Abstract
Steatosis is a characteristic morphological change of alcoholic liver disease, but its pathologic significance is still obscure. Regardless of cell types, intracellular lipid droplets are coated with a phospholipid monolayer, on which many kinds of lipid droplet-associated proteins are present. These proteins, such as the perilipin family of proteins and the cell death inducing DNA fragmentation factor (DFF) 45-like effectors, are recognized to play important roles in lipid metabolism in the physiological settings. In addition, recent lipidology studies have revealed that expression of the lipid droplet-associated proteins possibly participate in the pathologic processes of many metabolic disorders, including fatty liver and insulin resistance. Hence, controlling protein expressions is expected to offer novel therapeutic options. In this review, we summarize collected data concerning the potential contribution of the lipid droplet-associated proteins to the development of alcoholic fatty liver. Without exception, existing data indicates that the lipid droplet-associated proteins, especially the perilipin family proteins, are important factors in alcoholic fatty liver. These proteins exert a prosteatotic effect, and their expression is closely associated with lipotoxicity based on endoplasmic reticulum stress and oxidative injury. Although suppression of their expression may be beneficial, careful consideration is required because these proteins simultaneously function as protective factors against lipotoxicity.
Keywords: Alcoholic liver disease, steatosis, lipid droplet-associated protein, lipotoxicity, endoplasmic reticulum stress, oxidative stress
Introduction
In most East-Asian countries, especially in Japan, alcoholic beverages have been accepted traditionally as “Hyakuyaku-No-Cho (a king of drugs)”. Certainly modest drinking is known to be beneficial in the prevention of cardiovascular diseases [1,2]. Meanwhile, excessive alcohol consumption is one of the leading causes of chronic liver disease that progress to cirrhosis, followed often by hepatocellular carcinoma [3]. Cessation of alcohol drinking is currently the most effective treatment for alcoholic liver disease (ALD). From this simplified point of view, all scientific efforts to develop novel therapies for ALD appear to be in vain. However, in many countries, ALD is an expanding public health problem that requires special treatment frequently associated with a substantial and additional economic burden. Detailed pathologic mechanisms of ALD are still the subject of scientific investigation in order to establish time- and cost-effective therapy and prevention.
Hepatocellular steatosis is a characteristic morphological change in ALD. In summary, its process is explained as follows [4-6]: Hepatic metabolism of ethanol and its highly toxic metabolite, acetaldehyde, impairs mitochondrial function and the function of the endoplasmic reticulum (ER), affects lipid and protein metabolism, and disturbs fatty acid oxidation and lipoprotein secretion. As a result, excess intracellular lipid forms as lipid droplets (LDs). Oxidative stress and ER stress, which are generated in association with this abnormal metabolic condition, are recognized as one possible origin of cellular injury in ALD. Hepatosteatosis has tended to be considered as an accompanying phenomenon without an active contribution to the progression of the disease (so-called as an innocent bystander). In fact, steatosis in ALD is diminished after a relatively short period of abstinence. However, since the recent definition of nonalcoholic fatty liver disease as an independent entity [7], the pathologic significance of hepatosteatosis has been renewed as a relevant and possibly critical subject of current research.
Advanced lipidology has revealed that intracellular LDs are not immobilized and dormant lipid pools (or nutrition stores) but organelles that are involved in many physiological functions [8]. LDs of adipocytes and hepatocytes, which are covered with a phospholipid monolayer, store mainly triglyceride (TG). In addition, various LD-associated proteins are present on their surfaces, and these play key roles in cellular metabolism, such as LD formation/maintenance, and TG lipolysis depending on energy demand [9,10]. In association with step-by-step discoveries of LD-associated proteins and their functions, LDs have been gradually recognized as a vigorous player in cellular energy homeostasis.
The roles for LD-associated proteins are quite diverse. In addition to the physiological functions described above, they appear to contribute to the development of metabolic disorders caused by ER stress, oxidative stress, and insulin resistance (IR) related to lipotoxicity [8,11-14]. Accumulating evidence has suggested that LD-associated proteins potentially participate in pathologic mechanisms of ALD [15-17]. In this review, cellular biological features of each LD-associated protein expressed in hepatocytes were scrutinized on the basis of recently published data. We especially focused on the relationship between lipotoxicity and the generation of ER stress and oxidative stress, and we discuss the pathologic significance of hepatosteatosis in ALD.
