Abstract
Heat stroke (HS) has been shown to induce intestinal barrier dysfunction during whole body hyperthermia. HS-induced intestinal permeability change may result from modulation of aquaporin (AQP) expression, which subsequently regulates water homeostasis. This study aimed to evaluate AQP expression in the intestine of rats with HS at different recovery time points. Sprague-Dawley (SD) rats were exposed to an ambient temperature of 40 ± 0.5°C until a maximum core temperature of 40.5°C was attained. The small intestine was surgically removed and histologically examined, and AQP expression was determined by reverse transcription polymerase chain reaction and immunohistochemical staining. H&E staining revealed those intestinal villi were destroyed from HS0 to HS1 and rebuilt from HS3 to HS12. We further stain with activated caspase 3 found expressed at HS0 and back to normal at HS3. Investigation of AQP mRNA expression identified 10 genes. PCR results of AQP1, 3, 7, 8, and 11 transcripts were significantly higher in the HS group than in the sham group. Immunohistochemical staining showed a more than 11-fold increase in AQP3 and 11 expressions at HS0. AQP1 and 8 increased at HS1 and AQP7 increased at HS3 compared with those in the sham group. In this study, we found HS induced jejunum damage and cell apoptosis. AQPs were upregulation/downregulation after HS in different time point suggested that water/glycerol transport was important when hyperthermia occurred. Furthermore, the biological function of the AQP needs more exploration in response to HS.
Keywords: Aquaporins, heat stroke, intestine
Introduction
Heat stroke (HS), a rapid increase in core temperature (Tco) to more than 40°C, is a critical illness characterized by various neurological signs and multiple organ failure [1]. After prolonged exercise and strenuous physical exertion, ultramarathon runners with HS have been found to have intestinal barrier dysfunction, increased permeability, and increased levels gut-derived lipopolysaccharide [2]. In an animal and cell study, heat stress was found to induce tissue hyperthermia [3] and decrease intestinal blood flow, which might subsequently cause derangement of tight junctions and pro-inflammatory cytokine release [4,5]. The small intestine protects against bacterial invasion [6] and is involved in water regulation and solute transport [7]. It absorbs massive quantities of water and contributes to body fluid homeostasis. Net water movement across membranes occurs by osmosis through paracellular and transcellular pathways [8].
Aquaporins (AQPs) are a large family of integral membrane proteins that form pores, regulate water flow, and allow small molecules to pass through biological cell membranes [9-11]. AQPs play an important role in water reabsorption in the kidney, and AQP-mediated transcellular water movement has been identified in the small intestine of rats. In mammals, AQPs are divided into 3 subfamilies. The AQP subfamily consists of 6 members of water-selective pores that enable high permeation, and the aquaglyceroporin subfamily has 4 members that are permeable to water and low-molecular-weight solutes such as glycerol and urea. The members of these 2 families are located in the plasma membrane and are involved in osmoregulation and transcellular solute transport. AQP11 and 12 belong to the third subfamily but not well studied to date [12].
Furthermore, AQP1 and AQP3-8 have been identified in the small intestine of rats [13-18]. AQP6-8 is restricted to the apical membrane of the villous epithelium. AQP3 and 4 are expressed on the basolateral membrane of the villous epithelium; AQP4 on the basolateral membrane of crypt epithelial cells; AQP5 on the apical membrane of duodenal glands; and AQP1 on the central lacteal epithelium. Higher expression levels of AQP6-8 proteins observed at the apical pole of superficial epithelial cells suggests their involvement in rapid fluid movement across the villous epithelium.
We hypothesized that the diversity of AQP distribution and expression possibly indicated its differential functions in the regulation of fluid movement in response to external stimuli. This study aimed to evaluate AQP expression in the intestine of rats with HS at various recovery time points. We used a rat model of HS [19] by exposing anesthetized rats to elevated ambient temperature (Ta) (40°C) until their Tco approached 40.5°C (HS onset). The rats were then immediately removed from the heating chamber, allowed to recover for various time periods, and sacrificed for histological and biochemical studies.
Materials and methods
Experimental animals
Sprague-Dawley (SD) rats (weight, 250-300 g) were used in this study. Animal care and handling were performed in accordance with the National Institutes of Health guidelines and were approved by the Institutional Committee of Animal Care at the National Defense Medical Center. Male SD rats were anesthetized, and the experimental methods followed the protocol reported by Liu et al [19]. In brief, the male SD rats were anesthetized with urethane (0.6 g/kg) and pentobarbitol (30 mg/kg) then were randomly assigned to (A) normal thermic sham group (n=5), (B) Heatstroke (HS) group (n=5). At predetermined time intervals after recovery, the animals were sacrificed to obtain intestinal tissues.
Physiological indices
The experimental methods and physiology recording were followed the protocol reported by Liu et al [19]. In brief, rats were placed a cannula in the right femoral artery and a thermocouple probe was used for continuous recording of body Tco from the rectum. Initially, the animal Tco was set at 36 ± 0.1°C by an infrared light lamp. Then, Tco was manipulated to Ta of 40 ± 0.5°C and maintained for 1 h within an incubator to induce HS. The rats were expected to recover from HS at Ta of 25 ± 1°C.
