Abstract
Background
Charleston Harbor has elevated concentrations of PFAS in dolphins, but local human exposure data are limited.
Objectives
We sought to describe PFAS serum concentrations’ temporal trends among Gullah African American residents of coastal South Carolina.
Methods
Longitudinal measures of PFAS in blood serum from a Gullah clinical sample, without lupus, were examined using spaghetti plots and visit-to-visit change scores (e.g., differences in concentrations between visits) among the 68 participants with repeated measures available. We also modeled population-level trends among the 71 participants with any data using proportionate percentile models, accounting for clustering through robust standard errors. In a post-hoc analysis we examined heterogeneity of temporal trends by age through mixed-effects models for the log-transformed PFAS compounds.
Results
Population concentrations of PFOS dropped approximately 9 (95% CI: 8, 10) percent each year over 2003–2013. This was concordant with individual PFOS trajectories (median PFOS change score −21.7 ng/g wet weight, interquartile range of PFOS change scores: −32.8, −14.9) and reports for other populations over this time period. Several other compounds including PFOA, PFHxS, and PFuNDA also showed a population-level decrease. However, examination of individual trajectories suggested substantial heterogeneity. Post-hoc analyses indicated that PFAS trajectories were heterogeneous by age.
Conclusions
Many PFAS compounds are decreasing in a sample of Gullah African Americans from coastal South Carolina. There may be age differences in the elimination kinetics of PFASs. The possible role of age as a modifier of PFAS serum trends merits further research.
Keywords: PFAS, Gullah, PFOA, PFOS, biomonitoring, contaminant trends
INTRODUCTION
Perfluoroalkyl substances (PFAS) (Buck et al., 2011), although commercially useful for their surfactant properties (Lehmler, 2005), have spread around the globe and into foods and may have important consequences for the environment and human health (Armitage et al., 2009; Butt et al., 2010; Domingo, 2012; Giesy and Kannan, 2001; Ludwicki et al., 2015; Perez et al., 2014). The epidemiology and toxicology of these chemicals is still emerging, but PFAS exposures may have several health implications (Lau, 2012; Post et al., 2012; Steenland et al., 2010). Recent reviews have found that PFOA is negatively associated with fetal growth (Bach et al., 2014; Johnson et al., 2014). PFOA’s associations with cancer are the focus of an IARC monograph (Benbrahim-Tallaa et al., 2014), although a recent critical review found that overall the evidence was not supportive of PFOA or PFOS as carcinogens (Chang et al., 2014). There are differences by compound in the distribution of PFAS across tissues (Pérez et al., 2013). Although the health implications are still being assessed and are generally compound-specific, as a class these chemicals may be relevant for public health. It is therefore important to understand population exposures over time.
A possible route of exposure is through consumption of contaminated foods including fish (Perez et al., 2014). PFASs accumulate in marine food webs resulting in high concentrations in high trophic-level marine mammals serving as sentinel species (Fair et al., 2012a; Giesy and Kannan, 2001). Some of the highest PFASs found globally in marine mammals are in bottlenose dolphins in Charleston, South Carolina (Fair et al., 2012a; Houde et al., 2005). Concentrations of PFASs measured in the Charleston dolphins are on the same order of magnitude as occupationally exposed humans (Fair et al., 2012a; Fair et al., 2012b). The high PFAS concentrations in these dolphins alerted us to investigate the potential for environmental contamination possible human exposure. Further investigations have documented elevated concentrations of PFASs in the dolphin food web (Houde et al., 2006) and this current study focuses on exposures of the Gullah African Americans in the Charleston region. Local seafood is a dietary staple in the Gullah African American population (Ellis et al., 2014).
Many biomarker studies of PFASs to date are cross-sectional surveys collectively offering an oblique portrait of changing congener profiles across time and space (Apelberg et al., 2007; Frisbee et al., 2009; Gump et al., 2011; Guruge et al., 2005; Holzer et al., 2008; Kannan et al., 2004; Kato et al., 2014; Maisonet et al., 2012; Olsen and Zobel, 2007; Pinney et al., 2014; Schecter et al., 2012; Shaw et al., 2013; Tao et al., 2008a; Tao et al., 2008b; Tao et al., 2008c; von Ehrenstein et al., 2009; Zhang et al., 2013a; Zhang et al., 2010; Zhou et al., 2014). Several studies have examined trends across years in the same place, allowing more direct insights into temporal trends. Studies in Norway have shown decreases in perfluorooctane sulfonate (PFOS) and perfluorononanoic acid (PFOA) since 2000 (Haug et al., 2009; Nost et al., 2014); and decreases in women’s PFOS and PFOA concentrations after phase-out in commercial products (Berg et al., 2014). In Sweden, there were decreases over 1996–2010 in blood serum PFOA and PFOS, but increases in perfluorononanoate (PFNA), perfluorohexanesulfonate (PFHxS), and perfluorobutanesulfonate (PFBS) (Glynn et al., 2012). There were also decreases in PFOA and PFOS in breastmilk in Sweden 2001–2008 (Sundstrom et al., 2011). One study in Germany supports a decrease in serum PFOS concentrations during 2001–2010, and a decrease in PFHxS concentrations during 2005–2010 (Schroter-Kermani et al., 2013). However, another German survey of samples from two cities, Halle and Munster, collected over 1982–2009 did not find differences in PFHxS, but did see decreases in PFOS and PFOA, as well as increases in PFNA, PFDA and perfluoroundecanoic acid (PFuNDA) (Yeung et al., 2013a; Yeung et al., 2013b). In Japan, one study found apparent declines of 8.4% per year of PFOS and 3.1% per year of PFOA in umbilical cord blood samples collected over 2003–2011, but increases in PFNA and PFDA (Okada et al., 2013). Another study in Japan found increases in serum concentrations of perfluoroalkyl acids with chains longer than 8-carbons in three cities over 2002–2009 (Harada et al., 2011). In a study in South Korea, serum concentrations of PFNA, PFuNDA and perfluorotridecanoic acid (PFTrDA) were higher in 2007–2008 than in 1994 (Harada et al., 2011). Australian samples also showed a decrease in PFOS and PFOA during 2002–2011 (Toms et al., 2014).
Several studies have compared PFAS concentrations across years within the United States. One study of umbilical cord blood samples collected in New York over 1997–2007 found decreases in concentrations of PFOS, PFOA, PFHxS and perfluorooctane sulfonamide (PFOSA) after the year 2000, coinciding with the domestic phase-out of PFOA (Spliethoff et al., 2008). In a sample of blood donors from the American Red Cross, there was a decrease in 2010 concentrations compared to 2000–2001 concentrations for PFOS, PFOA and PFHxS (Olsen et al., 2012) The National Health and Nutrition Examination Survey also found lower concentrations of PFOS, PFOA, and PFHxS in 2003–2004 compared to 1999–2000 (Calafat et al., 2007), and over 2003–2008 while PFOS continued to decline, concentrations of PFOA remained subsequently unchanged, and the decline in PFHxS reversed in 2007–2008 (Kato et al., 2011). These apparent trends in the United States coincide with 3M’s commercial phase-out of PFOS announced in 2000 and begun in 2002 (Paul et al., 2008). However, the United States ‘temporal trend’ studies are almost all ecological comparisons of different participants recruited in different years. To our knowledge, the only previous longitudinal comparisons of same-subject serum concentrations in the United States were in highly exposed populations such as residents of the Mid-Ohio Valley before-and-after water filtration was introduced as an exposure intervention (Bartell et al., 2010; Fitz-Simon et al., 2013). The objective of this study was to summarize temporal trends of PFAS in serum collected from Gullah African Americans participating in a longitudinal study from 2003–2013.