Perilipin (Plin) family proteins
Plin is a representative LD-associated protein, which was discovered on the phospholipid monolayer of LD in early 1990s [18]. To date, five proteins exhibiting amino acid-sequence homology have been reported (Figure 1). Each of them has a specific nomenclature, but recently they were unified as the ‘Plin family proteins’ [19]. All Plin family proteins play important roles in LD metabolism, but they have slight differences in expression and function in different cell types. Plin 4 localizes to cholesterol-rich LDs, and largely contributes to the early phase of LD maturation and steroid-hormone metabolism [20-22]. Plin 1, Plin 2, Plin 3 and Plin 5 localize to TG-rich LDs in adipocytes and hepatocytes, and control LD size and lipolysis in cooperation with comparative gene identification-58 (CGI-58), adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) (details described later). As a result of changes in LD maturation and size, Plins expressed on the LD surface change from Plin 4, Plin 3, Plin 5 (small LDs) to Plin 2 (small to medium-sized LDs) or Plin 1 (large LDs) [22,23]. A similar alteration of pattern expression is noted in the process of hepatosteatosis (Figure 2) [11,12,24]. In this section, Plin 1, Plin 2 and Plin 5, and particularly their pathophysiological roles in ALD are described.
Figure 1.
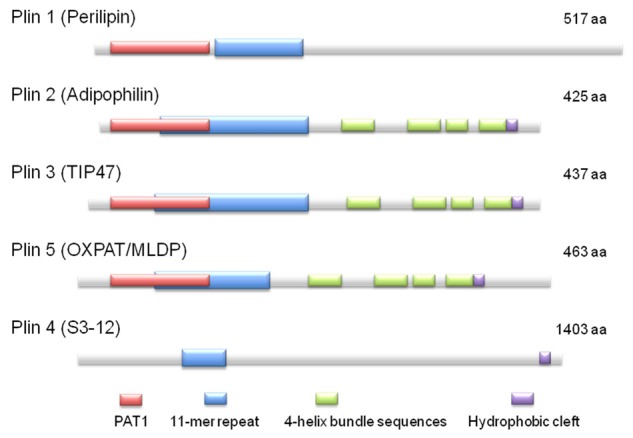
Schematic diagram of the structural features and the amino acid sequence similarities of PAT family proteins [9]. (aa, amino acids).
Figure 2.
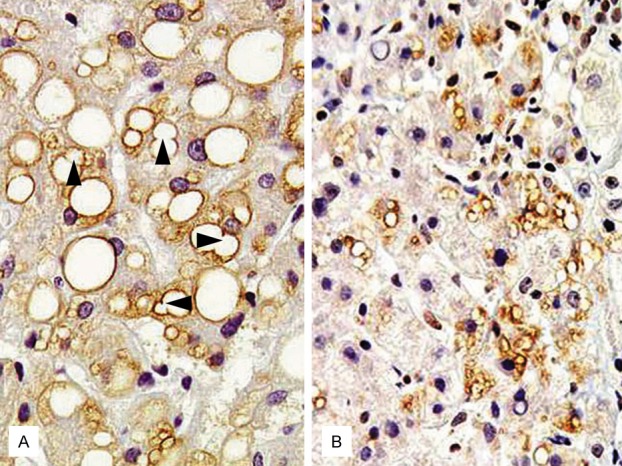
Immunolocalization of Plin 1 (A) and Plin 2 (B) in human steatotic liver [11]. (A) Plin 1 expression is seen predominantly in large-sized LDs in steatotic hepatocytes. Note LD-LD fusion portions (arrows) (B) Plin 2 expression is clearly shown in small-sized LDs.
Plin 1
Plin 1 is expressed primarily in mature adipocytes. It controls lipolysis, and contributes to the LD-LD fusion process through interaction with cell-death-inducing DNA-fragmentation-factor 45-like effector (CIDE)-C (Figure 2A) [25]. Once, Plin 1 was believed to localize very selectively to adipocyte LDs. We first reported Plin 1 expression in steatotic hepatocytes, which was later confirmed by another study group [26,27]. Plin 1 preferentially coats large LDs, and the same tendency is reported in hepatocyte LDs [11,12,23,24]. Probably, Plin 1 directs LD fusion to compose large unilocular LD via small LD-LD fusion and to stabilize LD maturation and size. Expression of Plin 1 is synchronized to the amount of TG stored and the expression of sterol regulatory element-binding protein (SREBP)-1c [28]. Together with enhanced SREBP-1c expression in ALD [29], these facts suggest that Plin 1 positively contributes to form large LDs in the livers of ALD.