Chemicals
All chemicals were prepared immediately before use. Tris-base and sodium chloride were obtained from Mallinckrodt Chemicals (Mallinckrodt Inc., Paris, KY, USA). Other chemicals were obtained from Sigma Chemicals (Sigma-Aldrich Chemie GmbH, Germany). All chemicals were of reagent grade.
Histological examination of intestinal tissues
Biopsy of the intestine was performed after completion of the experiment. The obtained intestinal specimens were excised and fixed in 4% paraformaldehyde. Paraffin-embedded intestine tissues were cut at a thickness of 2-5 µm. Some of part of these tissues were stained with hematoxylin and eosin for histological examination, and the other tissues were used for immunohistochemical staining (NovoLink polymer detection system; Leica Biosystems Newcastle Ltd, UK). A semiquantitative study of immunohistochemical staining was performed using image software (Image scope Ver. 10.2.2.2319; Aperio Technologies, Inc., Vista, CA, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the rat intestine tissues using the TriZol kit (Invitrogen, Carlsbad, CA, USA). Routine RT-PCR was performed according to the standard procedures recommended for the MMLV reverse-transcriptase kit (Epicentre, Madison, WI, USA). According to the protocol described by Tenckhoff et al [20], 11 sets of specific primers for AQPs were applied to amplify their specific segments that can play roles in the qualitative analysis of AQPs. Semiquantitative RT-PCR analysis was performed using Alpha Innotech software (Alpha Imager Ver. 5.5; Alpha Innotech, San Leandro, CA, USA).
Immunohistochemical staining
The paraffin-embedded intestine tissues were cut into 4 µm slices, dried at 60°C for at least 8 hours, deparaffinized in xylene for four times, rehydrated in graded alcohols, microwaved in EDTA buffer solution to retrieve tissue antigen, and incubated in 5% H2O2 solution for ten minutes to eliminate endogenous peroxidase activity. The slices were incubated in anti-AQP and anti-caspase 3 rabbit polyclonal antibody (AQP1, 1:1500; AQP3, 1:1000; AQP7, 1:500; AQP8, 1:500; AQP11, 1:1500; and caspase 3, 1:50., SANTA CRUZ BIOTECHNOLOGY, Inc.) at 25°C for 120 minutes and next in anti-rabbit secondary immunoglobulin G antibody solution labeled by horse radish peroxidase (DAKO, Denmark) at 25°C for 30 minutes in the same humidified chamber. Staining was visualized using diaminobenzidine (DAKO, Denmark) staining, followed by hematoxylin nuclear counterstaining. Finally, the slices were dehydrated by graded alcohols and mounted by neutral transparent gum.
Statistical analysis
Experimental data are expressed as mean ± SEM. P less than 0.05 was considered significant. Comparison of experimental data among 3 or more groups was performed using one-way analysis of variance and Tukey’s procedure for post hoc comparisons.
Results
Physiological alterations during heat stress onset and recovery periods
The animals were placed in an incubator at Ta of 40°C for 1 h to induce the status of heatstroke. Then, these rats were placed at room temperature. Tco was recorded at predetermined periods (Figure 1). Blood pressure and other physiological indices were recorded as described previously [19].
Figure 1.
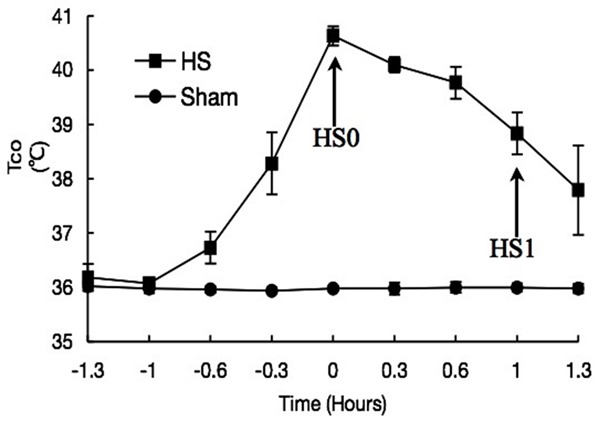
Time-course of Tco in HS-treated rats (n=5).
Time-course changes in jejunum histology after HS treatment
Time-course changes in jejunum histology after HS treatment are illustrated in Figure 2. Lamina propria cells were slugged immediately after heat stress treatment. Villous epithelial cells were seriously damaged.
Figure 2.
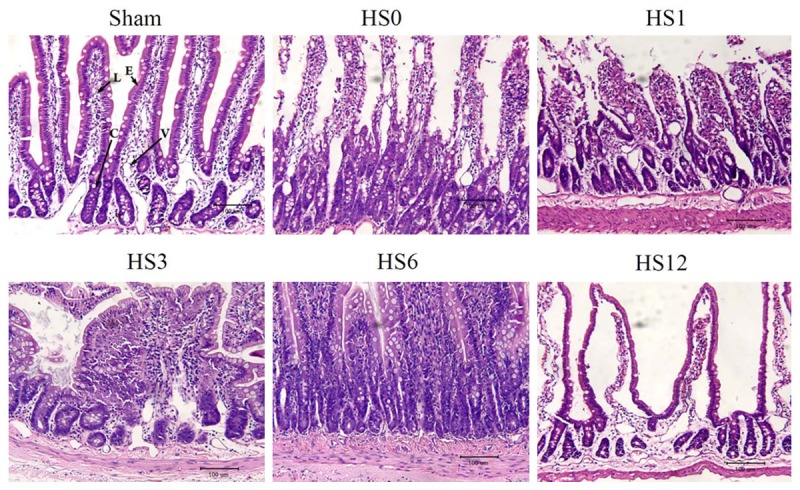
Representative photomicrographs (H&E stain) of the jejunum of rats before and after HS treatment at predetermined periods (HS0, HS1, HS3, HS6, and HS12). Lamina propria cells were slugged immediately after heat stress treatment. Villous epithelial cells were seriously damaged and showed morphology of cell death. Scale bar: 100 μm. V: Vessel; C: Crypt; L: Lacteal; E: Intestinal epithelium.
