Abstract
Objective: This study aimed to evaluate the relationship between forkhead box P3 (Foxp3) expression and clinicopathological characteristics of cervical cancer and to explore the influence of Foxp3 on the biological behaviors of cervical cancer cells. Methods: In this study, immunohistochemistry, lentivirus mediated transfection, Transwell assay; CCK-8 assay, real-time PCR and flow cytometry were employed to confirm the roles of Foxp3 in the occurrence and development of cervical cancer. Results: Foxp3 and p16INK4a were highly expressed in the cervical cancer and their expressions were related to the FIGO stage, tumor size, lymph node metastasis and serum SCC. Foxp3 had a high expression in the cervical cancer cells, tumor interstitium and metastatic lymph nodes. Foxp3 expression was positively related to p16INK4a expression in the cervical cancer. Foxp3 expression in the cervical cancer was negatively related to the prognosis: high Foxp3 expression predicted a poor prognosis. Silencing of Foxp3 was able to inhibit the proliferation and invasiveness of cervical cancer cells, promote their apoptosis, and induce the change in cell cycle. Silencing of Foxp3 also reduced the mRNA and protein expressions of p16INK4a in cervical cancer cells. Conclusion: Foxp3 is highly expressed in the cervical cancer, and able to facilitate the proliferation and invasiveness of cervical cancer, change cell cycle and inhibit their apoptosis, resulting in the occurrence, development and metastasis of cervical cancer.
Keywords: Cervical cancer, Foxp3, regulatory T cells, p16INK4a, cell apoptosis, human papillomavirus
Introduction
Cervical cancer is the second common malignancies in women [1] and significantly threatens the health and life of women. In the general population, the incidence of cervical cancer shows a decreasing tendency, but the morbidity and mortality of cervical cancer are still increasing in the developing countries, and the cervical cancer patients becomes younger [2]. Cervical cancer is an immunogenic malignancy, and high-risk human papilloma virus (HPV) subtypes may cause cervical cancer through several steps: Cervical intraepithelial neoplasia (CIN), carcinoma in situ of cervix, invasive carcinoma of cervix and cervical cancer metastasis. Persistent infection of high-risk HPV subtypes (such as HPV16) may significantly facilitate the development of CIN (especially the CIN2 and CIN3) and has been confirmed as a major risk factor of CIN2, CIN3 and cervical cancer. For healthy subjects with HPV infection confirmed by cervical cytology, more than 50% of them are diagnosed with transient HPV infection by re-examination at 12 months [3]. Whether HPV infection occurs after exposure to HPV and the outcome after HPV infection is closely related to the immune response of the host (especially the local immune response).
Regulatory T cells (Treg cells) are a group of mature T cells generated in the thymus following the induction of peripheral naïve T cells [4]. Treg cells are indispensable for the maintenance of non-response of host to autoantibodies and the inhibition of immune overreaction induced damage. On the other hand, over-production of Treg cells may block the protective immune response to infection and tumors. Multiple molecules of T cells are related to the specific functions of Treg cells. Forkhead/winged-helix transcription factor box P3 (Foxp3) is a member of forkhead/winged-helix family and a transcription factor specifically expressed on Treg cells. Human Foxp3 gene is mapped to Xp1 1.23 and includes 11 exons and 10 introns. Its cDNA is 1869 bp in length and it may produce a full-length mRNA and an mRNA without the 3rd exon [5]. Foxp3 plays crucial roles in the development and functions of Treg cells and especially Treg cells with high Foxp3 expression may significantly inhibit the immune response [4,6-10]. Dysfunction of Treg cells (such as Foxp3 gene mutation) may cause severe autoimmune immune diseases, immune pathology and allergic reaction [6]. Foxp3 gene mutation may result in X-linked recessive hereditary inflammatory disease in scurfy mice and Immune dysregulation, polyendocrinopathy, enteropathy and X-linked (IPEX) in humans [4,7]. In recent years, studies reveal that Foxp3 is not only specifically expressed in Treg cells naturally generated in the thymus, but in the cytoplasm and/or nucleus of cancer cells, and Foxp3 expression is closely related to the progression and prognosis of cancers [11-15]. For example, Foxp3 expression is aberrant in the gastric cancer [12], melanoma [13], liver cancer [14] and breast cancer [15] and closely associated with the occurrence, development and prognosis of cancers. However, the specific mechanism underlying the role of Foxp3 in the pathogenesis of cancers is still unclear. On one hand, Foxp3 positive Treg cells may facilitate the occurrence, development and metastasis of cancers; on the other hand, abnormal Foxp3 expression may also inhibit or facilitate the occurrence and development of cancers.
p16INK4a is a cyclin-dependent kinase inhibitor and may deactivate CD4K4/CDK6 to block the phosphorylation of retinoblastoma protein (pRB), which may cause the aging of normal cells [16]. p16INK4a expression is very low in normal cells and negatively regulated by the product of pRB gene. In cervical cancer cells, high-risk HPV E7 oncogene may inactivate pRB gene and thus p16INK4a expression increases significantly in the cervical cancer [16,17]. In the present study, the expressions of Foxp3 and p16INK4a were detected in cervical cancer, and the proliferation, apoptosis and invasiveness of cervical cancer cells were evaluated and the protein and mRNA expressions of p16INK4a were detected following Foxp3 silencing in cervical cancer cells.
Materials and methods
Subjects
A total of 148 cervical tissues were collected from inpatients and outpatients who received therapy in the Affiliated Shengjing Hospital of China Medical University between 2010 and 2011. There were 97 cervical cancer tissues, 15 metastatic lymph nodes, 5 non-metastatic lymph nodes, 31 CIN, and 20 normal cervical tissues. These patients had complete clinical information.
