Abstract
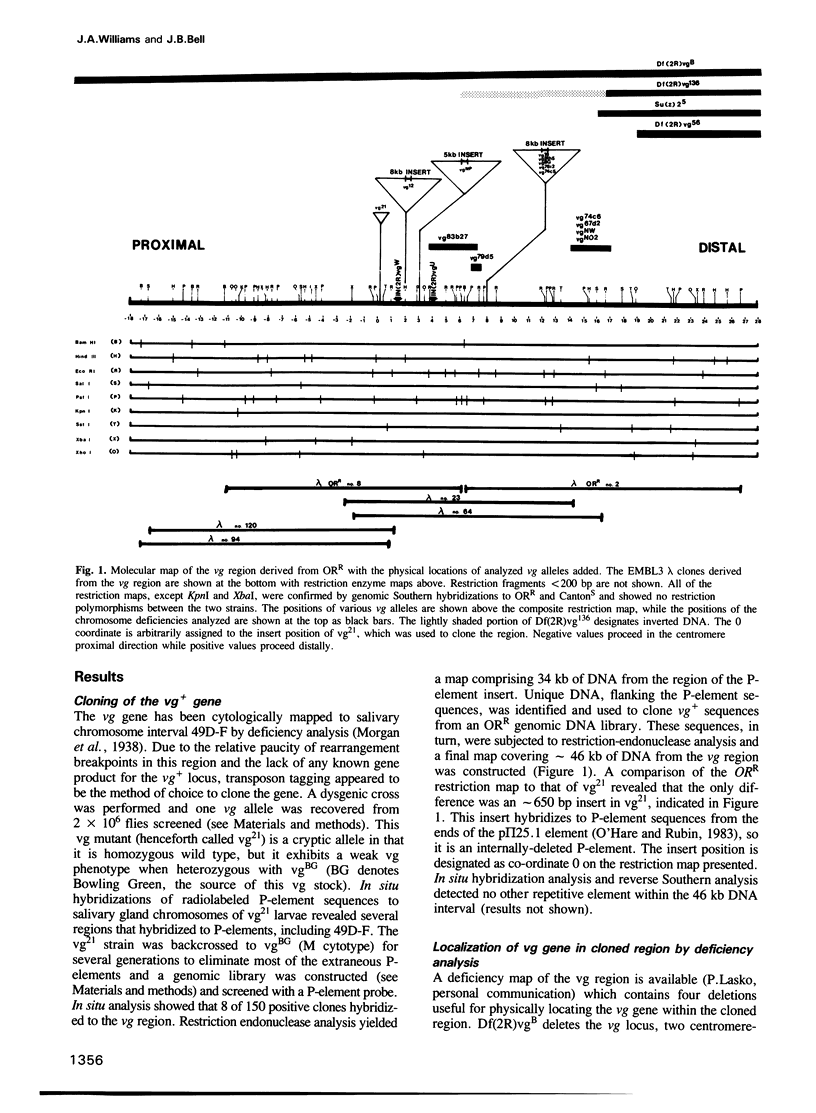
The vestigial (vg) locus of Drosophila melanogaster is involved in wing margin development. In the absence of a vg+ gene, extensive cell death occurs in third instar imaginal discs which results in a complete loss of adult wing margin structures. P-element tagging was used to obtain a molecular clone of the vg locus, which led to the molecular characterization of approximately 46 kb of DNA from the region. Deficiency analysis and molecular mapping identified sequences, spanning approximately 20 kb of DNA within the larger region, which are necessary for vg function. The molecular map was oriented with respect to a pre-existing genetic fine structure map of the locus. The centromere distal limits of the locus were defined by deficiency analyses while the proximal end has not yet been conclusively established. However, three transcripts, that are apparently unrelated to vg, provide circumstantial evidence for the proximal limits of the vg locus. The nature of the molecular lesions for several extant recessive or lethal vg alleles was determined, and these were placed on the vg molecular map. The characterization of the lesions associated with two dominant vg alleles and one complex vg allele imply interesting regulatory mechanisms for this locus. As well, a revertant of a 412 insertion mutant allele was shown to have resulted from a further insertion of a roo element into the 412 element.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W., Akam M., Karch F., Beachy P. A., Peifer M., Spierer P., Lewis E. B., Hogness D. S. Molecular Genetics of the Bithorax Complex in Drosophila melanogaster. Science. 1983 Jul 1;221(4605):23–29. doi: 10.1126/science.221.4605.23. [DOI] [PubMed] [Google Scholar]
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Balcells L., Villares R., Carramolino L., García-Alonso L., Modolell J. Excess function hairy-wing mutations caused by gypsy and copia insertions within structural genes of the achaete-scute locus of Drosophila. Cell. 1986 Jan 31;44(2):303–312. doi: 10.1016/0092-8674(86)90764-6. [DOI] [PubMed] [Google Scholar]
- Collins M., Rubin G. M. Structure of the Drosophila mutable allele, white-crimson, and its white-ivory and wild-type derivatives. Cell. 1982 Aug;30(1):71–79. doi: 10.1016/0092-8674(82)90013-7. [DOI] [PubMed] [Google Scholar]
- Coté B., Bender W., Curtis D., Chovnick A. Molecular mapping of the rosy locus in Drosophila melanogaster. Genetics. 1986 Apr;112(4):769–783. doi: 10.1093/genetics/112.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fristrom D. Cellular degeneration in the production of some mutant phenotypes in Drosophila melanogaster. Mol Gen Genet. 1969;103(4):363–379. doi: 10.1007/BF00383486. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Hodgetts R. B. An analysis of dopa decarboxylase expression during embryogenesis in Drosophila melanogaster. Dev Biol. 1985 Jan;107(1):142–155. doi: 10.1016/0012-1606(85)90383-5. [DOI] [PubMed] [Google Scholar]