Plin 2
Plin 2 is ubiquitously expressed in every somatic cell, and regulated by peroxisome proliferator-activated receptor (PPAR)-alpha [30]. As well as Plin 1, Plin 2 is expressed based on the amount of TG stored [15,31]. Growing interest has focused on the pathological significance of Plin 2 in metabolic disorders, including hepatosteatosis. It was reported that chronic alcohol consumption-induced IR and hepatosteatosis were closely associated with enhanced Plin 2 expression [16]. Our previous study also indicated a relationship between Plin 2 expression, hepatocellular ballooning and oxidative injury (Figure 3) [11]. In addition, it was elucidated by various methods that suppression of Plin 2 expression could prevent hepatosteatosis and IR [13,32,33]. The same result was obtained in a mouse-model of alcoholic fatty liver [17]. Plin 2 suppression also induces extrahepatic effects, such as prevention of macrophage foamy degeneration [34]. However, recent research results indicate that Plin 2 suppression with antisense oligonucleotide might induce profibrotic effects and Plin 2 and Plin 3 double-knockout actually induced IR [35,36]. These results suggest the necessity of giving very careful consideration of the potential for negative effects before attempting to develop similar therapeutics.
Figure 3.
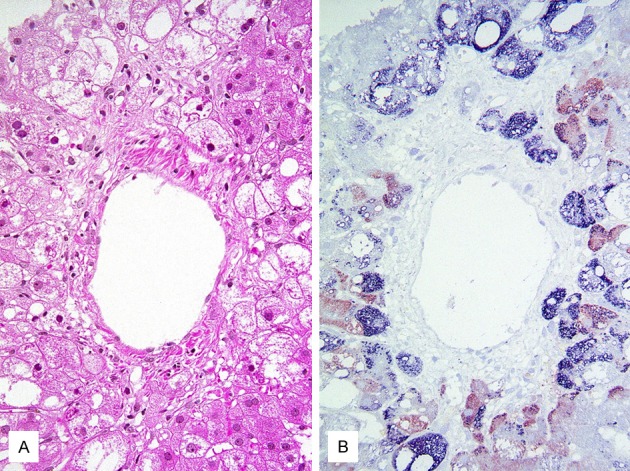
The colocalization/coexpression of Plin 2 and oxidized phospholipid in ballooned hepatocytes [11]. A. Hematoxylin-eosin stained section of fatty liver with marked ballooning degeneration. B. Immunohistochemical double staining for Plin 2 (blue) and oxidized phospholipid (red). Most ballooned hepatocytes are positive for Plin 2 and/or oxidized phospholipid.
Plin 5
Plin 5 is the latest member to be identified of the PAT family proteins [37]. Its expression is, like Plin 2, regulated by PPAR-alpha, and is seen mainly in oxidative tissues/organs, such as liver, heart and skeletal muscle. This cell type-specific expression is closely related to its function as a fuel switch. To prevent excessive FA oxidation in the mitochondria, Plin 5 sequesters FA in LDs and stabilizes LDs, but when sufficient energy supply is required, Plin 5 facilitates a linkage between LD and mitochondria to release FA to mitochondria [38]. Recent studies further revealed its cell-protective effect on cardiomyocytes and hepatocytes [39,40]. It can diminish ER stress and oxidative injury by the avoidance of excessive FA oxidation. The roles of Plin 5 in ALD pathophysiology remain to be explored.
Cell-death-inducing DNA fragmentation-factor 45-like effector (CIDE) proteins
CIDE proteins, as may be surmised from the name, possess an apoptosis-inducing property, but their actual roles in apoptosis are obscure. Alternatively, like Plin family proteins, CIDE proteins locate on the surfaces of LDs and ER, and play important roles in regulation of lipid metabolism and energy expenditure and homeostasis [41]. Their expression is closely associated with metabolic disorders including fatty liver diseases [42]. To date, three CIDE proteins sharing homologous DNA/amino acid-sequences have been reported. Prior investigations concerning CIDE proteins in humans, have aimed entirely at metabolic disorders and their hepatic manifestation: nonalcoholic fatty liver disease. However, in light of their functional effects on LDs, the pathological significance of CIDE proteins in ALD are suspected to be significant and comparable with those in metabolic disorder-related fatty liver.
CIDE-A
CIDE-A, of which expression is regulated by SREBP-1c, is one of the factors in hepatosteatosis caused by high fat diet [43,44]. The association of obesity with increased risk of ALD supports a likely association but additional studies are needed.
CIDE-B
CIDE-B is constitutively expressed in liver, and contributes to lipogenesis and lipidation of lipid moieties to very low-density lipoprotein (VLDL) [45,46]. In controlling VLDL lipidation, CIDE-B and Plin 2 exert opposite functions [46].