Immunohistochemistry
Neutral formalin-fixed tissues were embedded in paraffin with standard protocol. Samples were cut into 4 μm sections and mounted on glass slides. Sections were de-paraffinized with dimethylbenzene and rehydrated through a graded alcohol series, followed by blocking of the endogenous peroxidase activity by using hydrogen peroxidase (3%). The slides were then heated by microwave for antigen retrieval in citrate buffer (pH 6.0). After being cooled down in this buffer to room temperature, sections were covered with nonspecific serum, placed in a wet box and incubated at 37°C. Primary rabbit anti-p16INK4a at 1:300 (Bioword Technology Inc., MN, USA) and mouse anti-Foxp3 at 1:50 (Abcam, USA) were respectively added on the same slides in the wet box, and then incubated at 4°C overnight. Following 3 washes in PBS, the slides were incubated at 37°C with addition of biotinylated secondary antibodies. They were again washed thrice in PBS before adding streptococcus avidin-peroxidase (Zhongshan-Golden Bridge Biotechnology Ltd., Beijing, China) and incubated at 37°C for 30 minutes. Staining was developed with DAB chromogen (Zhongshan-Golden Bridge Biotechnology Ltd., Beijing, China). They were counterstained with hematoxylin, dehydrated with ethanol gradient, and sealed. Known positive tissue slides were used as positive controls, and PBS were used as the negative control instead of primary antibodies.
The immunostained sections were evaluated in a blinded manner by two independent researchers. The staining was evaluated as indicating expression through ten high-power fields per sample. The expression intensity was classified into four grades as follows: no staining visible was scored as 0; weak staining was scored as 1; moderate staining was scored as 2; strong staining was scored as 3. Scores for the positive cells were as follows: (score 0) means less than or equal 5% positive cells; (score 1) means 6-25% positive cells; (score 2) means 26-50% positive cells; and (score 3) means 51-75% positive cells; and (score 4) means more than or equal 76% positive cells. The scores of percentage and intensity reflect the sums of scores, with total scores of 0 indicated as (-); total scores of 1-2 as (+); total scores of 3-5 as (++); total scores of 6-7 as (+++).
Cell culture
Human cervical cancer cells (Hela cells, Siha cells and Caski cells) were purchased from the Shanghai Institute of Cell of Chinese Academy of Science. Hela cells were maintained in DMEM containing 10% fetal bovine serum (FBS), and Siha cells and Caski cells were cultured in MEM containing 10% FBS. Cells were digested with 0.25% trypsin in 0.1% EDTA and then centrifuged. Cells were harvested and re-suspended, followed by incubation in an environment with 5% CO2 at 37°C. Cells in logarithmic growth phase were harvested for further experiments.
Western blot assay
Western blot assay was employed to detect Foxp3 expression, and cervical cancer cells with Foxp3 silencing were screened. At 48 h after lentivirus mediated transfection, cells were harvested and total protein was extracted. The protein concentration was determined. Total protein was subjected to 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA). After blocking with 5% non-fat dry milk in TBS, the membrane was probed with primary monoclonal antibody specific to Foxp3 (1:200, Abcam, USA), p16INK4a (1:1000, Bioword Technology Inc., MN, USA) or β-actin (1:1000, Beyotime) as an internal control for protein. Then, the membrane was incubated with secondary antibody at 1:3000, and protein bands were scanned. The optical density (OD) of bands was analyzed with the Gel-Pro-Analyzer. Each experiment was conducted three times, and data were averaged.
Lentivirus mediated intervence in siha cells
Lentivirus expressing Foxp3-siRNA was prepared in the Shanghai Genepharma Biotech Co., Ltd. Cells were divided into 3 groups: blank control group, negative control group and Foxp3 siRNA group. Cells were seeded into 6-well plates at a density of 3×105 cells/well, and then incubated in an environment with 5% CO2 at 37°C for 24 h. Cells were transfected with blank control lentivirus, negative control lentivirus and lentivirus expressing Foxp3-siRNA for 48 h, and then observed under a fluorescence microscope. Cells were harvested for the detection of mRNA and protein expressions of target genes. Western blot assay was employed to screen cervical cancer cells with high Foxp3 expression, and Foxp3 expression was silenced in these cells. After silencing of Foxp3, Western blot assay and real-time PCR were employed to detect the protein and mRNA expression of Foxp3 and p16INK4a, respectively. Each experiment was conducted three times, and data were averaged.
Detection of cell proliferation in vitro
Cells with Foxp3 silencing were collected and seeded into five 96-well plates, followed by incubation for 24 h. Cells in 4 plates were processed for lentivirus mediated transfection, and cells in the remaining plate were processed for MTT assay (0 h). At 24 h, the medium was refreshed, and cells in 1 plate were processed for MTT assay (24 h). Cells in remaining 3 plates were maintained for 48 h, 72 h and 96 h, respectively, and then processed for MTT assay. Each experiment was conducted three times, and data were averaged.
Detection of cell invasion in vitro
Transwell chambers were added to 24-well plates and then pre-coated with diluted Matrigel (50 μl per chamber), followed by incubation at 37°C for 2 h. The medium was removed, and cells were washed in PBS thrice and digested in 0.25% trypsin. When the cells became round, excess trypsin was removed, and the pre-coated Transwell chambers were added to 24-well plates. After fixation in 4% paraformaldehyde at room temperature for 20 min, 0.5% crystal violet staining was performed for 5 min. After washing, cells were observed under an inverted microscope at 200×, and the migrating cells were counted. Five fields were randomly selected, and migrating cells were counted, and an average was obtained. Each experiment was conducted three times, and data were averaged.
Detection of cell apoptosis by flow cytometry
Cells were mixed with 5 μl of Annexin V-Light and 5 μl of propidium iodide and then subjected to flow cytometry. Cells located in 4 quadrants: cells in the upper left quadrant were necrotic cells; cells in lower left quadrant were normal cells; cells in upper right quadrant were late apoptotic cells; cells in lower right quadrant were early apoptotic cells. The proportion of different cells was calculated. Each experiment was conducted three times, and data were averaged.
Statistical analysis
Statistical analysis was performed with SPSS version 17.0. Chi square test was employed to evaluate the relationship of clinicopathological characteristics of cervical cancer with protein expressions of Foxp3 and p16INK4a. Spearman rank correlation analysis was used to test the relationship between Foxp3 expression and p16INK4 expression. A value of P<0.05 was considered statistical analysis. Quantitative data are expressed as mean ± standard deviation and were compared with analysis of variance. Qualitative data were compared with chi square test.