Immunohistochemical analysis of caspase-3 activity and significant AQP mRNA expression in the jejunum after HS treatment
The amount and distribution of AQPs with significant protein expression were quantified and evaluated by immunohistochemical staining. The expression pattern of caspase-3, a programmed cell death marker, was also assayed to quantitatively analyze the severity of cell damage (Figure 3).
Figure 3.
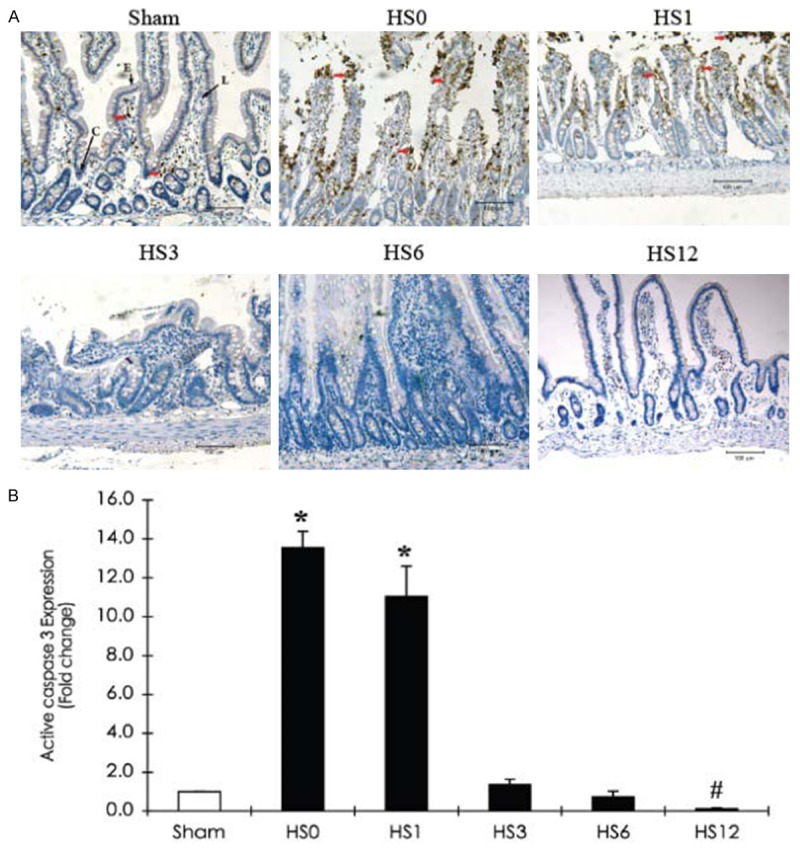
Immunohistochemical staining of caspase-3 expression in the jejunum of a sham rat and HS-treated rats (sacrificed at predetermined periods: HS0, HS1, HS3, HS6, and HS12). A. Representative photographs of active caspase-3 expression. Red arrows indicate positive caspase-3 cells. Scale bar: 100 μm. L: Lacteal; C: Crypt; E: Intestinal epithelium. B. Quantification of active caspase-3 expression obtained from 10 images at ×100 magnification (n=5). *Compared with sham, P < 0.05.
HS-induced AQP mRNA expression in rat jejunum
We performed semiquantitative RT-PCR to determine mRNA expression of 10 AQPs in the jejunum of rats with or without HS treatment. The samples were normalized to the amount of GAPDH, an internal standard. A representative agarose gel electrophoresis of RT-PCR products is shown in Figure 4A. A relative consistent finding was no detection of AQP2, 4, 6, and 9 in the jejunum of the sham and HS groups. Quantitative RT-PCR analyses showed that AQP1, 3, 5, 7, 8, and 11 were expressed in the jejunum of the sham or HS group, with variable expression levels during the recovery periods.
Figure 4.
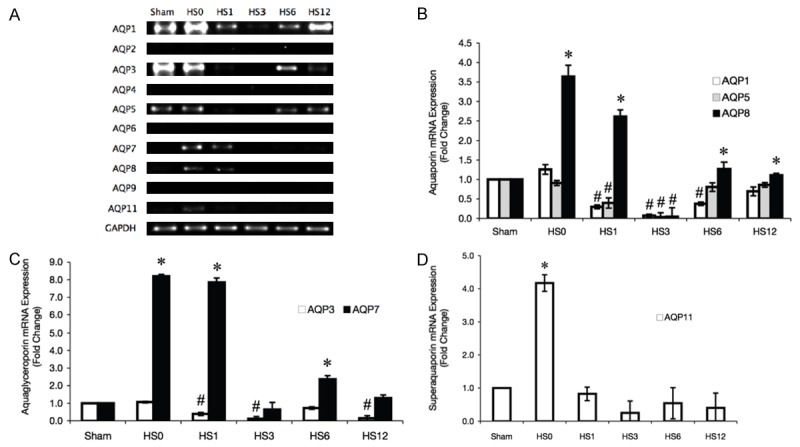
RT-PCR analysis of AQP mRNA expression in HS-treated rat jejunum tissue samples at predetermined time intervals. (A) Representative agarose gel electrophoresis of RT-PCR products. Quantification of (B) aquaporin, (C) aquaglyceroporin, and (D) super aquaporin subfamilies was performed by densitometrical scanning. The fold change was normalized to the GAPDH mRNA level. Data from at least 3 experiments are shown. *Compared with sham, P < 0.05.
