish whether the participants had access to a provider in their vicinity who offered anal Pap testing or whether it is currently possible to receive a free provider-based test. Both of these factors might reasonably influence men’s willingness to undergo such testing relative to a self-collected test. Furthermore, our surveys did not specify whether the home tests were self-collected anal Pap tests, HPV tests, or a combination of the two. If men perceive HPV testing to be more accurate, this might affect their willingness to undergo screening.
Conclusions
In conclusion, the majority of gay and bisexual men were willing to self-administer an anal cancer screening test at home. If anal Pap tests are shown to be an effective means of reducing incidence and mortality from invasive anal cancer, allowing men the option of home testing could improve screening uptake. This study identified three potentially modifiable factors associated with willingness to use a self-test and potential concerns men have with using a self-test that could be targeted in future public health campaigns to increase screening rates.
Supplementary Material
Acknowledgments
Source of Funding: Supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. Additional support provided by the American Cancer Society (MSRG-06-259-01-CPPB) and the National Cancer Institute at the National Institutes of Health (R25 CA57726 and P30CA016058).
Abbreviations
- AIDS
acquired immune deficiency syndrome
- HIV
human immunodeficiency virus
- HPV
human papillomavirus
- MSM
men who have sex with men
- OR
odds ratio
- Pap
Papanicolaou test
- PCP
primary care provider
- S.D
standard deviationl
Footnotes
IRB Status: This study was approved by the institutional review board of the University of North Carolina – Chapel Hill.
Declaration of Interests
NTB has received other grants from GlaxoSmithKline and Merck Sharp & Dohme Corp. and served on paid advisory boards for Merck Sharp & Dohme Corp. PLR has also received additional grants from Merck Sharp & Dohme Corp.
Conflict of Interest: A research grant to NTB from Merck Sharp & Dohme Corp. funded Study I. Merck Sharp & Dohme Corp. played no role in the study design, planning, implementation, analysis, or reporting of the findings. NTB has received other grants from GlaxoSmithKline and Merck Sharp & Dohme Corp. and served on paid advisory boards for Merck Sharp & Dohme Corp. PLR has also received additional grants from Merck Sharp & Dohme Corp. and a research grant from Cervical Cancer-Free America, via an unrestricted educational grant from GlaxoSmithKline.
References
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MA: National Cancer Institute; 2014. [Google Scholar]
- 2.van der Zee RP, Richel O, de Vries HJC, Prins JM. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med. 2013;71(8):401–411. [PubMed] [Google Scholar]
- 3.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncology. 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 4.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124(10):2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 5.Goldstone S, Palefsky JM, Giuliano AR, et al. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J Infect Dis. 2011;203(1):66–74. doi: 10.1093/infdis/jiq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palefsky JM, Rubin M. The epidemiology of anal human papillomavirus and related neoplasia. Obstet Gynecol Clin North Am. 2009;36(1):187–200. doi: 10.1016/j.ogc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Bulletins-Gynecology. ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222–1238. doi: 10.1097/aog.0b013e318277c92a. [DOI] [PubMed] [Google Scholar]
- 8.Cachay ER, Agmas W, Mathews WC. Relative accuracy of cervical and anal cytology for detection of high grade lesions by colposcope guided biopsy: a cut-point meta-analytic comparison. PLoS One. 2012;7(7):e38956. doi: 10.1371/journal.pone.0038956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyczek P, Singh AE, Romanowski B. Anal intraepithelial neoplasia: review and recommendations for screening and management. Int J STD AIDS. 2013;24(11):843–851. doi: 10.1177/0956462413481527. [DOI] [PubMed] [Google Scholar]
- 10.Park IU, Palefsky JM. Evaluation and management of anal intraepithelial neoplasia in HIV-negative and HIV-positive men who have sex with men. Curr Infect Dis Rep. 2010;12(2):126–133. doi: 10.1007/s11908-010-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 12.Landstra JM, Ciarrochi J, Deane FP. Psychosocial aspects of anal cancer screening: a review and recommendations. Sex Health. 2012;9(6):620–627. doi: 10.1071/SH11169. [DOI] [PubMed] [Google Scholar]