Results
Clinicopathological characteristics
Immunohistochemistry was done in 148 cervical tissues including 97 cervical cancer tissues, 31 CIN and 20 normal cervical tissues. All the patients had complete clinical and clinicopathological information (Table 1). The age of cervical cancer patients ranged from 27 years to 70 years (median: 44 years). Cervical cancer was staged according to the FIGO2009 criteria.
Table 1.
Clinicopathological characteristics of cervical tissues
| Characteristics | Number of cases (%) |
|---|---|
| Samples | |
| NSCES | 20 (13.5) |
| CIN | 31 (20.9) |
| ICC | 97 (65.5) |
| Differentiation | |
| WICC | 21 (21.7) |
| MICC | 49 (50.5) |
| PICC | 27(27.8) |
| Histology | |
| SCC | 88 (90.7) |
| ACC | 9 (9.3) |
| FIGO Stage | |
| I | 58 (59.8) |
| II | 39 (40.2) |
| LN Metastasis | |
| Absent | 77 (79.4) |
| Present | 20 (20.6) |
| Tumor Size (cm) | |
| <4 | 46 (47.4) |
| ≥4 | 51 (52.6) |
Expression of Foxp3 and p16INK4a in different cervical tissues
Immunohistochemistry was performed to detect the Foxp3 expression in normal cervical epithelium, CIN and cervical cancer. As shown in Figure 1, Foxp3 protein was not observed in normal cervical epithelium, CIN had a weak expression of Foxp3, and Foxp3 expression was observed as brown granules in the cytoplasm and nucleus of cervical cancer cells as well as in the interstitium of cervical cancer. In cervical cancer, the Foxp3 expression was significantly higher than that in normal cervical tissues and CIN. Significant difference was also observed in the Foxp3 expression between ICC group and NSCES group (χ2=87.174, P<0.001) and between ICC group and CIN group (χ2=72.072, P<0.001), but there was no marked difference between CIN group and NSCES group (χ2=4.641, P=0.098) (Table 2).
Figure 1.
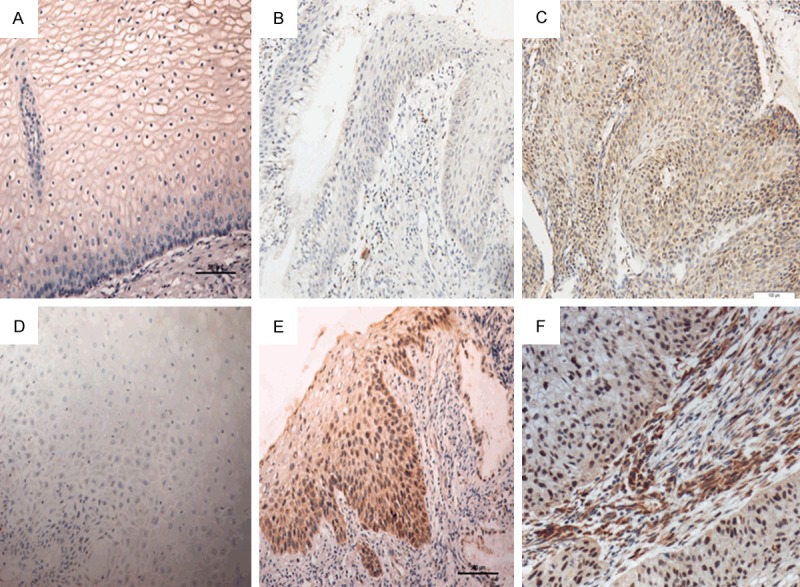
Expression of Foxp3 and p16INK4a in different cervical tissues. Foxp3 expression in normal cervical epithelium (A), CIN (B) and cervical cancer (C). p16INK4a expression in normal cervical epithelium (D), CIN (E) and cervical cancer (F) (200×).
Table 2.
Foxp3 expression in normal cervical tissues, CIN and ICC
| Characteristics | N | Foxp3 protein | |||
|---|---|---|---|---|---|
|
| |||||
| - | + | ++ | +++ | ||
| NSCES | 20 | 17 | 3 | 0 | 0 |
| CIN | 31 | 18 | 10 | 3 | 0 |
| ICC | 97 | 2 | 13 | 45 | 37 |
Correlation of Foxp3 and p16INK4a expressions with clinicopathological characteristics of cervical cancer
The Foxp3 and p16INK4a expressions were associated with some clinicopathological characteristics (FIGO stage, lymph node metastasis, tumor size and HPV infection) of cervical cancer patients, but had no relationship with differentiation degree, pathological type and age of cervical cancer patients (Table 3). In cervical cancer patients, p16INK4a expression was significantly different among ICC, CIN and NSCES (P<0.05) (Table 4). Moreover, in cervical cancer and CIN, Foxp3 expression was positively related to p16INK4a expression (Table 5).
Table 3.
Correlation between Foxp3 expression and clinicopathological characteristics of cervical cancer patients
| Characteristics | N | Foxp3 protein | P-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| - | + | ++ | +++ | |||
| Age (years) | ||||||
| ≤44 | 30 | 1 | 5 | 13 | 11 | 0.850 |
| >44 | 67 | 1 | 8 | 32 | 26 | |
| Differentiation | ||||||
| WICC | 21 | 0 | 5 | 8 | 8 | 0.189 |
| MICC | 49 | 0 | 4 | 25 | 20 | |
| PICC | 27 | 2 | 4 | 12 | 9 | |
| Histology | ||||||
| SCC | 88 | 2 | 12 | 42 | 32 | 0.706 |
| ACC | 9 | 0 | 1 | 3 | 5 | |
| FIGO Stage | ||||||
| I | 58 | 2 | 12 | 27 | 17 | 0.004 |
| II | 39 | 0 | 1 | 18 | 20 | |
| LN Metastasis | ||||||
| Absent | 77 | 1 | 12 | 40 | 24 | 0.022 |
| Present | 20 | 1 | 1 | 5 | 13 | |
| Tumor Size (cm) | ||||||
| <4 | 46 | 1 | 11 | 22 | 12 | 0.014 |
| ≥4 | 51 | 1 | 2 | 23 | 25 | |
| SCC (ng/ml) | ||||||
| <1.5 | 56 | 1 | 12 | 32 | 11 | <0.001 |
| ≥1.5 | 41 | 1 | 1 | 13 | 26 | |
Table 4.