Densitometrical analyses of the RT-PCR bands showed that the gene expression levels of AQP1, 7, 8, and 11 were higher at the instant recovery period (Figure 4B-D). Six genes (including AQP1, 3, 5, 7, 8, and 11) showed lower expression levels at 3 hrs after HS (HS3) compare with sham group, and revealed the significant different in AQP1, 3, 5 and 8.
HS-induced AQP protein expression in rat jejunum
Aquaporin subfamily
AQP1 is predominantly located in the villous vascular endothelium (Figure 5A), whereas AQP8 is expressed in the muscularis mucosae, lamina propria, and associated epithelial brush border (Figure 8A). HS induced a decrease in AQP1 levels in the villi but an increase in the crypt region (at HS0). From HS0 to HS12, sustained AQP1 expression was detected in lacteal regions, while AQP8 expression was persistent in the muscularis mucosae. At HS1, quantitative analysis revealed that AQP1 and 8 expression levels were more than 2.5-fold higher than those in the sham group (Figures 5B and 8B). No AQP5 was detected in the jejunum after HS treatment (data not shown).
Figure 5.
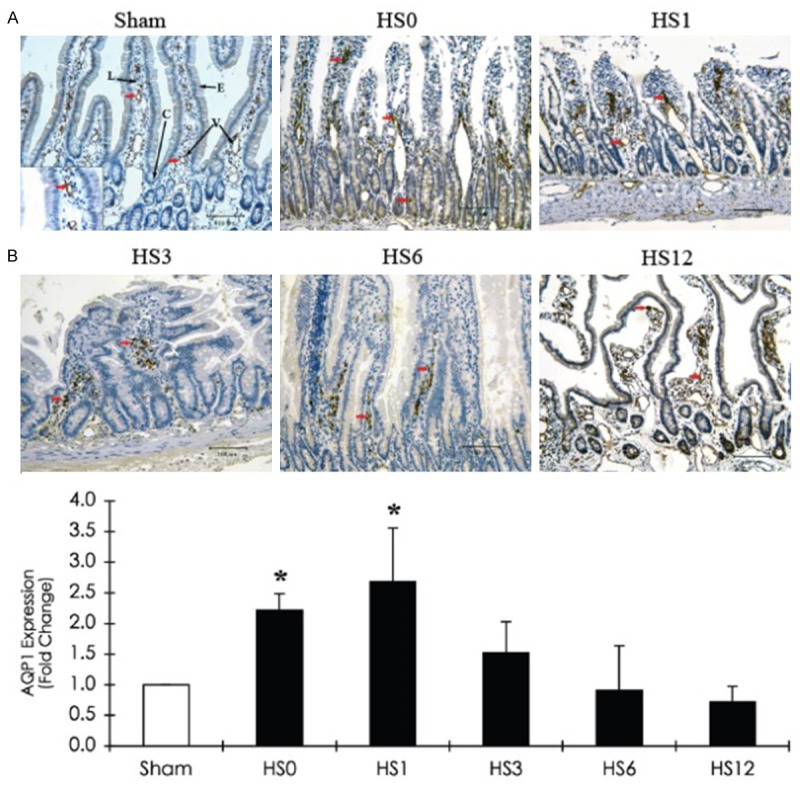
Immunohistochemical staining of AQP1 expression in the jejunum of a sham and HS-treated rats (sacrificed at predetermined periods: HS0, HS1, HS3, HS6, and HS12) A. Representative immunohistostaining photographs of AQP1 expression. Red arrows indicate positive AQP1 cells. Scale bar: 100 μm. V: Vessel; C: Crypt; L: Lacteal; E: Intestinal epithelium. B. Quantification of AQP1 expression obtained from 10 images at ×100 magnification (n = 5). *compared with sham, P < 0.05.
Figure 8.