METHODS
Study Population
The African American Gullah population is estimated to be between 100,000 and 300,000 and largely resides in the Sea Islands of South Carolina and Georgia. It is a unique community for defining environmental factors for autoimmune diseases due to its low non-African genetic admixture, environmental-geographic homogeneity within the Sea Island region, and high prevalence of antinuclear antibody (ANA) positivity and of autoimmune diseases such as systemic lupus erythematosus (SLE). The SLE in Gullah Health (SLEIGH) study is a longitudinal cohort of Gullah African Americans started in 2003 to investigate potential genetic and environmental factors in the development of autoimmunity (Kamen et al., 2008). The SLEIGH study is conducted in cooperation with and approval from the Sea Island Families Project Citizen Advisory Committee (Spruill et al., 2013).
Gullah African Americans participating in the SLEIGH study were invited between April 2010 and July 2013 to participate in an additional exposure assessment visit. This study protocol was reviewed and approved by the Medical University of South Carolina Institutional Review Board for Human Subjects Research and all participants provided informed consent. Participants came to study visits at the Medical University of South Carolina in Charleston, South Carolina, where blood and urine samples were collected per a standardized protocol. Eighty SLEIGH study participants with or without lupus completed the exposure assessment visit.
Serum samples from the study participants’ baseline SLEIGH visit and subsequent exposure assessment visit were kept frozen at −80 degrees, then batch shipped on dry ice to the Organic Analytical Chemistry Laboratory of the Wadsworth Center of the New York State Department of Health in Albany, NY for PFAS analysis. For this analysis, we excluded lupus patients and examined biomarker temporal patterns between the first and second visit among 71 persons without lupus. Most (81%) participants were female; participants ranged in age from 6.1 to 77.6 years old at first visit.
PFAS Biomarker Measurements
Serum concentrations of PFASs were measured using high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) as described elsewhere (Gump et al., 2011; Zhang et al., 2010). Briefly, 0.5 mL of serum sample, 5 ng of internal standards (18O2-PFHxS, 13C4-PFOS, 13C2-PFHxA, 13C4-PFOA, 13C2-PFNA, 13C2-PFDA, 13C2-PFUnDA, and 13C2-PFDoDA), 2 mL of 0.25 M sodium carbonate/sodium bicarbonate buffer and 1 mL of 0.8 M tetrabutyl ammonium hydrogen sulfate solution (adjusted to pH 11), were added to a 15 mL polypropylene tube for extraction. After thorough mixing, 5 mL of methyl tert-butyl ether (MTBE) was added, and the mixture was shaken for 40 min. The organic and aqueous layers were separated by centrifugation at 3500 rpm for 3 min and an exact volume of MTBE (4 mL) was removed. The aqueous mixture was rinsed with 3 mL MTBE and separated twice; the rinses were combined in a second polypropylene tube. MTBE was allowed to evaporate under gentle nitrogen stream before being reconstituted in 0.5 mL of methanol. The sample was vortexed for 30 s and transferred into an autosampler vial prior to instrumental analysis.
Analyte separation was performed using an Agilent1100 series HPLC. Ten microliter of the extract was injected onto a 100 × 2.1 mm (5 µm; Thermo Electron Corporation, Bellefonte, PA) Betasil C18 column with 2mM ammonium acetate/methanol as the mobile phase starting at 10% methanol. At a flow rate of 300 µL/min, the gradient increased to 100% methanol at 10 min before reverting to original conditions at 12 min. For quantitative determination, the HPLC system was interfaced to an API 2000 triple-quadruple mass spectrometer (Applied Biosystems, Foster City, CA) operated in electrospray negative ionization mode. Instrumental parameters were optimized to transmit the [M-K]− ion before fragmentation to one or more product ions. Declustering potential and collision energies were optimized for each analyte and ranged from 35 to 90 V and 10 to 35 eV, respectively. Multiple reaction monitoring (MRM) was used for the confirmation of the qualitative mass transitions.
Quality Assurance and Quality Control
The 18O2-PFHxS, 13C4–PFOS, 13C2–PFHxA, 13C4–PFOA, 13C2-PFNA, 13C2-PFUnDA, and 13C2-PFDoDA (99% purity, Wellington Laboratories, Guelph, ON, Canada), 13C2–PFNA and13C2–PFDA (3 M Company, St. Paul, MN) were spiked as internal standards into each serum sample prior to the addition of reagents for extraction. PFC concentrations were calculated by isotopic dilution method, and further confirmed by matrix matched calibration standards. Recoveries of 18O2-PFHxS, 13C4–PFOS, 13C2–PFHxA, 13C4–PFOA,13C2–PFNA, 13C2–PFDA, 13C2-PFUnDA, and 13C2-PFDoDA were 100±5%, 76±9%, 82±7%, 104±7%, 108±9%, 99±8%, 93±14%, and 80±33%,, respectively. Matrix spike recoveries were tested by spiking native standards of all 12 target compounds into 6 randomly selected samples, at levels of 10 ng and 20 ng for each of the target compounds. All the matrix spike samples were analyzed in duplicate. Recoveries of native standards spiked in blood matrix were 99±10% for PFBS, 99±2% for PFHxS, 105±27% for PFOS, 84±2% for PFDS, 75±10% for PFOSA, 109±5% for PFHxA, 137±16% for PFHpA, 115±6% for PFOA, 106±6% for PFNA, 105±4% for PFDA, 106±5% for PFUnDA, and 99±17% for PFDoDA, respectively. The relative standard deviations (RSD) of duplicate analyses were less than 5% for PFBS, PFHxS, PFOS, PFDS, PFHxA, PFHpA, PFOA, PFNA, PFDA, and less than 10% for PFOSA, PFUnDA, and PFDoDA. Milli-Q water (18 MΩ) was analyzed through the entire procedure as a blank, for every batch of 20 samples. Procedure blanks were also spiked with native standards. Solvents and serum collection tubes were checked for the presence of the PFASs analyzed in this study. Concentrations in blanks are below limit of detection for all twelve PFASs. The limit of quantitation (LOQ) was determined based on the linear range of the ten-point calibration curve prepared at a concentration range of 0.05–100 ng/mL. Concentrations in samples which were at least 3-fold greater than the lowest acceptable standard concentration were considered to be valid. A curve point was deemed acceptable if (1) it was back-calculated to be within 30% of the theoretical value when evaluated versus the 1/x weighted quadratic curve (R2>0.999), and (2) the peak area of the standard was at least 3 times greater than that in the blank. Concentration/dilution factors were included in the calculation of the LOQ. The LOQ (ng/mL) was 0.22 for PFBS, 0.20 for PFHxS, 0.18 for PFOS, 0.44 for PFDS, 0.16 for PFHxA, 0.36 for PFHpA, 0.20 for PFOA, 0.20 for PFNA, 0.16 for PFDA, 0.16 for PFUnDA, 0.52 for PFDoDA, and 0.20 for PFOSA, respectively. Left-censored biomarker values were imputed as the limit of quantitation divided by the square root of 2. We excluded compounds with ≥ 40% of the samples < LOQ from the temporal trends analysis (Supplemental Table S1).