Correlation between p16INK4a expression and clinicopathological characteristics of cervical cancer patients
| Characteristics | N | p16INK4a protein | P-value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| - | + | ++ | +++ | |||
| NSCES | 20 | 15 | 4 | 1 | 0 | <0.05 |
| CIN | 31 | 0 | 11 | 15 | 5 | |
| ICC | 97 | 1 | 4 | 63 | 29 | |
| Age (years) | ||||||
| ≤44 | 30 | 1 | 1 | 17 | 11 | 0.331 |
| >44 | 67 | 0 | 3 | 46 | 18 | |
| Differentiation | ||||||
| WICC | 21 | 0 | 0 | 13 | 8 | 0.504 |
| MICC | 49 | 0 | 3 | 34 | 12 | |
| PICC | 27 | 1 | 1 | 16 | 9 | |
| Histology | ||||||
| SCC | 88 | 1 | 4 | 57 | 26 | 0.907 |
| ACC | 9 | 0 | 0 | 6 | 3 | |
| FIGO Stage | ||||||
| I | 58 | 0 | 3 | 47 | 8 | <0.001 |
| II | 39 | 1 | 1 | 16 | 21 | |
| LN Metastasis | ||||||
| Absent | 77 | 0 | 3 | 55 | 19 | 0.021 |
| Present | 20 | 1 | 1 | 8 | 10 | |
| Tumor Size (cm) | ||||||
| <4 | 46 | 1 | 3 | 36 | 6 | 0.005 |
| ≥4 | 51 | 0 | 1 | 27 | 23 | |
| SCC (ng/ml) | ||||||
| <1.5 | 56 | 1 | 4 | 42 | 9 | 0.003 |
| ≥1.5 | 41 | 0 | 0 | 21 | 20 | |
Table 5.
Correlation between Foxp3 expression and p16INK4a expression in cervical cancer
| Foxp3 | p16INK4a | Correlation coefficient | P | |
|---|---|---|---|---|
|
| ||||
| -/+ | ++/+++ | |||
| -/+ | 16 | 25 | 0.551 | <0.001 |
| ++/+++ | 0 | 87 | ||
Foxp3 expression in metastatic lymph nodes of cervical cancer patients
Immunohistochemistry was employed to detect the Foxp3 expression in metastatic lymph nodes and normal lymph nodes (Table 6). As shown in Figure 2, Foxp3 expression was mainly found as brown granules in the nucleus of lymphocytes and the Foxp3 expression in metastatic lymph nodes was significantly higher than in normal lymph nodes (P<0.05).
Table 6.
Foxp3 expression in metastatic lymph nodes and normal lymph nodes
| LN | Foxp3 | P | |
|---|---|---|---|
|
| |||
| -/+ | ++/+++ | ||
| Present | 4 | 11 | <0.05 |
| Absent | 4 | 1 | |
Figure 2.
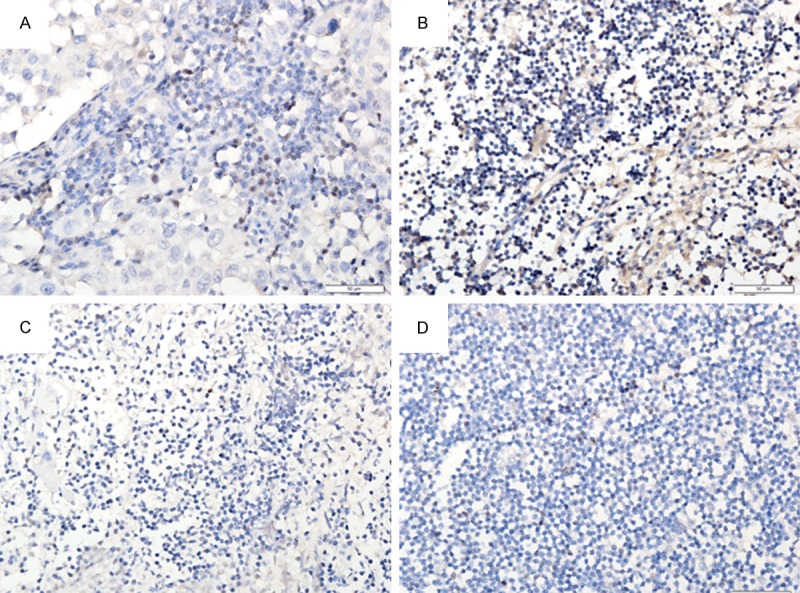
Foxp3 expression of metastatic lymph nodes (A, B) and normal lymph nodes (C, D) (400×).
Foxp3 expression in cervical cancer cells
As shown in Figure 3, Foxp3 expression was high in Siha cells, but low in Hela cells.
Figure 3.
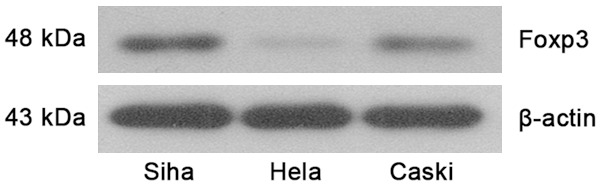
Foxp3 protein expression in cervical cancer cell lines.
Foxp3 expression in Siha cells after Foxp3 silencing
Cells with Foxp3 silencing (GFP/LV3-Foxp3-hemo-876) were selected, and Siha cells transfected with GFP/LV3-NC (negative control) and Siha cells without transfection (blank control) served as controls. Western blot assay and real-time PCR were used to detect the protein and mRNA expressions of Foxp3 and p16INK4a. Figure 2 shows the Foxp3 expression in Siha cells following lentivirus mediated RNA interference. As shown in Figures 3 and 4, the mRNA and protein expressions of Foxp3 and p16INK4a in cells after Foxp3 silencing reduced significantly when compared with blank control group and negative control group (P<0.05) (Figures 4, 5 and 6).
Figure 4.