CIDE-C
CIDE-C, also known as fat-specific protein 27 (FSP27), contributes to enlarging LDs in adipocytes as a cofactor of Plin 1 for LD-LD fusion (Figure 2A). Also it participates in controlling mitochondrial activity and insulin sensitivity [25,47,48]. CIDE-C is regulated by PPAR-alpha/gamma, and induces hepatosteatosis [44,49].
LD formation and lipolysis
Lipid storage is a physiological function of the liver. The amount of hepatic fat is determined by a balance between influx of FA, oxidation of FA and export of VLDL. In patients with ALD, FA-release from visceral adipocytes is increased because of enhanced lipolysis and suppressed by lipogenesis caused by acetaldehyde [50,51]. Excess FA is transported to the liver and stored as the TG of LDs in hepatocytes. Plin family proteins induced according to the stored TG amount serve as a stabilizer of LDs. An interaction between Plin 1 and CIDE-C induces LD-LD causing fusion and formation of large unilocular LD in hepatocytes. Plin family proteins also control lipolysis in LDs that have been composed via these processes in order to supply FA to mitochondrial beta-oxidation. Following this process, LD-associated proteins additionally participate in the lipolysis process in LDs.
Comparative gene identification-58 (CGI-58) and adipose triglyceride lipase (ATGL)
Both CGI-58 and ATGL interact with the Plin family proteins, and contribute to lipolysis in LDs as shown in the schematic diagram (Figure 4). ATGL is a rate-limiting enzyme of lipolysis, and CGI-58 acts as a cofactor [52]. CGI-58 binds to Plin family proteins to stabilize LDs in a non-lipolytic phase. In response to catecholamine-stimulation, CGI-58 is released from Plin proteins and binds to ATGL. The CGI-58/ATGL binding initiates lipolysis, which is further accelerated by the action of hormone-sensitive lipase [40,53]. It has been suggested that Plin 5 can bind directly to ATGL initiating lipolysis [54]. Raised lipolysis in adipocytes in patients with ALD is considered to be mediated by up-regulation of ATGL [50,55].
Figure 4.
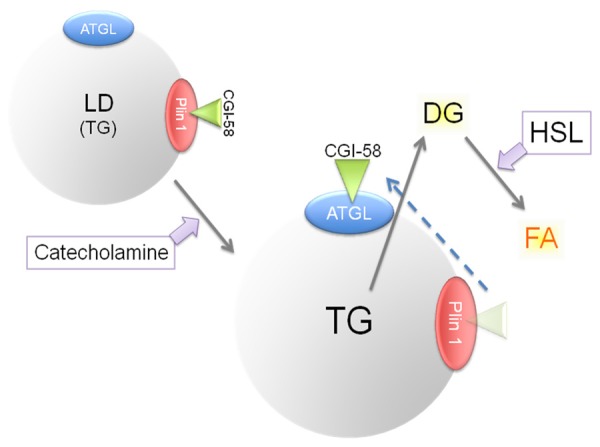
A present understanding of the process of lipolysis. Plin 1, binding with CGI-58, functions as a suppressor of lipolysis (upper left). Under catecholamine stimulation, ATGL binds with CGI-58 and evokes lipolysis (center). HSL further progresses lipolysis to release FA (upper right). DG, diacylglycerol.
Ras-related proteins in brain GTPases (Rab)-18
Rab proteins are known to function in membrane trafficking and fusion [56]. Rab-18 localizes to LDs and ER, and participates in lipid mobilization. Rab-18 and Plin 2 are reciprocally expressed on LDs in association with lipolysis status [57,58]. Inversely, Rab-18 expression on LDs in ethanol-fed mice is suppressed by increased LD size and Plin 2 expression [59]. Further studies are required, but impaired Rab-18 expression may be pivotal in alcoholic fatty liver.
Lipotoxicity, ER stress and oxidative injury
In livers of ALD, the metabolic capacity of hepatocytes is considerably occupied by ethanol/acetaldehyde metabolism, and hence, hepatocytes cannot appropriately utilize FA as a fuel for beta-oxidation [3]. Increased adipocyte-derived FA by excessive ethanol consumption further exacerbates the hepatic FA overload [50,51,55], and the excess FA is thought to injure the hepatocytes [60]. These pathologic processes are recognized as lipotoxicity, which is conducted chiefly via ER stress and oxidative injury caused by excess FA [61-65].