Statistical Analysis
Skewed distributions such as serum PFAS can be approximated using parametric distributions including lognormal or Weibull, both of which are special cases of the generalized gamma distribution (Cox et al., 2007; Gribble et al., 2013; Pierce et al., 2011). We summarized the population-level trends in PFAS concentrations using Weibull, generalized gamma or lognormal parametric quantile regression with year as a predictor variable, with and without adjustment for age and sex. We did not have adequate sample size for stable estimation of random effects in the parametric quantile regression models (Crowther et al., 2014), so to account for repeated measures we instead used robust sandwich standard errors clustering on each person’s visits. We allowed possible nonlinearity in the temporal pattern for PFHxS through inclusion of a quadratic term for year. Adequacy of the population-average trend for summarizing individual trajectories was explored visually (e.g., “spaghetti plots”), and through individual changes in concentrations between visits (e.g., change scores). Any individual who was <LOQ at both visits for a congener was excluded from the change score estimates for that congener. Missing data were handled by listwise deletion. All analyses were conducted in Stata/SE 13.1..
In post-hoc analyses, we used mixed-effect linear regression models for log-transformed perfluorochemical concentrations to test for interactions of calendar year by age. In sensitivity analyses, we restricted these tests to female participants only (118 observations from 60 participants).
RESULTS
Baseline visit levels of PFAS are summarized in Table 1. There were no samples < LOQ for PFOA and PFOS, and only one sample < LOQ for PFHxS or PFNA. For PFDA, three participants had one sample each < LOQ. Therefore, change scores will not be biased by comparing LOQ for any of these compounds, as the censored data are still informative about possible trends (left-censoring implies a low concentration). The only compound which may have individual change scores misestimated for some individuals by both visits being < LOQ is PFUnDA, which only one individual had both visits < LOQ. This person’s samples had the same LOQ <0.16 both times so the estimate for that individual is no change. In addition to this one person censored at both visits, there were five people with a single visit censored who are still informative.
Table 1.
Baseline levels of serum PFAS in n=71 Gullah African Americans from South Carolina. Concentrations are reported as ng/g wet weight.
| Compound | Median (IQR) | Mean (SD) |
|---|---|---|