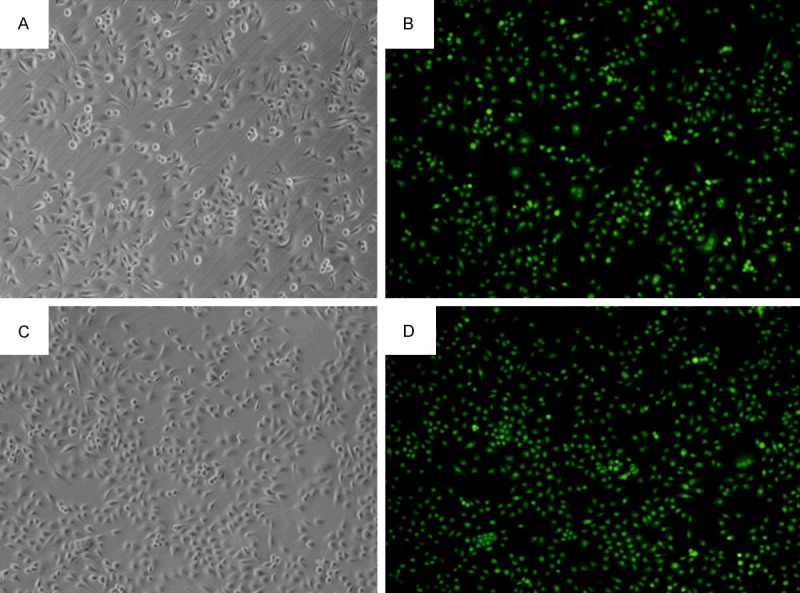
Foxp3 expression in Siha cells of different groups (100×). A, B: Foxp3 silencing group; C, D: Negative control group.
Figure 5.
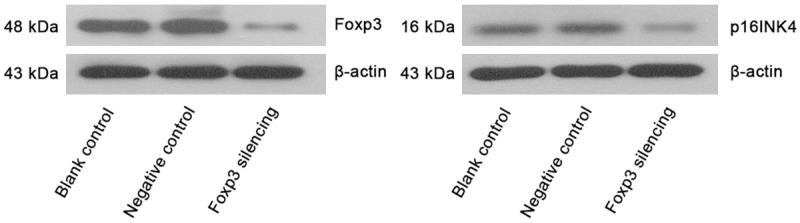
Expressions of Foxp3 and p16INK4a in Siha cells of different groups (Western blot assay).
Figure 6.
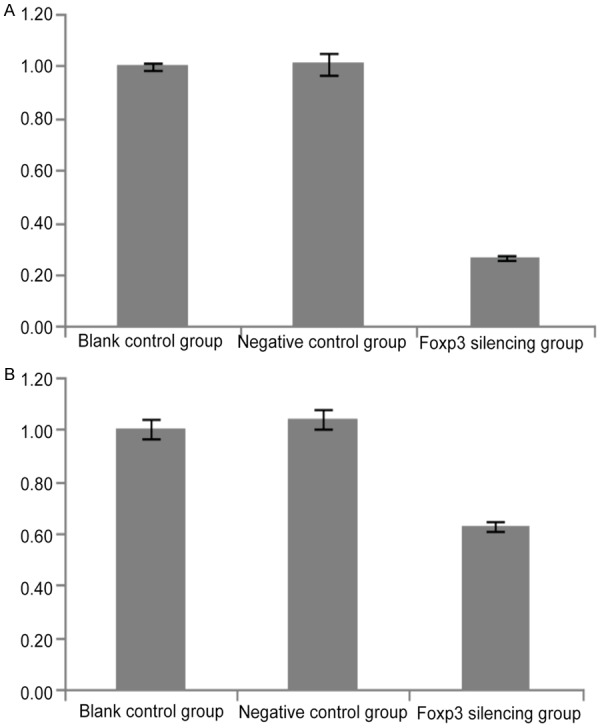
Expression of Foxp3 and p16INK4a in Siha cells of different groups (real-time PCR).
Detection of cell proliferation in Siha cells following Foxp3 silencing by CCK-8 assay
In this study, CCK-8 assay was employed to detect the cell proliferation in Siha cells following Foxp3 silencing, Siha cells of negative control group and Siha cells of blank control group. The absorbance in difference groups is shown in Table 7. In Siha cells with Foxp3 silencing, the number of cells reduced significantly at 24, 48, 72 and 96 h when compared with negative control group and blank control group (P<0.05). This suggests that Foxp3 silencing may inhibitthe proliferation of Siha cells (Figure 7).
Table 7.
Absorbance of Siha cells in different groups at distinct time points
| Group | Absorbance | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 h | 24 h | 48 h | 72 h | 96 h | |
| Blank control | 0.314±0.022 | 0.489±0.060 | 0.806±0.089 | 1.127±0.121 | 1.206±0.126 |
| Negative control | 0.307±0.043 | 0.450±0.048 | 0.773±0.078 | 1.090±0.105 | 1.169±0.120 |
| Foxp3 silencing | 0.305±0.046 | 0.355±0.053* | 0.617±0.070* | 0.899±0.090* | 0.982±0.118* |
P<0.05 vs. negative control group and blank control.
Figure 7.
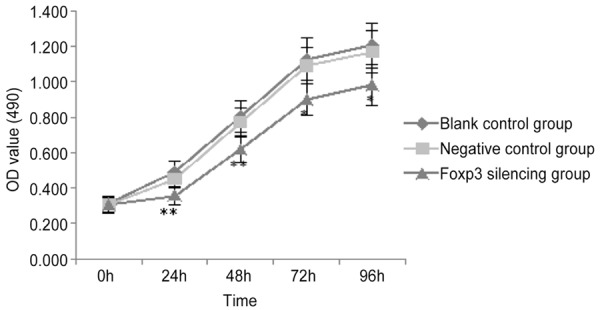
Proliferation of cells in different groups.
Effect of Foxp3 on the invasiveness of cervical cancer cells
Transwell chambers were employed to evaluate the invasiveness of cervical cancer cells. Results showed Siha cells after Fopx3 silencing showed a significantly reduced invasiveness as compared to blank control group and negative control group (45.60±5.41 vs. 72.40±8.20 and 73.60±7.89, P<0.001) (Table 8; Figures 8 and 9).
Table 8.