Since the early 2000’s, processes of cellular injury based on ER stress have been considered to be one of the critical factors in the pathology of ALD [66,67]. First of all, acetaldehyde is a robust ER stress inducer [68]. Additionally, because studies of ceramide-related ER stress are currently in progress [65,69], the contribution of excessive FA-induced ER stress to ALD remains obscure. However, past experimental evidence has provided sufficient insight into ER stress caused in the hepatocytes of ALD by excess FA [61-63]. Plin 2-knockout mice can escape from ceramide accumulation and oxidative injury, suggesting also that excess lipid accumulation and imbalanced lipolysis deeply contribute to ER stress in ALD.
Excess FA-induced oxidative injury has been recognized to constitute a large part of the pathological mechanisms of ALD. In other words, over-oxidation in mitochondria and ER due an excess of FA generates reactive oxygen species (ROS), which are considered a cause of severe cellular damage [70]. However, an association between the LD formation and lipolysis process and oxidative injury has been obscure although a long-standing theme of ALD research. Recent studies of LD-associated proteins have opened a new way to understand the pathophysiology of ALD. Currently, innovative findings concerning the relationship between LD metabolism and oxidative injury are increasing. A line of our previous joint work provided substantial progress to this field by demonstrating the association between LD formation/Plin protein expression and oxidative injury in ballooned hepatocytes [11,71-73].
The results collectively suggested that hepatocellular ballooning is associated with the destruction of the cytoskeleton and with intracellular accumulation of Plin-coated micro-LDs and peroxidation of the LD surface phospholipid (Figure 5). Orlicky et al. [12] further demonstrated the concomitant expressions of Plin proteins and oxidized lipid (4-hydroxynonenal) on LDs, further suggesting a close association between LD metabolism and oxidative injury.
Figure 5.
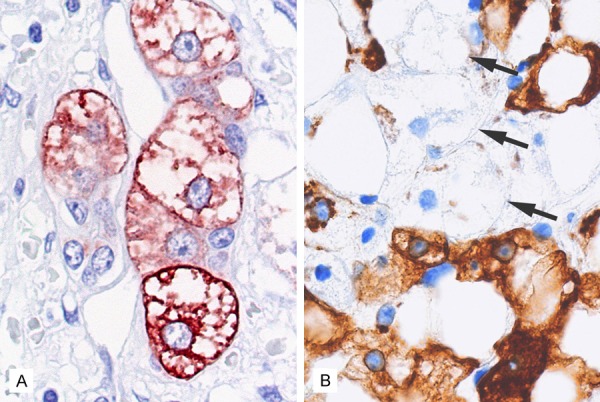
Evidence of cytoskeleton destruction due to oxidative injury in ballooned hepatocytes. A. Ballooned hepatocytes often show immunoreactivity with an antibody against oxidized phospholipid, a marker for oxidative cellular injury [72]. B. Ballooned hepatocytes are always negative for cytokeratin 18 (arrows), indicating cytoskeleton destruction in the damaged cells [73].
Hepatosteatosis in ALD: An enemy, a savior, or a good Samaritan?
Based on these comments, the ultimate question is whether hepatocellular LD formation is itself pathological or not. Forced Plin family protein suppression, which leads to attenuation of hepatosteatosis, by drugs or genetic manipulation is expected to be a novel therapeutic target [13,17,32-34,74]. Controversially, several experimental facts have suggested that Plin family protein suppression is not a benefical strategy. Plin 2 and Plin 3 double-knockout mice demonstrate induction of IR [36], Plin 2 suppression potentially exacerbates hepatic fibrosis [35], and finally, Plin 5 may prevent lipotoxicity by controlling lipolysis [39,40].
Through the results obtained in many previous studies, it can be interpreted that hepatosteatosis itself is not pathogenic but the involved mechanisms carry risk. Plin family protein expression seems to be a physiological response (or a kind of an adaptation) to a temporary increase in intracellular free FA, which is more toxic than LDs. Plin family protein expression on LD surface appears to regulate sequestration of such toxic FA in the LDs. Several institutes are now trying to develop a therapy for hepatosteatosis targeting Plin 2. However, careful consideration regarding its local and systemic effects, as well as balancing of benefit and risk will be required to minimize potentially detrimental effects.