| PFOA | 5.6 (4.5, 5.6) | 6.0 (2.8) |
| PFOS | 41.1 (28.0, 41.1) | 53.3 (36.5) |
| PFHxS | 2.6 (1.5, 2.6) | 5.8 (16.9) |
| PFNA | 1.9 (1.5, 1.9) | 2.3 (1.4) |
| PFDA | 0.9 (0.5, 0.9) | 1.5 (1.3) |
| PFUnDA | 0.7 (0.3, 0.7) | 1.1 (1.2) |
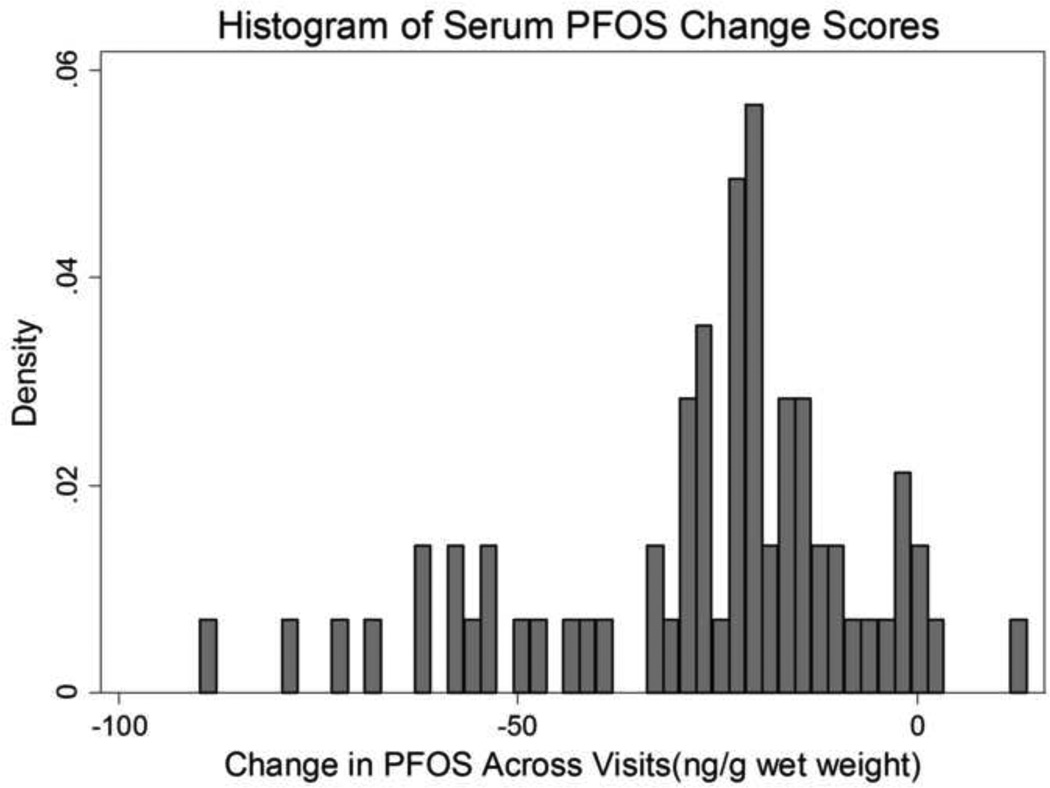
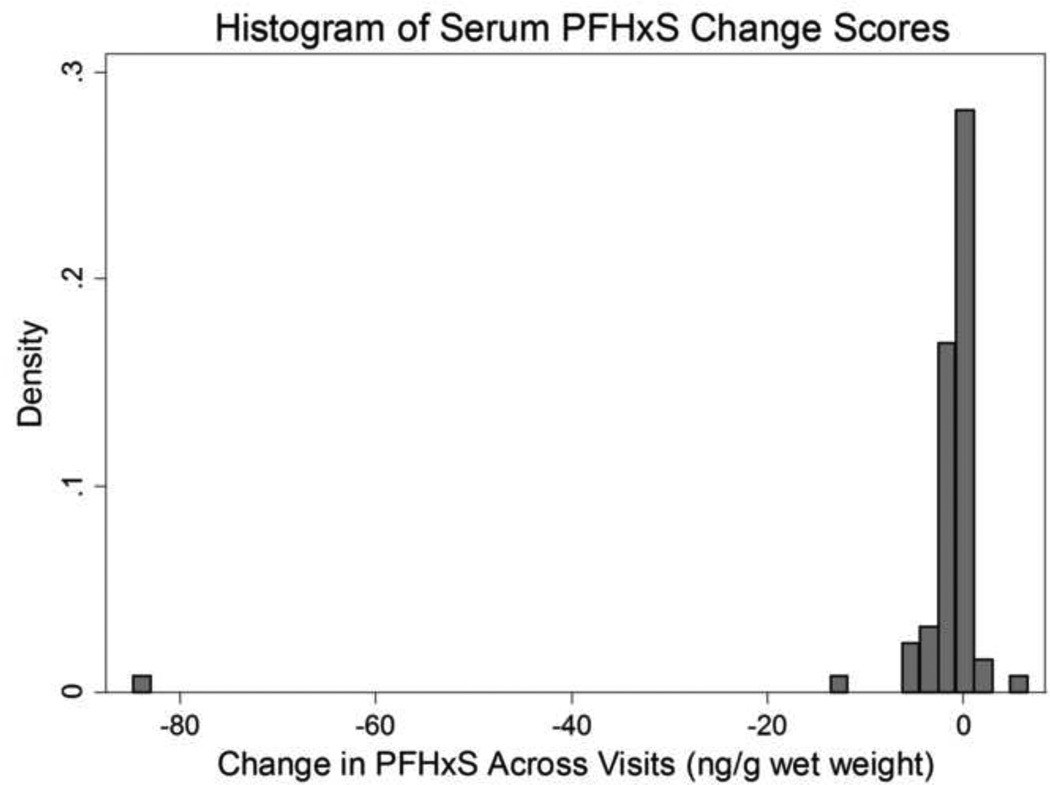
There was a sharp decrease in individual serum PFOS across visits for most participants, with a median decrease of −21.7 ng/g wet weight across visits (Table 2, Figure 1). The median change score indicated a slight decrease for PFOA, PFHxS, PFNA, and PFuNDA, and negligible change for PFDA. Many of these change score distributions, in particular PFHxS, were skewed, with some participants reporting a changes in serum concentrations between visits far greater than their contemporaries; histograms of these distributions are provided in the Figures 1, 3 and the Supplemental Material (Supplemental Figures S2, S5, S7, S9). A few participants had positive increases for PFHxS although most were negative changes (Figure 3). The PFUnDA distribution summary (Table 1) was the same to one decimal place with and without the person who had both visits < LOQ.
Table 2.
Distributions of individual visit-to-visit differences in concentration of serum perfluorochemicals (ng/g wet weight). The mean time elapsed between visits was 7.3 (SD 1.8) years.
| Chemical | 25th %ile | Median | 75th %ile | Mean | SD |
|---|---|---|---|---|---|
| PFOA | −2.8 | −2.2 | −0.9 | −2.2 | 2.1 |
| PFOS | −32.8 | −21.7 | −14.9 | −26.9 | 20.6 |
| PFHxS | −1.6 | −0.6 | −0.1 | −2.3 | 10.4 |
| PFNA | −0.7 | −0.3 | 0.2 | −0.3 | 0.9 |
| PFDA | −0.3 | 0.0 | 0.4 | 0.1 | 0.9 |
| PFUnDA | −0.3 | −0.1 | 0.1 | −0.1 | 0.6 |
Figure 1.
Longitudinal decrease in individual serum perfluorooctane sulfonate across study visits in a sample of n=68 Gullah African Americans, 2003–2013.
Figure 3.
Heterogeneity of individual declines in perfluorohexane sulfonate in serum from a sample of n=71 Gullah African Americans, 2003–2013.
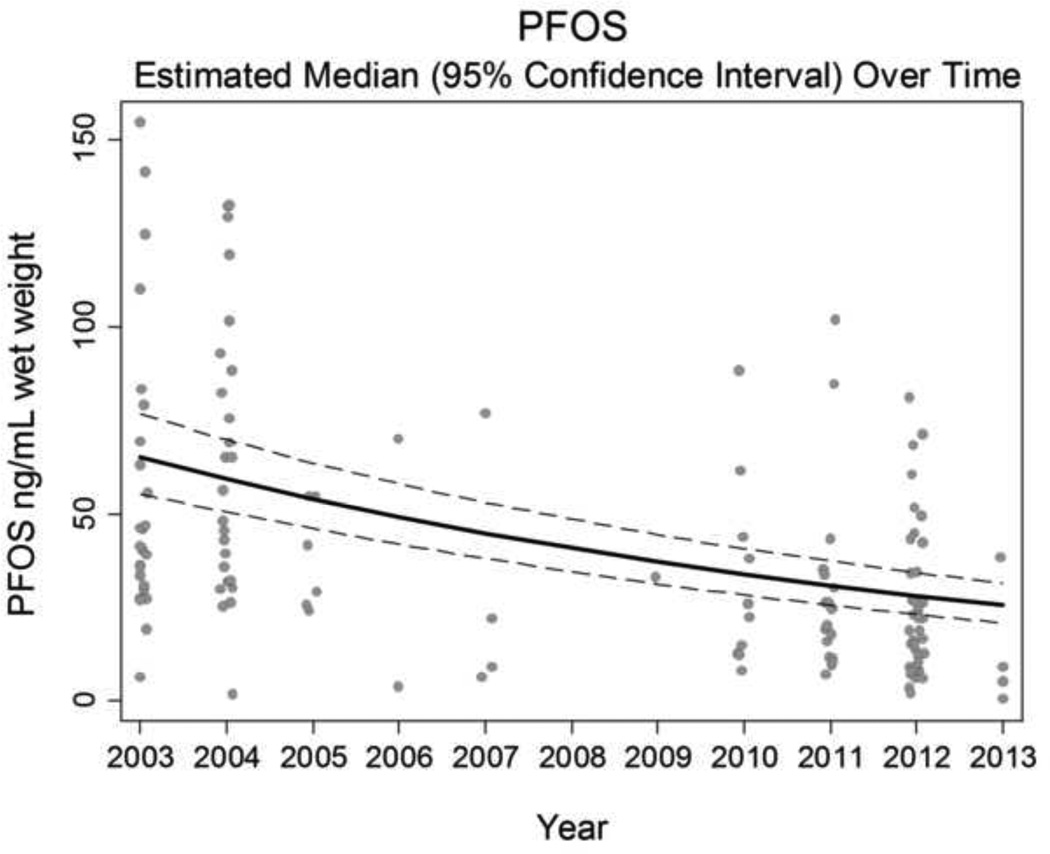
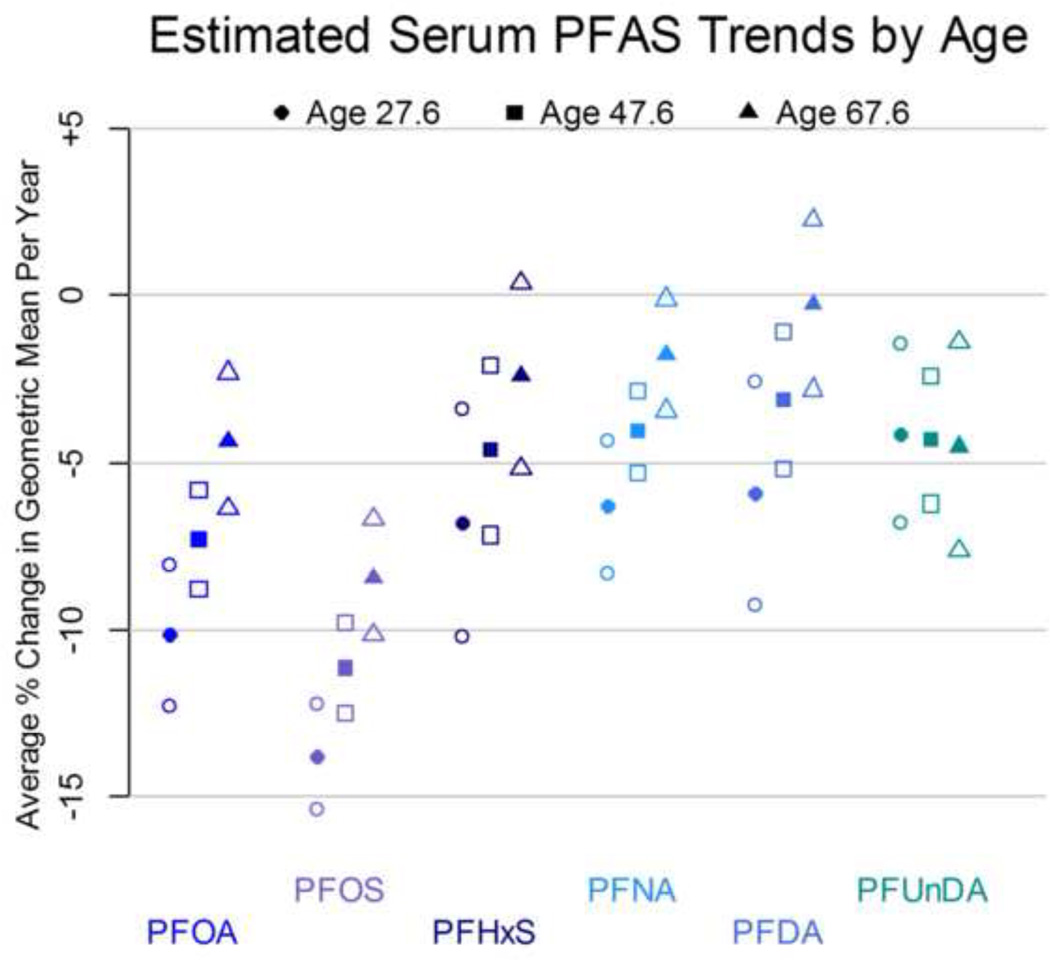
At the population level, there appeared to be an overall decrease in the serum concentrations of PFOA, PFOS, PFHxS, and PFuNDA in this population over 2003–2013 using models with a linear term for time (Table 3, Figure 2, and Supplemental Material – Supplemental Figures S3, S4, S6, S8, S10). The strongest decreases were for PFOS concentrations, which dropped approximately 9% (95% CI: 8, 10) each year (Table 3, Figure 2). These trends persisted after regression adjustment for age and sex. There was slightly better model fit allowing for a nonlinear pattern, with slight increase