Number of migrating Siha cells in different groups
| Groups | Cell No |
|---|---|
| Blank control | 73.60±7.89 |
| Negative control | 72.40±8.20 |
| Foxp3 silencing | 45.60±5.41* |
P<0.01 vs. blank control group and negative control group.
Figure 8.
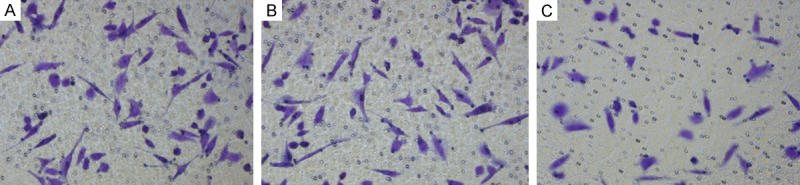
Cells migrating across the membrane in different groups (200×). A: Blank control group; B: Negative control group; C: Foxp3 silencing group.
Figure 9.
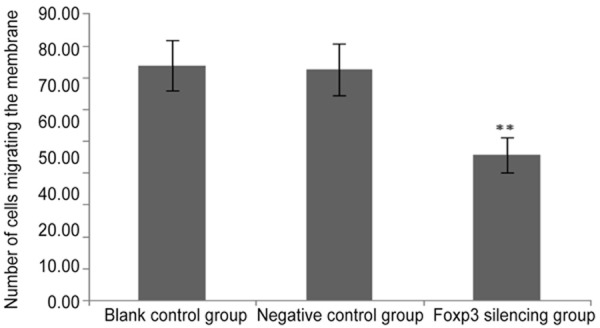
Number of cells migrating the membrane in three groups. **P< 0.01 vs. blank control group and negative control group.
Effect of Foxp3 on the apoptosis of cervical cancer cells
After PI staining, cells were subjected to flow cytometry. The apoptosis rate is shown in Table 9. The apoptosis rate in Siha cells after Foxp3 silencing increased significantly when compared with blank control group and negative control group (Figure 10).
Table 9.
Apoptosis rate in three groups
| Groups | Apoptosis rate (%) |
|---|---|
| Blank control | 3.68±0.13 |
| Negative control | 3.58±0.23 |
| Foxp3 silencing | 26.02±1.28* |
P<0.01 vs. blank control group and negative control group.
Figure 10.
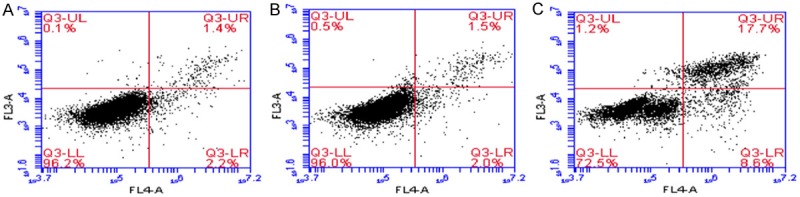
Normal cells, necrotic cells and cells at different stages of apoptosis (flow cytometry). A: Blank control group; B: Negative control group; C: Foxp3 silencing group.
Effect of Foxp3 on the cell cycle of cervical cancer cells
After AnnexinV-Light staining, flow cytometry was performed to detect cells in different phases of cell cycle. As shown in Table 10, cells in S phase and G2 phase reduced significantly and those in G1 phase increased markedly after Foxp3 silencing when compared with blank control group and negative control group (P<0.01). This suggests that Foxp3 silencing reduces cells in S phase and G2 phase, but blocks cells in G1 phase (Figure 11).
Table 10.
Cells in different phases of cell cycle in different groups
| Groups | G1 (%) | S (%) | G2 (%) |
|---|---|---|---|
| Blank control | 61.60±1.80 | 15.40±1.00 | 22.31±0.83 |
| Negative control | 62.59±1.11 | 14.72±0.37 | 21.72±0.82 |
| Foxp3 silencing | 76.29±1.09* | 12.34±0.47* | 11.49±0.87* |
P<0.01 vs. blank control group and negative control group.
Figure 11.
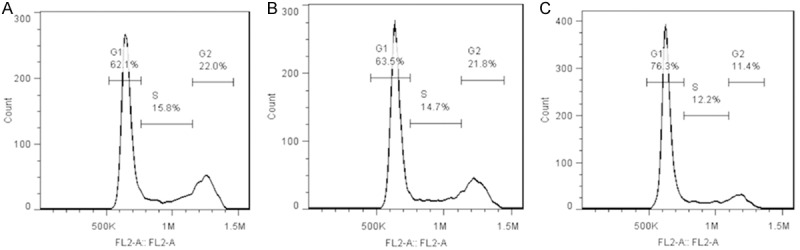
Cells in different phases of cell cycle (flow cytometry). A: Blank control group; B: Negative control group; C: Foxp3 silencing group.
Discussion
Foxp3 has been regarded as a specific marker of Treg cells and plays important roles in the development and functions of Treg cells [8]. Foxp3 has three discernible domains: C2H2 zinc finger domain at N terminal, leucine zipper motif (LeuZip) and forkhead DNA binding domain at C terminal (FKH). The C2H2 zinc finger domain and LeuZip are conservative in the FOX family and can bind to regulatory DNA to induce the formation of dimmers. FKH domain is a shared characteristic of FOX family and crucial for the nuclear localization and DNA binding [5]. In recent years, Foxp3 expression is found in not only T cells, but in the peripheral blood, lymph nodes and tumor microenvironment of tumor patients. The Foxp3 positive Treg cells are abundant in the liver cancer, and Foxp3 has been found to facilitate cancer metastasis and be associated with a poor prognosis of cancer patients [14]. In the local lymph nodes of ovarian cancer patients, Foxp3 over-expression was observed in Treg cells and closely related to the poor prognosis [18]. Ma et al found that Foxp3 was highly expressed in the gastric cancer cells and Foxp3 could activate the apoptosis pathway to induce the apoptosis of gastric cancer cells and inhibit the cancer progression, and high Foxp3 expression predicted a better prognosis [12,19]. Above findings suggest that Foxp3 gene in cancer cells may serve as a tumor suppressor gene to inhibit cancer progression, or Foxp3 possesses activities similar to those of Foxp3 positive cells to suppress the immune mediated killing of cancer cells. The roles of Foxp3 in the cancer cells are still conflicting, and the specific mechanism is unclear. p16INK4a is a marker of HPV infection. In tonsillar squamous cell carcinoma, p16INK4a expression is related to Foxp3 expression and predicts a better prognosis [20].