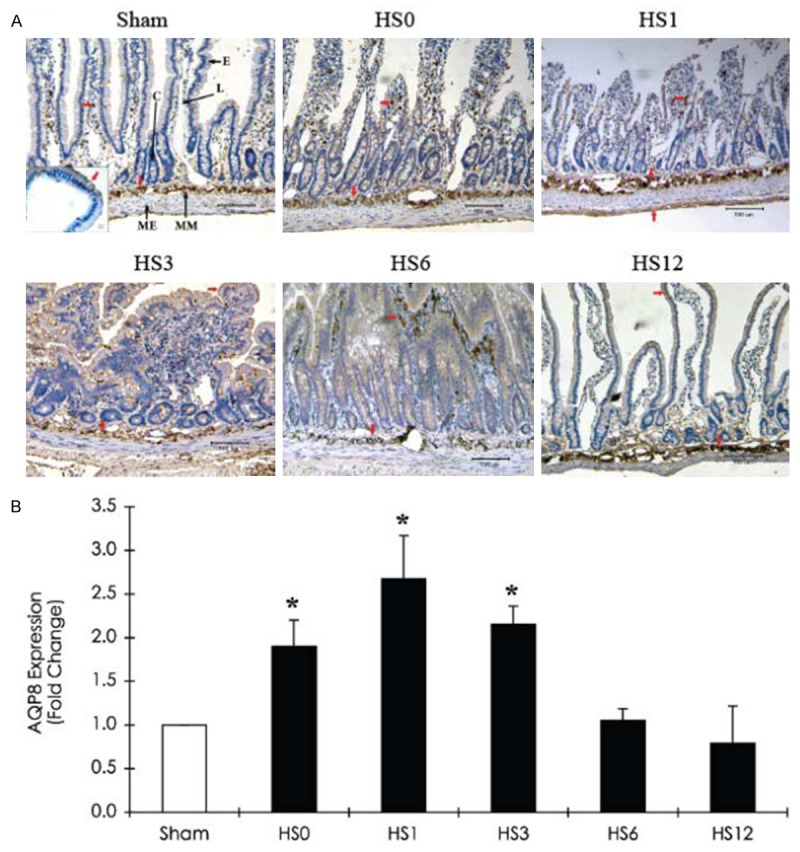
Immunohistochemical staining of AQP8 expression in the jejunum of a sham and HS-treated rats (sacrificed at predetermined periods: HS0, HS1, HS3, HS6, and HS12) A. Representative immunohistostaining photographs of AQP8 expression. Red arrows indicate positive AQP8 cells. Scale bar: 100 μm. L: Lacteal; C: Crypt; E: Intestinal epithelium; MM: Muscularis mucosae; ME: Muscularis externa. B. Quantification of AQP8 expression obtained from 10 images at ×100 magnification (n = 5). *Compared with sham, P < 0.05.
Aquaglyceroporin subfamily
The expression of AQP3, largely expressed in the lamina propria, was instantly induced in the crypt following HS treatment. Subsequently, protein levels declined at HS1 and demonstrated a 15-fold increase at HS3 (Figure 6). AQP7, located in vessel endothelial cells and villous epithelia, was observed to decrease in the villous epithelial cells at HS0 and HS1. The AQP7 level was then restored and increased at HS3 (Figure 7).
Figure 6.
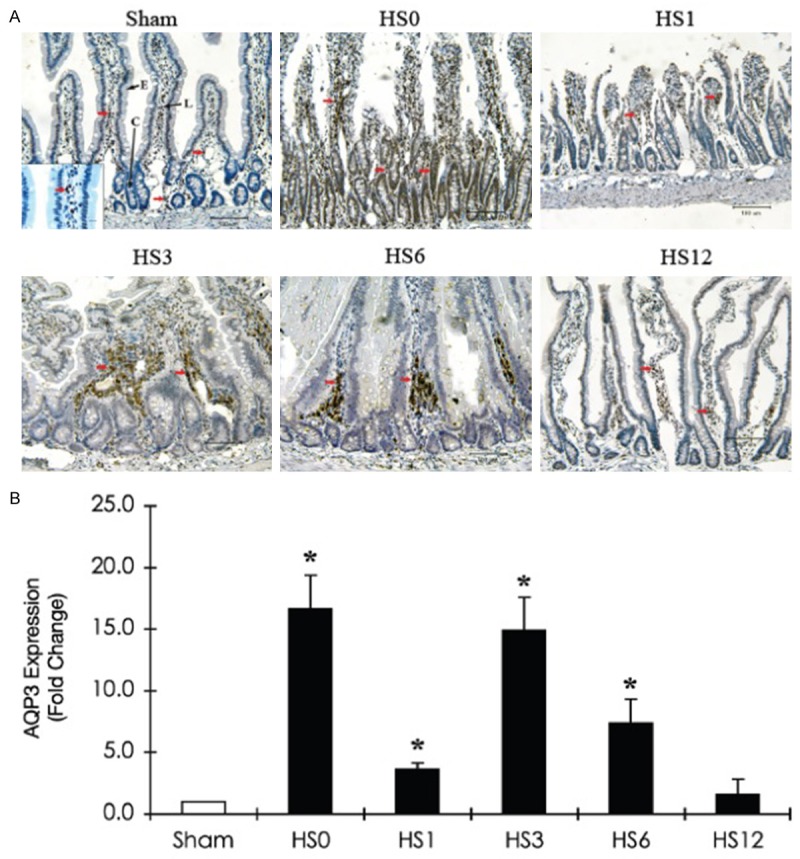
Immunohistochemical staining of AQP3 expression in the jejunum of a sham rat and HS-treated rats (sacrificed at predetermined periods: HS0, HS1, HS3, HS6, and HS12) A. Representative immunohistostaining photographs of AQP3 expression. Red arrows indicate positive AQP3 cells. Scale bar: 100 μm. L: Lacteal; C: Crypt; E: Intestinal epithelium. B. Quantification of AQP3 expression obtained from 10 images at ×100 magnification (n=5). *Compared with sham, P < 0.05.
Figure 7.