in PFHxS in later years (Supplement S4). Post-hoc analyses found evidence for heterogeneity by age in the temporal trends of log-transformed compounds, in robust mixed-effect linear regression models adjusted for gender. These interactions between mean-centered age and mean-centered calendar year for log-serum concentrations were significant for (log-transformed) PFOA (P < 0.001), PFOS (P <0.001), PFHxS (P=0.02), PFNA (P=0.001), and PFDA (P=0.01), but not significant for PFUnDA (P=0.86). The estimated temporal trends (and 95% CI) for persons of mean age, 20 years younger and 20 years older are shown in Figure 4, adjusting for sex and allowing age-year interaction terms for all compounds regardless of statistical significance. Sensitivity analyses restricting to female participants yielded similar inferences regarding age interactions for temporal trends of (log-transformed) PFOA (P < 0.001), PFOS (P<0.001), PFHXS (P=0.03), and PFNA (P=0.003), but the interaction was no longer significant but qualitatively similar for PFDA (P = 0.09). There was no interaction among females for PFUnDA (P = 0.63).
Table 3.
Percent change per year in concentration of perfluoronated compounds in serum over 2003–2013, from ‘linear time’ models with or without adjustment for age and sex. Data come from 139 observations, total from 71 participants (some participants seen only once).
| Chemical | Distribution | Unadjusted Change per Year |
Adjusted Change per Year |
|---|---|---|---|
| PFOA | Weibull | −5 (−7, −4)% | −7 (−8, −5)% |
| PFOS | Weibull | −9 (−10, −8)% | −11 (−12, −10)% |
| PFHxS | Lognormal | −6 (−8, −3) % | −6 (−8, −3)% |
| PFNA | Weibull | −2 (−3, 0)% | −4 (−6, −3)% |
| PFDA | Lognormal | −1 (−3, +1)% | −4 (−6, −2)% |
| PFUnDA | Lognormal | −3 (−5, −1)% | −5 (−7, −3)% |
Figure 2.
Population average decrease in serum perfluorooctane sulfonate from a sample of n=71 Gullah African Americans, 2003–2013. Estimated population median and 95% confidence interval controlling for repeated measures, shown along with observed biomarker concentrations (with jitter).
Figure 4.
Heterogeneity of temporal trends by age. Parameter estimates are from log-transformed perfluorochemical outcome mixed-effect linear regression models (n=71) adjusted for gender, with robust standard errors. Shapes correspond to estimates for different ages: circles show estimated trend for an ‘average’ 27.6-year-old (20 years younger than mean age), squares for an ‘average’ 47.6-year-old (mean age), and triangles for an average 67.6-year-old (20 years older than mean age). Filled dots are point estimates and empty dots are 95% confidence interval bounds.