Conclusion
We have reviewed the potential pathological significance of LD-associated proteins in ALD on the basis of the latest reports of relevant studies. Hepatosteatosis, which is a pathognomonic morphological change, together with LD-associated protein expression, appears to be closely associated with ER stress and oxidative injury of hepatocytes. LD formation may be implicated in the cellular protective mechanism for escaping from the toxic effects of free FA. Expression of LD-associated proteins seems to contribute to the stabilization of LDs, which are fragile chambers accommodating TG to prevent releasing toxic FA. Intervention on LD-associated protein expression is a promising novel therapy for fatty liver diseases including ALD. However, it should be emphasized that further investigations are needed to assure the efficacy and safety.
Acknowledgements
The authors thank Ms Jessie Kitaguchi for her assistance in the preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology. 1990;1:342–8. doi: 10.1097/00001648-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner B. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–50. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM, Neuschwander-Tetri BA, Burt AD. Alcoholic liver disease. In: Burt AD, Portmann BC, Ferrell LD, editors. MacSween’s Pathology of the Liver. 6th edition. Amsterdam: Elsevier; 2012. pp. 299–318. [Google Scholar]
- 4.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:E10–E16. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30:378–90. doi: 10.1055/s-0030-1267538. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Zhong W, Qiu Y, Kang X, Sun X, Tan X, Zhao Y, Sun X, Jia W, Zhou Z. Preservation of hepatocyte nuclear factor-4α contributes to the beneficial effect of dietary medium chain triglyceride on alcohol-induced hepatic lipid dyshomeostasis in rats. Alcohol Clin Exp Res. 2013;37:587–98. doi: 10.1111/acer.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 8.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–10. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–59. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Khor VK, Shen WJ, Kraemer FB. Lipid droplet metabolism. Curr Opin Clin Nutr Metab Care. 2013;16:632–7. doi: 10.1097/MCO.0b013e3283651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii H, Ikura Y, Arimoto J, Sugioka K, Iezzoni JC, Park SH, Naruko T, Itabe H, Kawada N, Caldwell SH, Ueda M. Expression of perilipin and adipophilin in nonalcoholic fatty liver disease; relevance to oxidative injury and hepatocyte ballooning. J Atheroscler Thromb. 2009;16:893–901. doi: 10.5551/jat.2055. [DOI] [PubMed] [Google Scholar]
- 12.Orlicky DJ, Roede JR, Bales E, Greenwood C, Greenberg A, Petersen D, McManaman JL. Chronic ethanol consumption in mice alters hepatocyte lipid droplet properties. Alcohol Clin Exp Res. 2011;35:1020–33. doi: 10.1111/j.1530-0277.2011.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai Y, Varela GM, Jackson MB, Graham MJ, Crooke RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–54. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Aon MA, Bhatt N, Cortassa SC. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 2014;5:282. doi: 10.3389/fphys.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mak KM, Ren C, Ponomarenko A, Cao Q, Lieber CS. Adipose differentiation-related protein is a reliable lipid droplet marker in alcoholic fatty liver of rats. Alcohol Clin Exp Res. 2008;32:683–9. doi: 10.1111/j.1530-0277.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 16.Carr RM, Dhir R, Yin X, Agarwal B, Ahima RS. Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol Clin Exp Res. 2013;37:1091–9. doi: 10.1111/acer.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr RM, Peralta G, Yin X, Ahima RS. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One. 2014;9:e97118. doi: 10.1371/journal.pone.0097118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–6. [PubMed] [Google Scholar]
- 19.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–71. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolins NE, Skinner JR, Schoenfish MJ, Tzekov A, Bensch KG, Bickel PE. Adipocyte protein S3-12 coats nascent lipid droplets. J Biol Chem. 2003;278:37713–21. doi: 10.1074/jbc.M304025200. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh K, Lee YK, Londos C, Raaka BM, Dalen KT, Kimmel AR. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-ester-specific intracellular lipid storage droplets. J Cell Sci. 2012;125:4067–76. doi: 10.1242/jcs.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–55. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 23.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–91. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 24.Pawella LM, Hashani M, Eiteneuer E, Renner M, Bartenschlager R, Schirmacher P, Straub BK. Perilipin discerns chronic from acute hepatocellular steatosis. J Hepatol. 2014;60:633–62. doi: 10.1016/j.jhep.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Gong J, Wu H, Xu W, Wu L, Xu D, Gao J, Wu JW, Yang H, Yang M, Li P. Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat Commun. 2013;4:1594. doi: 10.1038/ncomms2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell SH, Ikura Y, Iezzoni JC, Liu Z. Has natural selection in human populations produced two types of metabolic syndrome (with and without fatty liver)? J Gastroenterol Hepatol. 2007;22(Suppl 1):S11–S19. doi: 10.1111/j.1440-1746.2006.04639.x. [DOI] [PubMed] [Google Scholar]