Foxp3 expression increases in the gastric cancer cells, is able to inhibit the proliferation of gastric cancer cells and facilitate their apoptosis by activating apoptosis pathway and may serve as a predictor of good prognosis [12]. In melanoma SM-MEL-28 cells, no more than 1% of cells are positive for Foxp3; in cells with stable high Foxp3 expression, the proliferation is inhibited, cell colonies reduce in vitro and tumor growth reduces in vivo [13]. In melanoma cells, Foxp3 is not a key protein that is able to facilitate or inhibit the cancer progression. Some studies show that Foxp3 may inhibit the occurrence and development of cancers and its expression is positively related to the survival of cancer patients, but others reveal Foxp3 has no relationship with the occurrence and development of cancer. The exact reason for this discrepancy is still poorly understood. In studies on breast cancer, the roles of Foxp3 are conflicting: some show it is able to promote the development of breast cancer, but others reveal it inhibits the development of breast cancer. In breast cancer with HER2 over-expression, patients with Foxp3 positive cancer have improved recurrence free survival and overall survival. Foxp3 expression in breast cancer cells is an independent prognostic factor of HER2 positive breast cancer patients, and Foxp3 expression is helpful to identify high risk patients who require additional therapy after neoadjuvant chemotherapy [15].
Abnormal p16INK4a expression is closely related to HR-HPV infection. Integration of HPV DNA into DNA of the host is crucial for the stability and its maintenance of virus in the host [21]. p16INK4a is a marker indicating that HPV-DNA integrates into host cells and induce the abnormal cell cycle [16,17,22].
In our study, the expression of Foxp3 and p16INK4a was detected in 97 cervical cancer tissues, 31 CIN and 20 normal cervical tissues. Results showed expression of Foxp3 and p16INK4a was mainly found in cervical cancer tissues, normal cervical tissues had no or weak expression of Foxp3 and P16INK4a, strong Foxp3 and P16INK4a expression was found in lesioned cervical tissues, and CIN had intermediate Foxp3 and p16INK4a expression. Significant differences were observed in the Foxp3 and p16INK4a expression among these tissues. This indicates that Foxp3 and P16INK4a are activated in cervical cancer, and their expression shows an increasing tendency with the progression of cervical lesions. Thus, we speculate that the up-regulated Foxp3 and p16INK4a expression play an important role in the progression of normal cervical tissues into CIN or even cervical cancer. Immunohistochemistry was performed to detect the Foxp3 expression in metastatic lymph nodes of 15 patients and normal lymph nodes of 5 patients. Results showed the Foxp3 expression in metastatic lymph nodes was significantly higher than in normal lymph nodes. This implies that Foxp3 plays an important role in the lymph node metastasis of cervical cancer.
Clinical stage, pathological grade, lymph node metastasis and tumor size can be used to predict the invasiveness of cervical cancer and the prognosis of cervical cancer patients. The higher the clinical stage and the higher the pathological grade, the more potent the invasiveness and metastasis of cervical cancer are. Under this condition, cervical cancer cells are susceptible to infiltrate the cervical interstitium and invade the blood vessels and lymphatic space, resulting in early metastasis. To investigate whether Foxp3 and p16INK4a expressions can be used to predict the malignancy and metastasis of cervical cancer, the expression of Foxp3 and p16INK4a was detected and their relationship with clinicopathological characteristics was further evaluated. Results showed Foxp3 and p16INK4a expression was closely related to the FIGO stage, tumor size, lymph node metastasis and serum SCC. The higher the FIGO stage, the higher the Foxp3 and p16INK4a expression was. This suggests that the up-regulated Foxp3 and p16INK4a expression is related to a poor prognosis of cervical cancer. However, there were no correlations of Foxp3 and p16INK4a expression with the differentiation degree, pathological type and age. On the above findings, we postulate that the up-regulated Foxp3 and p16INK4a expression play pivotal roles in the occurrence and development of cervical cancer. Moreover, Foxp3 expression was high in cervical cancer cells, cancer interstitium and metastatic lymph nodes, but the influence of Foxp3 on the behaviors of cancer cells is required to be further studied. Foxp3 plays crucial roles in the occurrence and development of cervical cancer, and thus it is of great importance to further explore the roles of Foxp3 in cervical cancer.
The treatments of cervical cancer are multi-disciplinary and include surgery, radiotherapy and chemotherapy. Surgery and radiotherapy are traditional modalities used for the treatment of cervical cancer, but immunotherapy is an alternative for patients who are intolerable to surgery and/or radiotherapy due to poor disease condition (cachexia). In recent years, immunotherapy has been a new adjunctive therapy of cancers and a focus in studies on cancers. Immunotherapy has fewer side effects as compared to chemotherapy. Several vaccines have been developed against cervical cancer (such as E6/E7 polypeptide and gene vaccine) and used in animal studies and clinical trials. Treg cells are able to inhibit the immune response of the host against the tumor and enhance the escape of the tumor from immune clearance, suggesting that Treg cells may be a key missing part in the immunotherapy of cancers [23]. The role of Treg cells in the anti-tumor activity of immune system and Treg cells becoming a new target for the immunotherapy of cancers make Treg cells become a focus in studies on immunotherapy. In this study, the Foxp3 expression was detected in cervical cancer cell lines, lentivirus vector expressing Foxp3 siRNA was constructed to silence Foxp3 expression in Siha cells, and CCK-8 assay, Transwell assay and flow cytometry were employed to explore the roles of Foxp3 in the proliferation, apoptosis and invasion of Siha cells. Our findings will provide evidence for the new adjunctive immunotherapy of cancers. Our results showed lentivirus mediated transfection was effective to silence Foxp3 in Siha cells. Under an invert microscope, the growth reduced and the number of viable cells also decreased following Foxp3 silencing as compared to blank control group and negative control group. CCK-8 assay showed Foxp3 silencing inhibited the proliferation of Siha cells, indicating that Foxp3 silencing affects the proliferation of Siha cells. Transwell chamber was employed to evaluate the influence of Foxp3 on the invasiveness of Siha cells. Results revealed that Foxp3 silencing significantly reduced the invasiveness of Siha cells as compared to blank control group and negative control group. This implies that Foxp3 silencing also affects the invasiveness of cervical cancer cells. Apoptosis analysis showed the apoptosis rate of Siha cells reduced dramatically after Foxp3 silencing, suggesting that Foxp3 silencing may reduce the number of apoptotic cells and also indicating that Foxp3 is involved in the proliferation of cervical cancer cells. Cell cycle is basic physiological process of cells. Our findings indicated that the proportion of cells in S phase and G2 phase reduced markedly and that of cells in G1 phase increased significantly after Foxp3 silencing, indicating that Foxp3 is able to facilitate the growth of cervical cancer cells. After silencing of Foxp3, the mRNA and protein expression of p16INK4a also decreased markedly in cervical cancer cells, further confirming that Foxp3 expression is positively related to p16INK4a expression, but the specific mechanism is required to be further elucidated. Above findings demonstrate that Foxp3 may facilitate the proliferation, reduce the apoptosis, promote the cell cycle progression and increase the cell invasion to affect the proliferation and invasion of cervical cancer cells and enhance their malignancy.