DISCUSSION
This study, chronicling the decline of several PFAS in serum over 2003–2013 in a sample of Gullah African Americans, further advances the global understanding of recent temporal trends in PFAS concentrations. Our study demonstrated strong population-level decreases in serum concentrations of many PFAS compounds in Gullah African Americans residing in coastal SC over 2003–2013, in particular PFOS, which also decreased at the level of individual change scores. The sharp decrease in PFOS over the decade of observation is consistent with what has been reported in many other populations (Berg et al., 2014; Glynn et al., 2012; Haug et al., 2009; Nost et al., 2014; Okada et al., 2013) (Calafat et al., 2007; Olsen et al., 2008; Spliethoff et al., 2008). The population-level decrease in several other compounds, however, might be more of a local phenomenon. Concentrations of long-chain perfluoroalkyl acids (including PFNA, PFDA, and PFuNDA) appear to be increasing in Scandinavia (through 2010), Asia (through 2011), and the United States (through 2008) (Calafat et al., 2007; Glynn et al., 2012; Harada et al., 2011; Kato et al., 2011; Nost et al., 2014; Okada et al., 2013), but there were weak decreases in PFHxS, PFNA and PFuNDA, and no significant change in PFDA in this study population (Table 3). However, a comparison of NHANES summary statistics for 2003–2004 vs. 2011–2012 among non-Hispanic black participants suggests a similar decrease in median serum concentrations for PFHxS, PFOA, PFOS, and PFNA over a time period comparable to our study window (Centers for Disease Control and Prevention, 2015). Most individuals showed very little change in PFAS between visits. The possible U-shape population-level temporal pattern of serum PFHxS is unusual compared to many geographic locations but may match the pattern of the United States general population (Kato et al., 2011). It is also consistent with our individual-level finding that some of the participants had a large positive mean change score for PFHxS between visits. The hazards of PFHxS could include developmental neurotoxicity (Lee and Viberg, 2013) or endocrine-disrupting potential (Kjeldsen and Bonefeld-Jorgensen, 2013; Long et al., 2013; Wen et al., 2013), so it is important to determine whether there is a new source of PFHxS exposure and to further characterize this compound’s relevance for human health. The possible change in trajectory of PFHxS suggested at the population level but not corroborated by most individual trajectories could also be a result of aggregation bias.
Although it is likely that the decrease in serum PFOS in all recent biomonitoring studies reflects the phase-out from commerce, the reasons for observed trends in some of the other PFASs are less clear. Serum concentrations at any given time reflect a complex combination of environmental contamination, exposure behaviors, and physiological processes such as renal elimination (Han et al., 2012); each of these characteristics are likely to have varied over time (Bartell et al., 2004). The implications of the commercial chemical phase-out for contamination of environmental matricies by PFASs is an active area of research (Land et al., 2015). Additional data on local sources of exposure could help clarify the relationship between intake and biomarkers. Research in a highly exposed population (Watkins et al., 2013) as well as in the general population (Shankar et al., 2011) suggests that serum PFAS concentrations may be highly sensitive to kidney function; for example, age-related declines in glomerular filtration rates (Glassock and Winearls, 2009) should affect PFAS biomarker trends in longitudinal studies by lengthening PFAS residence times in the body during the course of follow-up. There also may be differences between isomers of the same compound in elimination half-lives (Zhang et al., 2013b). The importance of renal versus other routs of elimination may vary by compound as well, with urine mattering more for PFOA elimination and less for PFOS, PFHxS, and the longer-chain PFAAs (Zhang et al., 2013b).
Our sensitivity analysis exploring potential heterogeneity of temporal trends by age through linear regression models may have involved some model misspecification (i.e. residuals were skewed for some models, indicating that errors might not be normally distributed) as a practical compromise for stability of estimation. Therefore, the ‘significance’ or ‘non-significance’ of a particular trend is preliminary and not definitive. Nevertheless, it is of interest that so many PFASs appeared to have possibly different trajectories depending on age. This is consistent with a previous report of slower elimination of serum PFOA at higher ages after an exposure intervention in Germany (Brede et al., 2010). There may also be differences by age in exposure to compounds that may interact with transporters in the kidney important for renal elimination and reabsorption of PFAS (Epel et al., 2008; Han et al., 2012; Kudo et al., 2002). The determinants of PFAS toxicokinetics are not fully understood (Ng and Hungerbühler, 2014), so predicting exposure-exposure interactions relevant for PFAS toxicokinetics may be challenging. Although organic anion trasnporters are known to be important (Han et al., 2012; Kudo et al., 2002), previous work in a mussel gill tissue toxicological model suggested that PFAS may not be major substrates for verapamil-sensitive transporters such as P-glycoprotein (Stevenson et al., 2006). In addition to the possibility that age-related pharmaceuticals could interactively affect PFAS kinetics, some other xenobiotics with possible impacts on efflux transporters such as synthetic musk fragrances may be more abundant in older than younger women (Hutter et al., 2010; Luckenbach et al., 2004; Luckenbach and Epel, 2005). Another possible explanation is that it is possible that younger women, but not post-menopausal women, were losing some PFAS through menstruation (Harada et al., 2005; Lorber et al., 2015) and the majority of this study sample was female. Recent work suggests that elimination half-lives may be different between younger women vs. males and older women, although all these compounds have long half-lives (Zhang et al., 2013b). Zhang et al’s recent estimates for younger women’s elimination geometric mean half-lives are 7.1 years for PFHxS, 6.0 years for PFOS (sum across isomers), 1.8 for PFOA (sum across isomers), 1.7 years for PFNA, 4.0 years for PFDA, and 4.4 years for PFUnDA. Their estimates for males and older women are 25 years for PFHxS, 18 years for PFOS (sum across isomers), 1.7 years for PFOA, 3.5 years for PFNA, 9.2 years for PFDA, and 7.7 years for PFUnDA. We tentatively interpret our observed interaction by age, and similar results in sensitivity analysis restricted to females, as a replication of the finding that there may be elimination differences in young women vs. older women.
Strengths of this study include the data to contrast individual-level with population-level trajectories of exposure; the flexible and interpretable modeling approach; and the low limits of quantitation for each PFAS. Limitations of this study include the sample size, narrow geographic scope, and potential non-representativeness of a sample selected to be without lupus for the broader Gullah African American community in coastal South Carolina. Also, because of our sample size limitation we opted for robust standard errors rather than hierarchical modeling. Future research in a larger sample might allow for more flexible modeling.
CONCLUSIONS
Serum concentrations decreased over 2003–2013 among Gullah African Americans in coastal South Carolina for PFOS and several other PFAS. No serum PFAS appeared to monotonically increase over this decade at the population level, and the general pattern of individual biomarker trajectories was generally consistent, although PFHxS might be increasing in recent years. Additional data are needed on whether PFHxS exposure is increasing in recent years in this setting. Age may be an important modifier of PFAS exposure trajectories; future studies should investigate possible mechanism by which age may modify these trajectories.
Supplementary Material
Highlights.
Most Many PFAS decreased in serum of Gullah African Americans from Charleston, SC over the years 2003–2013.
There was possible heterogeneity in individual serum PFAS trajectories by participant age.
Additional More research is needed into the PFAS routes of exposure and explanations for the apparent temporal patterns. of serum perfluoronated chemicals in these communities.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Numbers R21 ES017934 (DLK), P60 AR062755 (DLK) and UL1 RR029882 to MUSC. MOG was supported by an “Environmental Genomics” T32 training grant from the National Institute for Environmental Health Sciences (T32ES013678-07). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank all of the study participants for their time and commitment to the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors have no competing interests to declare.