- 13.Lampinen TM, Miller ML, Chan K, et al. Randomized clinical evaluation of self-screening for anal cancer precursors in men who have sex with men. CytoJournal. 2006;3:4. doi: 10.1186/1742-6413-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman AH, Bernstein KT, Kohn RP, Philip S, Rauch LM, Klausner JD. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38(11):1036–1039. doi: 10.1097/OLQ.0b013e318227713e. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Stephenson RB, White D, Sullivan PS. Acceptability and intended usage preferences for six HIV testing options among internet-using men who have sex with men. SpringerPlus. 2014;3:109. doi: 10.1186/2193-1801-3-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed AC, Reiter PL, Smith JS, Palefsky JM, Brewer NT. Gay and bisexual men’s willingness to receive anal Papanicolaou testing. Am J Public Health. 2010;100(6):1123–1129. doi: 10.2105/AJPH.2009.176446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter PL, McRee AL, Katz ML, Paskett ED. HPV vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105(1):96–102. doi: 10.2105/AJPH.2014.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazekas KI, Brewer NT, Smith JS. HPV vaccine acceptability in a rural Southern area. J Womens Health. 2008;17(4):539–548. doi: 10.1089/jwh.2007.0489. [DOI] [PubMed] [Google Scholar]
- 19.Reiter PL, Brewer NT, Gottlieb SL, et al. Parents’ health beliefs and HPV vaccination of their adolescent daughters. Soc Sci Med. 2009;69(3):475–480. doi: 10.1016/j.socscimed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Krane D, Witeck B, Combs W. Surveying among gays & lesbians. Harris Interactive. 2011 [Google Scholar]
- 21.Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of human papillomavirus vaccine initiation among adolescent girls in a high-risk geographic area. Sex Transm Dis. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiter PL, McRee AL, Pepper JK, et al. Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. Am J Public Health. 2013;103(8):1419–1427. doi: 10.2105/AJPH.2012.301189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, von Elm E, Altman DG, et al. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS medicine. 2007;4(10) doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Souza G, Rajan SD, Bhatia R, et al. Uptake and predictors of anal cancer screening in men who have sex with men. Am J Public Health. 2013;103(9):e88–95. doi: 10.2105/AJPH.2013.301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racey CS, Withrow DR, Gesink D. Self-collected HPV Testing Improves Participation in Cervical Cancer Screening: A Systematic Review and Meta-analysis. Can J Public Health. 2013;104(2):e159–e166. doi: 10.1007/BF03405681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert M, Kwag M, Mei W, et al. Feasibility of incorporating self-collected rectal swabs into a community venue-based survey to measure the prevalence of HPV infection in men who have sex with men. Sex Transm Dis. 2011;38(10):964–969. doi: 10.1097/OLQ.0b013e318222899d. [DOI] [PubMed] [Google Scholar]
- 27.Newman PA, Roberts KJ, Masongsong E, Wiley DJ. Anal Cancer Screening: Barriers and Facilitators Among Ethnically Diverse Gay, Bisexual, Transgender, and Other Men Who Have Sex With Men. J Gay Lesbian Soc Serv. 2008;20(4):328–353. doi: 10.1080/10538720802310733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? A meta-analysis of experimental studies. Psychol Bull. 2014;140(2):511–543. doi: 10.1037/a0033065. [DOI] [PubMed] [Google Scholar]
- 29.Gimeno Garcia AZ, Hernandez Alvarez Buylla N, Nicolas-Perez D, Quintero E. Public awareness of colorectal cancer screening: knowledge, attitudes, and interventions for increasing screening uptake. ISRN oncology. 2014;2014:425787. doi: 10.1155/2014/425787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rompalo AM, Hsieh YH, Hogan T, et al. Point-of-care tests for sexually transmissible infections: what do ‘end users’ want? Sex Health. 2013;10(6):541–545. doi: 10.1071/SH13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews BA, Nattinger AB, Venkatesan T, Shaker R. Colorectal cancer screening among midwestern community-based residents: indicators of success. J Community Health. 2007;32(2):103–120. doi: 10.1007/s10900-006-9038-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.