The growth, infiltration and metastasis of cancers have involvement of multiple signaling pathways and a lot of factors. More studies are required to elucidate the relationship between Foxp3 and cervical cancer, the role of Foxp3 in the occurrence and development of cervical cancer, and the specific mechanism underlying the role of Foxp3 in cervical cancer. In addition, whether the in vivo effects of Foxp3 on the cervical cancer are consistent with its in vitro effects on the cervical cancer cells and whether the cervical cancer can be treated by targeting Foxp3 are also required to be further studied.
In conclusion, the Foxp3 and p16INK4a expressions increase with the progression of cervical lesions, and Foxp3 expression is positively related to p16INK4a expression in cervical cancer. This suggests that the up-regulated expression of Foxp3 and p6INK4a may be one of mechanisms underlying the occurrence and development of cervical cancer. Foxp3 and p16INK4a expression is related to the FIGO stage, lymph node metastasis, tumor size and serum SCC-Ag. In the metastatic lymph nodes, Foxp3 expression is significantly higher than in normal lymph nodes, indicating that Foxp3 is associated with cervical cancer metastasis and may facilitate the metastasis of cervical cancer. Silencing of Foxp3 is able to significantly inhibit the proliferation and invasion of cervical cancer cells, reduce cells in S phase and G2 phase and increase the apoptosis rate of cervical cancer cells. Moreover, silencing of Foxp3 in cervical cancer cells also significantly reduces the mRNA and protein expression of p16INK4a, Foxp3 expression is positively related to p16INK4a expression, and Foxp3 is associated with HPV infection, but the specific mechanism is still poorly understood.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant #81372776) and the Free Researcher Project of Shengjing Hospital (grant #201302).
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Hildesheim A, Castle PE, Solomon D, Burk R. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100:513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 5.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 11.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, Barda AK, Gourgoulianis KI, Germenis AE. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma GF, Chen SY, Sun ZR, Miao Q, Liu YM, Zeng XQ, Luo TC, Ma LL, Lian JJ, Song DL. FoxP3 inhibits proliferation and induces apoptosis of gastric cancer cells by activating the apoptotic signaling pathway. Biochem Biophys Res Commun. 2013;430:804–809. doi: 10.1016/j.bbrc.2012.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Tan B, Anaka M, Deb S, Freyer C, Ebert LM, Chueh AC, Al-Obaidi S, Behren A, Jayachandran A, Cebon J, Chen W, Mariadason JM. FOXP3 over-expression inhibits melanoma tumorigenesis via effects on proliferation and apoptosis. Oncotarget. 2014;5:264–276. doi: 10.18632/oncotarget.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 15.Ladoire S, Arnould L, Mignot G, Coudert B, Rebe C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, Fumoleau P, Ghiringhelli F. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;125:65–72. doi: 10.1007/s10549-010-0831-1. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Albers AE, Qin J, Kaufmann AM. Prognostic significance of overexpressed p16INK4a in patients with cervical cancer: a meta-analysis. PLoS One. 2014;9:e106384. doi: 10.1371/journal.pone.0106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordi J, Sagasta A, Munmany M, Rodriguez-Carunchio L, Torne A, del Pino M. Usefulness of p16/Ki67 immunostaining in the triage of women referred to colposcopy. Cancer Cytopathol. 2014;122:227–235. doi: 10.1002/cncy.21366. [DOI] [PubMed] [Google Scholar]
- 18.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 19.Ma GF, Miao Q, Liu YM, Gao H, Lian JJ, Wang YN, Zeng XQ, Luo TC, Ma LL, Shen ZB, Sun YH, Chen SY. High FoxP3 expression in tumour cells predicts better survival in gastric cancer and its role in tumour microenvironment. Br J Cancer. 2014;110:1552–1560. doi: 10.1038/bjc.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park K, Cho KJ, Lee M, Yoon DH, Kim SB. Importance of FOXP3 in prognosis and its relationship with p16 in tonsillar squamous cell carcinoma. Anticancer Res. 2013;33:5667–5673. [PubMed] [Google Scholar]
- 21.Tsai HT, Wu CH, Lai HL, Li RN, Tung YC, Chuang HY, Wu TN, Lin LJ, Ho CK, Liu HW, Wu MT. Association between quantitative high-risk human papillomavirus DNA load and cervical intraepithelial neoplasm risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2544–2549. doi: 10.1158/1055-9965.EPI-05-0240. [DOI] [PubMed] [Google Scholar]
- 22.Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, De Marco L, Giorgi-Rossi P, Pontenani G, Rosso S, Sani C, Sintoni C, Segnan N, Zorzi M, Cuzick J, Rizzolo R, Ronco G. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2008;9:937–945. doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 23.Hou F, Ma D, Cui B. Treg cells in different forms of uterine cancer. Clin Chim Acta. 2013;415:337–340. doi: 10.1016/j.cca.2012.11.004. [DOI] [PubMed] [Google Scholar]