- 27.Straub BK, Stoeffel P, Heid H, Zimbelmann R, Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47:1936–46. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi Y, Shinoda A, Furuya N, Harada E, Arimura N, Ichi I, Fujiwara Y, Inoue J, Sato R. Perilipin-mediated lipid droplet formation in adipocytes promotes sterol regulatory element- binding protein-1 processing and triacylglyceride accumulation. PLoS One. 2013;8:e64605. doi: 10.1371/journal.pone.0064605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249–64. doi: 10.1515/BC.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006;47:931–43. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Masuda Y, Itabe H, Odaki M, Hama K, Fujimoto Y, Mori M, Sasabe N, Aoki J, Arai H, Takano T. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J Lipid Res. 2006;47:87–98. doi: 10.1194/jlr.M500170-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.McManaman JL, Bales ES, Orlicky DJ, Jackman M, MacLean PS, Cain S, Crunk AE, Mansur A, Graham CE, Bowman TA, Greenberg AS. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res. 2013;54:1346–59. doi: 10.1194/jlr.M035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu F, Wang C, Zhang L, Xu Y, Jang L, Gu Y, Cao X, Zhao X, Ye J, Li Q. Metformin prevents hepatic steatosis by regulating the expression of adipose differentiation-related protein. Int J Mol Med. 2014;33:51–8. doi: 10.3892/ijmm.2013.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Son SH, Goo YH, Chang BH, Paul A. Perilipin 2 (PLIN2)-deficiency does not increase cholesterol-induced toxicity in macrophages. PLoS One. 2012;7:e33063. doi: 10.1371/journal.pone.0033063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imai Y, Boyle S, Varela GM, Caron E, Yin X, Dhir R, Dhir R, Graham MJ, Ahima RS. Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiol Genomics. 2012;44:1125–31. doi: 10.1152/physiolgenomics.00045.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–45. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–40. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Sreenivasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res. 2011;52:2159–68. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, Matsumura S, Inoue K, Fushiki T, Kojima Y, Hashimoto T, Sakai F, Hirose F, Osumi T. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012;287:23852–63. doi: 10.1074/jbc.M111.328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Zhao Y, Gao X, Li L, Yuan Y, Liu F, Zhang L, Wu J, Hu P, Zhang X, Gu Y, Xu Y, Wang Z, Li Z, Zhang H, Ye J. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology. 2015;61:870–82. doi: 10.1002/hep.27409. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, Liu K, Zhou S, Tillison K, Wu Y, Smas CM. Assessment of fat-specific protein 27 in the adipocyte lineage suggests a dual role for FSP27 in adipocyte metabolism and cell death. Am J Physiol Endocrinol Metab. 2008;294:E654–E667. doi: 10.1152/ajpendo.00104.2007. [DOI] [PubMed] [Google Scholar]
- 42.Gong J, Sun Z, Li P. CIDE proteins and metabolic disorders. Curr Opin Lipidol. 2009;20:121–6. doi: 10.1097/MOL.0b013e328328d0bb. [DOI] [PubMed] [Google Scholar]
- 43.Wang R, Kong X, Cui A, Liu X, Xiang R, Yang Y, Guan Y, Fang F, Chang Y. Sterol-regulatory-element-binding protein 1c mediates the effect of insulin on the expression of Cidea in mouse hepatocytes. Biochem J. 2010;430:245–54. doi: 10.1042/BJ20100701. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, Xu L, Ye J, Li D, Wang W, Li X, Wu L, Wang H, Guan F, Li P. Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatology. 2012;56:95–107. doi: 10.1002/hep.25611. [DOI] [PubMed] [Google Scholar]