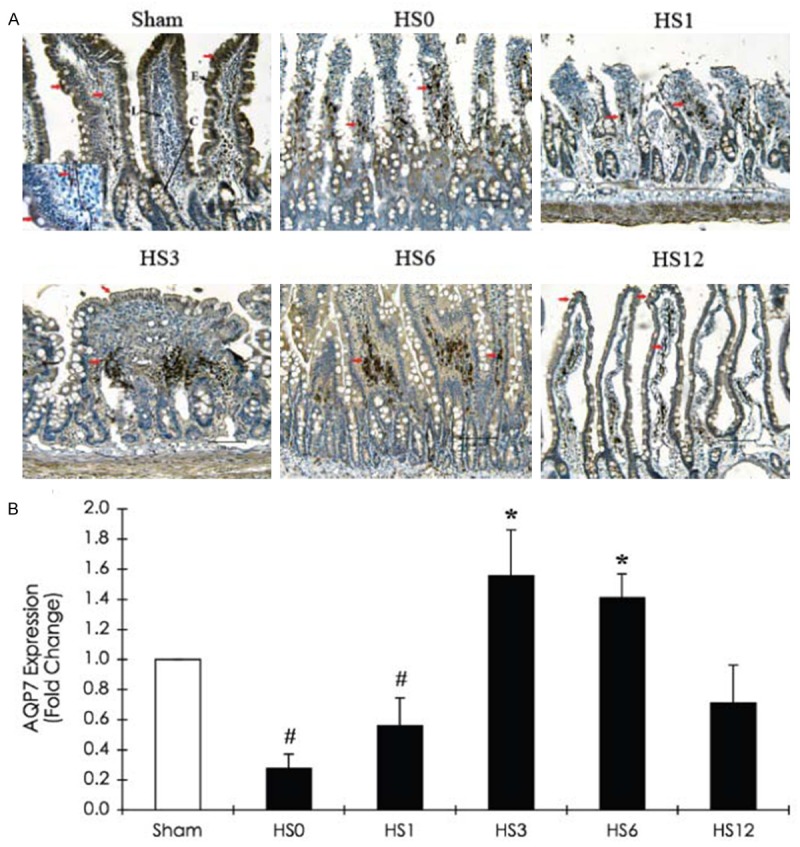
Immunohistochemical staining of AQP7 expression in the jejunum of a sham rat and HS-treated rats (sacrificed at predetermined periods: HS0, HS1, HS3, HS6, and HS12) A. Representative immunohistochemical staining photographs of AQP7 expression. Red arrows indicate positive AQP7 cells. Scale bar: 100 μm. L: Lacteal; C: Crypt; E: Intestinal epithelium. B. Quantification of AQP7 expression obtained from 10 images at ×100 magnification (n=5). *Compared with sham, P < 0.05.
Superaquaporin subfamily
Significant AQP11 expression was induced at the lamina propria immediately after HS treatment, but the expression levels returned to normal 3 h after recovery. The time course of the AQP11 expression level in HS-treated jejunum is illustrated in Figure 9.
Figure 9.
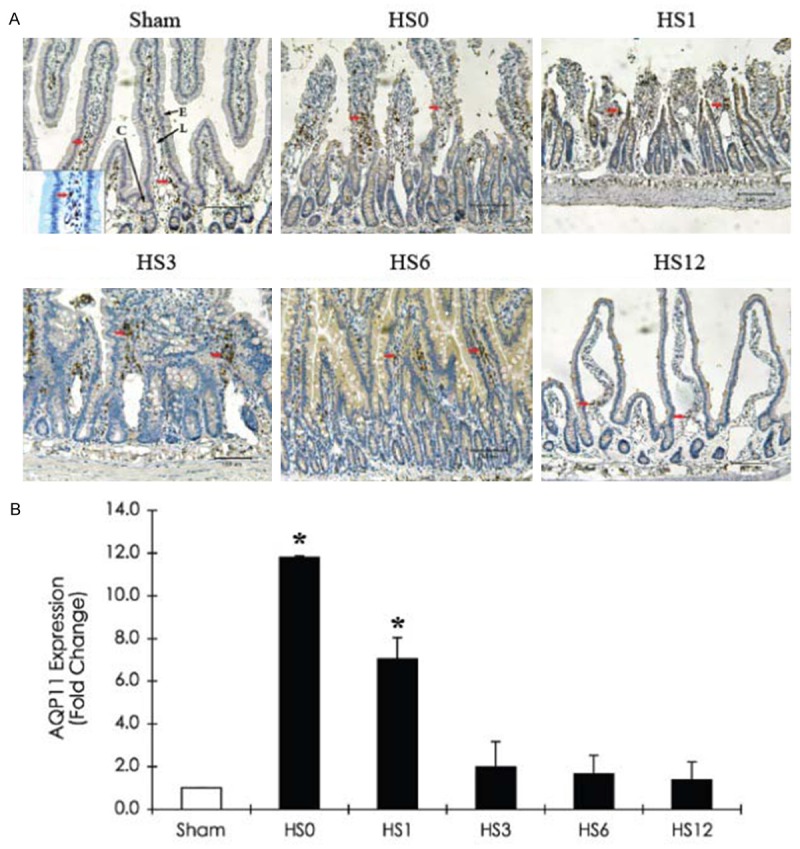
Immunohistochemical staining of AQP11 expression in the jejunum of a sham rat and HS-treated rats (sacrificed at predetermined periods: HS0, HS1, HS3, HS6, and HS12) A. Representative immunohistostaining photographs of AQP11 expression. Red arrows indicate positive AQP11 cells. Scale bar: 100 μm. L: Lacteal; C: Crypt; E: Intestinal epithelium; V: Vessel. B. Quantification of AQP11 expression obtained from 10 images at ×100 magnification (n = 5). *compared with sham, P < 0.05.