REFERENCES
- Apelberg BJ, et al. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ Sci Technol. 2007;41:3891–3897. doi: 10.1021/es0700911. [DOI] [PubMed] [Google Scholar]
- Armitage JM, et al. Comparative assessment of the global fate and transport pathways of long-chain perfluorocarboxylic acids (PFCAs) and perfluorocarboxylates (PFCs) emitted from direct sources. Environ Sci Technol. 2009;43:5830–5836. doi: 10.1021/es900753y. [DOI] [PubMed] [Google Scholar]
- Bach CC, et al. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: A systematic review. Crit Rev Toxicol. 2014:1–15. doi: 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- Bartell SM, et al. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, et al. Temporal error in biomarker-based mean exposure estimates for individuals. J Expo Anal Environ Epidemiol. 2004;14:173–179. doi: 10.1038/sj.jea.7500311. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, et al. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol. 2014;15:924–925. doi: 10.1016/s1470-2045(14)70316-x. [DOI] [PubMed] [Google Scholar]
- Berg V, et al. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int. 2014;69:58–66. doi: 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Brede E, et al. Two-year follow-up biomonitoring pilot study of residents' and controls' PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int J Hyg Environ Health. 2010;213:217–223. doi: 10.1016/j.ijheh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Buck R, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, et al. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ. 2010;408:2936–2965. doi: 10.1016/j.scitotenv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Calafat AM, et al. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ET, et al. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit Rev Toxicol. 2014;44(Suppl 1):1–81. doi: 10.3109/10408444.2014.905767. [DOI] [PubMed] [Google Scholar]
- Cox C, et al. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26:4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- Crowther MJ, et al. Multilevel mixed effects parametric survival models using adaptive Gauss-Hermite quadrature with application to recurrent events and individual participant data meta-analysis. Stat Med. 2014 doi: 10.1002/sim.6191. [DOI] [PubMed] [Google Scholar]
- Domingo JL. Health risks of dietary exposure to perfluorinated compounds. Environ Int. 2012;40:187–195. doi: 10.1016/j.envint.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Ellis JH, et al. A Qualitative Exploration of Fishing and Fish Consumption in the Gullah/Geechee Culture. J Community Health. 2014;39:1161–1170. doi: 10.1007/s10900-014-9871-5. [DOI] [PubMed] [Google Scholar]
- Epel D, et al. Efflux transporters: newly appreciated roles in protection against pollutants. Environ Sci Technol. 2008;42:3914–3920. doi: 10.1021/es087187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair PA, et al. Assessment of perfluorinated compounds (PFCs) in plasma of bottlenose dolphins from two southeast US estuarine areas: relationship with age, sex and geographic locations. Mar Pollut Bull. 2012a;64:66–74. doi: 10.1016/j.marpolbul.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Fair PA, et al. Association between plasma concentrations of perfluorochemicals (PFCs) and immune and clinical chemistry parameters in bottlenose dolphins (Tursiops truncatus) Environmental Toxicology & Chemistry. 2012b;32:736–746. doi: 10.1002/etc.2122. [DOI] [PubMed] [Google Scholar]
- Fitz-Simon N, et al. Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology. 2013;24:569–576. doi: 10.1097/EDE.0b013e31829443ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ, et al. The C8 health project: design, methods, and participants. Environ Health Perspect. 2009;117:1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419–428. [PMC free article] [PubMed] [Google Scholar]
- Glynn A, et al. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol. 2012;46:9071–9079. doi: 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- Gribble MO, et al. Body composition and arsenic metabolism: a cross-sectional analysis in the Strong Heart Study. Environ Health. 2013;12:107. doi: 10.1186/1476-069X-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, et al. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011;45:8151–8159. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruge KS, et al. Perfluorinated organic compounds in human blood serum and seminal plasma: a study of urban and rural tea worker populations in Sri Lanka. J Environ Monit. 2005;7:371–377. doi: 10.1039/b412532k. [DOI] [PubMed] [Google Scholar]
- Han X, et al. Renal elimination of perfluorocarboxylates (PFCAs) Chem Res Toxicol. 2012;25:35–46. doi: 10.1021/tx200363w. [DOI] [PubMed] [Google Scholar]
- Harada K, et al. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res. 2005;99:253–261. doi: 10.1016/j.envres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Harada KH, et al. Odd-numbered perfluorocarboxylates predominate over perfluorooctanoic acid in serum samples from Japan, Korea and Vietnam. Environ Int. 2011;37:1183–1189. doi: 10.1016/j.envint.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Haug LS, et al. Time Trends and the Influence of Age and Gender on Serum Concentrations of Perfluorinated Compounds in Archived Human Samples. Environmental Science & Technology. 2009;43:2131–2136. doi: 10.1021/es802827u. [DOI] [PubMed] [Google Scholar]
- Holzer J, et al. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ Health Perspect. 2008;116:651–657. doi: 10.1289/ehp.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, et al. Perfluoroalkyl compounds in relation to life-history and reproductive parameters in bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida, U.S.A. Environ Toxicol Chem. 2006;25:2405–2412. doi: 10.1897/05-499r.1. [DOI] [PubMed] [Google Scholar]
- Houde M, et al. Polyfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environ Sci Technol. 2005;39:6591–6598. doi: 10.1021/es0506556. [DOI] [PubMed] [Google Scholar]
- Hutter HP, et al. Higher blood concentrations of synthetic musks in women above fifty years than in younger women. International journal of hygiene and environmental health. 2010;213:124–130. doi: 10.1016/j.ijheh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Johnson PI, et al. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122:1028–1039. doi: 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen DL, et al. Autoantibody prevalence and lupus characteristics in a unique African American population. Arthritis Rheum. 2008;58:1237–1247. doi: 10.1002/art.23416. [DOI] [PubMed] [Google Scholar]
- Kannan K, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Kato K, et al. Changes in Serum Concentrations of Maternal Poly- and Perfluoroalkyl Substances over the Course of Pregnancy and Predictors of Exposure in a Multiethnic Cohort of Cincinnati, Ohio Pregnant Women during 2003–2006. Environ Sci Technol. 2014 doi: 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, et al. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45:8037–8045. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int. 2013;20:8031–8044. doi: 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- Kudo N, et al. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact. 2002;139:301–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Land M, et al. What is the effect of phasing out long-chain per- and perfluoroalkyl substances on the concentration of perfluoroalkyl acids and their precursors in the environment? A systematic review protocol. Environmental Evidence. 2015;4 [Google Scholar]
- Lau C. Perfluoroalkyl acids: Recent research highlights. Reproductive Toxicology. 2012;33:405–409. doi: 10.1016/j.reprotox.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Lee I, Viberg H. A single neonatal exposure to perfluorohexane sulfonate (PFHxS) affects the levels of important neuroproteins in the developing mouse brain. Neurotoxicology. 2013;37:190–196. doi: 10.1016/j.neuro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ. Synthesis of environmentally relevant fluorinated surfactants--a review. Chemosphere. 2005;58:1471–1496. doi: 10.1016/j.chemosphere.2004.11.078. [DOI] [PubMed] [Google Scholar]