- 45.Li JZ, Ye J, Xue B, Qi J, Zhang J, Zhou Z, Li Q, Wen Z, Li P. Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes. 2007;56:2523–32. doi: 10.2337/db07-0040. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Ye J, Zhou L, Gu W, Fisher EA, Li P. Opposing roles of cell death-inducing DFF45-like effector B and perilipin 2 in controlling hepatic VLDL lipidation. J Lipid Res. 2012;53:1877–89. doi: 10.1194/jlr.M026591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J, Yao H, Zhang Y, Xue B, Li Q, Yang H, Wen Z, Li P. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One. 2008;3:e2890. doi: 10.1371/journal.pone.0002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195:953–63. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsusue K, Kusakabe T, Noguchi T, Takiguchi S, Suzuki T, Yamano S, Gonzalez FJ. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–11. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, Sun X, Yin X, Sun X, Kim S, McClain CJ, Zhang X, Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, Zhong W, Sun X, Sun Q, Tan X, Li Q, Sun X, Zhou Z. Visceral white adipose tissue is susceptible to alcohol-induced lipodystrophy in rats: role of acetaldehyde. Alcohol Clin Exp Res. 2015;39:416–23. doi: 10.1111/acer.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Omatsu N, Morimoto E, Nakashima H, Ueno K, Tanaka T, Satouchi K, Hirose F, Osumi T. CGI-58 facilitates lipolysis on lipid droplets but is not involved in the vesiculation of lipid droplets caused by hormonal stimulation. J Lipid Res. 2007;48:1078–89. doi: 10.1194/jlr.M600493-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Bell M, Sreenivasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011;286:15707–15. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao YW, Jiang Y, Zhang DY, Zhang XJ, Hu YJ, Li P, Su H, Wan JB. The hepatoprotective effect of aqueous extracts of Penthorum chinense Pursh against acute alcohol-induced liver injury is associated with ameliorating hepatic steatosis and reducing oxidative stress. Food Funct. 2015;6:1510–7. doi: 10.1039/c5fo00098j. [DOI] [PubMed] [Google Scholar]
- 56.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–7. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci. 2005;118:2601–11. doi: 10.1242/jcs.02401. [DOI] [PubMed] [Google Scholar]
- 58.Martin S, Driessen K, Nixon SJ, Zerial M, Parton RG. Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J Biol Chem. 2005;280:42325–35. doi: 10.1074/jbc.M506651200. [DOI] [PubMed] [Google Scholar]
- 59.Rasineni K, McVicker BL, Tuma DJ, McNiven MA, Casey CA. Rab GTPases associate with isolated lipid droplets (LDs) and show altered content after ethanol administration: potential role in alcohol-impaired LD metabolism. Alcohol Clin Exp Res. 2014;38:327–35. doi: 10.1111/acer.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–7. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 62.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–37. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Kuo TF, Tatsukawa H, Matsuura T, Nagatsuma K, Hirose S, Kojima S. Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stress-stimulated PERK pathways. J Cell Physiol. 2012;227:1130–7. doi: 10.1002/jcp.22833. [DOI] [PubMed] [Google Scholar]
- 64.Setshedi M, Longato L, Petersen DR, Ronis M, Chen WC, Wands JR, de la Monte SM. Limited therapeutic effect of N-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol-induced steatohepatitis. Alcohol Clin Exp Res. 2011;35:2139–51. doi: 10.1111/j.1530-0277.2011.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Ruiz C, Mato JM, Vance D, Kaplowitz N, Fernández-Checa JC. Acid sphingomyelinase-ceramide system in steatohepatitis: a novel target regulating multiple pathways. J Hepatol. 2015;62:219–33. doi: 10.1016/j.jhep.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 66.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 67.Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol. 2004;10:1699–708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lluis JM, Colell A, García-Ruiz C, Kaplowitz N, Fernández-Checa JC. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124:708–24. doi: 10.1053/gast.2003.50089. [DOI] [PubMed] [Google Scholar]
- 69.Longato L, Ripp K, Setshedi M, Dostalek M, Akhlaghi F, Branda M, Wands JR, de la Monte SM. Insulin resistance, ceramide accumulation, and endoplasmic reticulum stress in human chronic alcohol-related liver disease. Oxid Med Cell Longev. 2012;2012:479348. doi: 10.1155/2012/479348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756–72. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caldwell SH, Redick JA, Chang CY, Davis CA, Argo CK, Al Osaimi KA. Enlarged hepatocytes in NAFLD examined with osmium fixation: does microsteatosis underlie cellular ballooning in NASH? Am J Gastroenterol. 2006;101:1677–8. doi: 10.1111/j.1572-0241.2006.00627_8.x. [DOI] [PubMed] [Google Scholar]
- 72.Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S, Kawada N, Arakawa T, Ueda M. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506–14. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 73.Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, Pramoonjago P, Simmons W, Scruggs H, Rosenbaum N, Wilkinson T, Toms P, Argo CK, Al-Osaimi AM, Redick JA. Hepatocellular ballooning in NASH. J Hepatol. 2010;53:719–23. doi: 10.1016/j.jhep.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langhi C, Marquart TJ, Allen RM, Baldán A. Perilipin-5 is regulated by statins and controls triglyceride contents in the hepatocyte. J Hepatol. 2014;61:358–65. doi: 10.1016/j.jhep.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