- Glew L., Lo R., Reece T., Nichols M., Söll D., Bell J. The nucleotide sequence, localization and transcriptional properties of a tRNALeuCUG gene from Drosophila melanogaster. Gene. 1986;44(2-3):307–314. doi: 10.1016/0378-1119(86)90195-2. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Pinchin S. M., Schedl P., Artavanis-Tsakonas S., Mirault M. E. Genetic and molecular analysis of the 87A7 and 87C1 heat-inducible loci of D. melanogaster. Cell. 1979 Dec;18(4):1351–1358. doi: 10.1016/0092-8674(79)90245-9. [DOI] [PubMed] [Google Scholar]
- James A. A., Bryant P. J. Mutations causing pattern deficiencies and duplications in the imaginal wing disk of Drosophila melanogaster. Dev Biol. 1981 Jul 15;85(1):39–54. doi: 10.1016/0012-1606(81)90234-7. [DOI] [PubMed] [Google Scholar]
- Levis R., Collins M., Rubin G. M. FB elements are the common basis for the instability of the wDZL and wC Drosophila mutations. Cell. 1982 Sep;30(2):551–565. doi: 10.1016/0092-8674(82)90252-5. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrokhi L. J., Obolenkova L. A., Priimägi A. F., Ilyin Y. V., Gerasimova T. I., Georgiev G. P. The nature of unstable insertion mutations and reversions in the locus cut of Drosophila melanogaster: molecular mechanism of transposition memory. EMBO J. 1985 Dec 30;4(13B):3781–3787. doi: 10.1002/j.1460-2075.1985.tb04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D., Bell J. Larval age and the pattern of DNA synthesis in polytene chromosomes. Can J Genet Cytol. 1968 Mar;10(1):82–90. doi: 10.1139/g68-011. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Scherer G., Tschudi C., Perera J., Delius H., Pirrotta V. B104, a new dispersed repeated gene family in Drosophila melanogaster and its analogies with retroviruses. J Mol Biol. 1982 May 25;157(3):435–451. doi: 10.1016/0022-2836(82)90470-3. [DOI] [PubMed] [Google Scholar]
- Searles L. L., Jokerst R. S., Bingham P. M., Voelker R. A., Greenleaf A. L. Molecular cloning of sequences from a Drosophila RNA polymerase II locus by P element transposon tagging. Cell. 1982 Dec;31(3 Pt 2):585–592. doi: 10.1016/0092-8674(82)90314-2. [DOI] [PubMed] [Google Scholar]
- Shepherd B. M., Finnegan D. J. Structure of circular copies of the 412 transposable element present in Drosophila melanogaster tissue culture cells, and isolation of a free 412 long terminal repeat. J Mol Biol. 1984 Nov 25;180(1):21–40. doi: 10.1016/0022-2836(84)90428-5. [DOI] [PubMed] [Google Scholar]
- Zachar Z., Bingham P. M. Regulation of white locus expression: the structure of mutant alleles at the white locus of Drosophila melanogaster. Cell. 1982 Sep;30(2):529–541. doi: 10.1016/0092-8674(82)90250-1. [DOI] [PubMed] [Google Scholar]