- Long M, et al. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int. 2013;20:8045–8056. doi: 10.1007/s11356-013-1628-7. [DOI] [PubMed] [Google Scholar]
- Lorber M, et al. The effect of ongoing blood loss on human serum concentrations of perfluorinated acids. Chemosphere. 2015;118:170–177. doi: 10.1016/j.chemosphere.2014.07.093. [DOI] [PubMed] [Google Scholar]
- Luckenbach T, et al. Fatal attraction: synthetic musk fragrances compromise multixenobiotic defense systems in mussels. Marine environmental research. 2004;58:215–219. doi: 10.1016/j.marenvres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Luckenbach T, Epel D. Nitromusk and polycyclic musk compounds as long-term inhibitors of cellular xenobiotic defense systems mediated by multidrug transporters. Environmental health perspectives. 2005;113:17–24. doi: 10.1289/ehp.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwicki JK, et al. Hazard quotient profiles used as a risk assessment tool for PFOS and PFOA serum levels in three distinctive European populations. Environ Int. 2015;74:112–118. doi: 10.1016/j.envint.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Maisonet M, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120:1432–1437. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CA, Hungerbühler K. Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environmental Science & Technology. 2014;48:4637–4648. doi: 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- Nost TH, et al. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from Northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environ Int. 2014;67:43–53. doi: 10.1016/j.envint.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Okada E, et al. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ Int. 2013;60:89–96. doi: 10.1016/j.envint.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Olsen GW, et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ Sci Technol. 2012;46:6330–6338. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- Olsen GW, et al. Decline in perfluorooctanesulfonate and other polyfluoroalkyl chemicals in American Red Cross adult blood donors, 2000–2006. Environ Sci Technol. 2008;42:4989–4995. doi: 10.1021/es800071x. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health. 2007;81:231–246. doi: 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- Paul AG, et al. A First Global Production, Emission, And Environmental Inventory For Perfluorooctane Sulfonate. Environmental Science & Technology. 2008;43:386–392. doi: 10.1021/es802216n. [DOI] [PubMed] [Google Scholar]
- Perez F, et al. Assessment of perfluoroalkyl substances in food items at global scale. Environ Res. 2014;135C:181–189. doi: 10.1016/j.envres.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Pérez F, et al. Accumulation of perfluoroalkyl substances in human tissues. Environment International. 2013;59:354–362. doi: 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Pierce CB, et al. Methods for characterizing differences in longitudinal glomerular filtration rate changes between children with glomerular chronic kidney disease and those with nonglomerular chronic kidney disease. Am J Epidemiol. 2011;174:604–612. doi: 10.1093/aje/kwr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SM, et al. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ Pollut. 2014;184:327–334. doi: 10.1016/j.envpol.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post GB, et al. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Schecter A, et al. Polyfluoroalkyl compounds in Texas children from birth through 12 years of age. Environ Health Perspect. 2012;120:590–594. doi: 10.1289/ehp.1104325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroter-Kermani C, et al. Retrospective monitoring of perfluorocarboxylates and perfluorosulfonates in human plasma archived by the German Environmental Specimen Bank. Int J Hyg Environ Health. 2013;216:633–640. doi: 10.1016/j.ijheh.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Shankar A, et al. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol. 2011;174:893–900. doi: 10.1093/aje/kwr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SD, et al. Persistent organic pollutants including polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans in firefighters from Northern California. Chemosphere. 2013;91:1386–1394. doi: 10.1016/j.chemosphere.2012.12.070. [DOI] [PubMed] [Google Scholar]
- Spliethoff HM, et al. Use of newborn screening program blood spots for exposure assessment: declining levels of perluorinated compounds in New York State infants. Environ Sci Technol. 2008;42:5361–5367. doi: 10.1021/es8006244. [DOI] [PubMed] [Google Scholar]
- Spruill IJ, et al. Successes, Challenges and Lessons Learned: Community-engaged research with South Carolina's Gullah population. Gateways: International Journal of Community Research and Engagement. 2013;6:150–169. doi: 10.5130/ijcre.v6i1.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, et al. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA) Environ Health Perspect. 2010;118:1100–1108. doi: 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CN, et al. New perspectives on perfluorochemical ecotoxicology: inhibition and induction of an efflux transporter in the marine mussel, Mytilus californianus. Environ Sci Technol. 2006;40:5580–5585. doi: 10.1021/es0602593. [DOI] [PubMed] [Google Scholar]
- Sundstrom M, et al. A temporal trend study (1972–2008) of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in pooled human milk samples from Stockholm, Sweden. Environ Int. 2011;37:178–183. doi: 10.1016/j.envint.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Tao L, et al. Biomonitoring of perfluorochemicals in plasma of New York State personnel responding to the World Trade Center disaster. Environ Sci Technol. 2008a;42:3472–3478. doi: 10.1021/es8000079. [DOI] [PubMed] [Google Scholar]
- Tao L, et al. Perfluorinated compounds in human milk from Massachusetts U.S.A. Environ Sci Technol. 2008b;42:3096–3101. doi: 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- Tao L, et al. Perfluorinated compounds in human breast milk from several Asian countries, and in infant formula and dairy milk from the United States. Environ Sci Technol. 2008c;42:8597–8602. doi: 10.1021/es801875v. [DOI] [PubMed] [Google Scholar]
- Toms LM, et al. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environ Int. 2014;71:74–80. doi: 10.1016/j.envint.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ehrenstein OS, et al. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod Toxicol. 2009;27:239–245. doi: 10.1016/j.reprotox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, et al. Exposure to Perfluoroalkyl Acids and Markers of Kidney Function among Children and Adolescents Living near a Chemical Plant. Environ Health Perspect. 2013;121:625–630. doi: 10.1289/ehp.1205838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen LL, et al. Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007–2010. J Clin Endocrinol Metab. 2013;98:E1456–E1464. doi: 10.1210/jc.2013-1282. [DOI] [PubMed] [Google Scholar]
- Yeung LW, et al. Part I. A temporal study of PFCAs and their precursors in human plasma from two German cities 1982–2009. Environ Sci Technol. 2013a;47:3865–3874. doi: 10.1021/es303716k. [DOI] [PubMed] [Google Scholar]
- Yeung LW, et al. Part II. A temporal study of PFOS and its precursors in human plasma from two German cities in 1982–2009. Environ Sci Technol. 2013b;47:3875–3882. doi: 10.1021/es4004153. [DOI] [PubMed] [Google Scholar]
- Zhang T, et al. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ Sci Technol. 2013a;47:7974–7981. doi: 10.1021/es400937y. [DOI] [PubMed] [Google Scholar]
- Zhang T, et al. Perfluorinated compounds in whole blood samples from infants, children, and adults in China. Environ Sci Technol. 2010;44:4341–4347. doi: 10.1021/es1002132. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Biomonitoring of Perfluoroalkyl Acids in Human Urine and Estimates of Biological Half-Life. Environmental Science & Technology. 2013b;47:10619–10627. doi: 10.1021/es401905e. [DOI] [PubMed] [Google Scholar]
- Zhou Z, et al. Highly elevated serum concentrations of perfluoroalkyl substances in fishery employees from Tangxun lake, china. Environ Sci Technol. 2014;48:3864–3874. doi: 10.1021/es4057467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.