Discussion
Almost all fluids that pass through the gastrointestinal system are absorbed. Failure of intestinal absorption can lead to rapid dehydration and electrolyte imbalance. Heat stress leads to many physiological and metabolic changes, including altered intestinal membrane integrity [21-24]. The damage in the membranes results in increased permeability to toxic ions [23]. The response to water deficiency and HS may alter the synthesis and distribution of water channels. AQPs are ion and water channels that play an important role in osmoregulation and water reabsorption. Analysis of AQP mRNA expression revealed that only 6 genes were expressed (AQP1, 3, 5, 7, 8, and 11), which is consistent with previous findings. AQP1, 3, and 5 mRNA expression downregulated immediately after heat stress, while AQP7, 8, and 11 transcripts were upregulated. In this study, the intestine membranes of HS rats, with ambient temperature of 40.5°C maintained for 60 mins, had been destroyed with the pathological characteristics of enterocytes detached from their basal membrane.
Our results showed that the mRNA expression levels of both AQP1 and 3 declined gradually from HS0 to HS3 and returned to the previous levels from HS3 to HS12. At HS3, the mRNA expression levels of both AQP1 and 3 were equal to the trough levels. AQP1 immunohistochemical staining showed that the protein was predominantly located around the villous vessels and lymphatic ducts, whereas AQP3 was located at the lamina propria. Both AQP1 and 3 have been shown to play roles in angiogenesis, cell migration, and differentiation [25-30]. Whether the instant increase in AQP1 and 3 protein expression repaired intestinal membrane functionality merits further investigation. AQP8 mRNA expression increased by 2.5-fold at HS0 and HS1, whereas the intensity of AQP8 immunohistochemical staining increased by 2-fold. On the other hand, AQP7 mRNA expression increased by 8-fold, but the intensity of AQP7 immunohistochemical staining decreased at HS0 and HS1. AQP7, a channel for water, glycerol, and urea, has been identified from the villous epithelia, testis, and adipose tissues [11,31]. The change in mRNA expression of the uncoupling protein AQP7 may indicate that visceral adipose tissues were prone to damage after HS. In addition, a significant increase in AQP11 mRNA expression and immunohistochemical staining was observed in the early response to heat stress treatment. AQP11 has been shown to be localized in the endoplasmic reticulum and to be involved in unfolding protein transportation [32,33]. A previous study demonstrated that AQP11 could ameliorate damage to the proximal tubules in acute renal failure [34]; however, it remains unknown whether AQP11 has the same function in intestinal villi damage. This will be the subject of our future study.
Conclusions
mRNA expression of only 6 AQP genes (AQP1, 3, 5, 7, 8, and 11) was identified in the rat intestine. In response to HS, AQP1, 3 and 5 mRNA expressions was downregulated, while AQP7, 8, and 11 transcriptions was upregulated. However, the biological function of the AQP time-course protein expression needs further exploration in response to HS.
Acknowledgements
This work was supported by grants from the National Science Council (NSC 101-2314-B-038-058), Taipei Medical University (TMU99-AE1-B19) and from the Taipei Medical University-Wan Fang Hospital (100TMU-WFH07), Taiwan.
Disclosure of conflict of interest
None.
References
- 1.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 2.Brock-Utne JG, Gaffin SL, Wells MT, Gathiram P, Sohar E, James MF, Morrell DF, Norman RJ. Endotoxaemia in exhausted runners after a long-distance race. S Afr Med J. 1988;73:533–6. [PubMed] [Google Scholar]
- 3.Chao CM, Cheng BC, Chen CY, Lin MT, Chang CP, Yang ST. Proteomic analysis of hypothalamic injury in heatstroke rats. Proteomics. 2015;15:1921–34. doi: 10.1002/pmic.201400492. [DOI] [PubMed] [Google Scholar]
- 4.Du Y, Meng Y, Lv X, Guo L, Wang X, Su Z, Li L, Li N, Zhao S, Zhao L, Zhao X. Dexamethasone attenuates LPS-induced changes in expression of urea transporter and aquaporin proteins, ameliorating brain endotoxemia in mice. Int J Clin Exp Pathol. 2014;7:8443–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87(Suppl 14):E101–8. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Sun X, Tang J, Tang Y, Tong H, Wen Q, Liu Y, Su L. Intestinal inflammation and tissue injury in response to heat stress and cooling treatment in mice. Mol Med Rep. 2011;4:437–43. doi: 10.3892/mmr.2011.461. [DOI] [PubMed] [Google Scholar]
- 7.Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685–94. doi: 10.1097/00075197-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Ma T, Verkman AS. Aquaporin water channels in gastrointestinal physiology. J Physiol. 1999;517:317–26. doi: 10.1111/j.1469-7793.1999.0317t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du KX, Dong Y, Zhang Y, Hou LW, Fan DX, Luo Y, Zhang XL, Jia TM, Lou JY. Effects of dexamethasone on aquaporin-4 expression in brain tissue of rat with bacterial meningitis. Int J Clin Exp Pathol. 2015;8:3090–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi K, Koike S, Kondo S, Hara S, Tanaka Y. The role of a group III AQP, AQP11 in intracellular organelle homeostasis. J Med Invest. 2009;56(Suppl):312–7. doi: 10.2152/jmi.56.312. [DOI] [PubMed] [Google Scholar]
- 11.Laforenza U, Gastaldi G, Grazioli M, Cova E, Tritto S, Faelli A, Calamita G, Ventura U. Expression and immunolocalization of aquaporin-7 in rat gastrointestinal tract. Biol Cell. 2005;97:605–13. doi: 10.1042/BC20040090. [DOI] [PubMed] [Google Scholar]
- 12.Ishibashi K, Kondo S, Hara S, Morishita Y. The evolutionary aspects of aquaporin family. Am J Physiol Regul Integr Comp Physiol. 2011;300:R566–76. doi: 10.1152/ajpregu.90464.2008. [DOI] [PubMed] [Google Scholar]
- 13.Elkjaer ML, Nejsum LN, Gresz V, Kwon TH, Jensen UB, Frøkiaer J, Nielsen S. Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am J Physiol Renal Physiol. 2001;281:F1047–57. doi: 10.1152/ajprenal.0158.2001. [DOI] [PubMed] [Google Scholar]
- 14.Koyama Y, Yamamoto T, Tani T, Nihei K, Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K, Kihara I. Expression and localization of aquaporins in rat gastrointestinal tract. Am J Physiol. 1999;276:C621–7. doi: 10.1152/ajpcell.1999.276.3.C621. [DOI] [PubMed] [Google Scholar]
- 15.Laforenza U, Gastaldi G, Polimeni M, Tritto S, Tosco M, Ventura U, Scaffino MF, Yasui M. Aquaporin-6 is expressed along the rat gastrointestinal tract and upregulated by feeding in the small intestine. BMC Physiol. 2009;9:18. doi: 10.1186/1472-6793-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuzaki T, Tajika Y, Suzuki T, Aoki T, Hagiwara H, Takata K. Immunolocalization of the water channel, aquaporin-5 (AQP5), in the rat digestive system. Arch Histol Cytol. 2003;66:307–15. doi: 10.1679/aohc.66.307. [DOI] [PubMed] [Google Scholar]
- 17.Chang H, Shi YH, Talaf TK, Lin C. Aquaporin-8 mediates human esophageal cancer Eca-109 cell migration via the EGFR-Erk1/2 pathway. Int J Clin Exp Pathol. 2014;7:7663–71. [PMC free article] [PubMed] [Google Scholar]
- 18.Ramírez-Lorca R, Vizuete ML, Venero JL, Revuelta M, Cano J, Ilundáin AA, Echevarría M. Localization of aquaporin-3 mRNA and protein along the gastrointestinal tract of Wistar rats. Pflugers Arch. 1999;438:94–100. doi: 10.1007/s004240050884. [DOI] [PubMed] [Google Scholar]
- 19.Liu TT, Hu CH, Tsai CD, Li CW, Lin YF, Wang JY. Heat stroke induces autophagy as a protection mechanism against neurodegeneration in the brain. 2010;34:643–8. doi: 10.1097/SHK.0b013e3181e761c1. [DOI] [PubMed] [Google Scholar]
- 20.Tenckhoff S, Hollborn M, Kohen L, Wolf S, Wiedemann P, Bringmann A. Diversity of aquaporin mRNA expressed by rat and human retinas. Neuroreport. 2005;16:53–6. doi: 10.1097/00001756-200501190-00013. [DOI] [PubMed] [Google Scholar]
- 21.Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290:G204–12. doi: 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol. 2001;280:H509–21. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- 23.Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87:E101–8. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- 24.Soderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2001;280:G7–13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- 25.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2008;86:221–31. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 26.Tu XH, Huang SX, Li WS, Song JX, Yang XL. Mesenchymal stem cells improve intestinal integrity during severe acute pancreatitis. Mol Med Rep. 2014;10:1813–20. doi: 10.3892/mmr.2014.2453. [DOI] [PubMed] [Google Scholar]
- 27.Monzani E, Bazzotti R, Perego C, La Porta CA. AQP1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PLoS One. 2009;4:e6167. doi: 10.1371/journal.pone.0006167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–92. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 30.Verkman AS. Physiological importance of aquaporin water channels. Ann Med. 2002;34:192–200. [PubMed] [Google Scholar]
- 31.Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, Marumo F, Sasaki S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem. 1997;272:20782–6. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- 32.Morishita Y, Matsuzaki T, Hara-chikuma M, Andoo A, Shimono M, Matsuki A, Kobayashi K, Ikeda M, Yamamoto T, Verkman A, Kusano E, Ookawara S, Takata K, Sasaki S, Ishibashi K. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol. 2005;25:7770–9. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada S, Misaka T, Tanaka Y, Matsumoto I, Ishibashi K, Sasaki S, Abe K. Aquaporin-11 knockout mice and polycystic kidney disease animals share a common mechanism of cyst formation. FASEB J. 2008;22:3672–84. doi: 10.1096/fj.08-111872. [DOI] [PubMed] [Google Scholar]
- 34.Morishita Y, Sakube Y, Sasaki S, Ishibashi K. Molecular mechanisms and drug development in aquaporin water channel diseases: aquaporin superfamily (superaquaporins): expansion of aquaporins restricted to multicellular organisms. J Pharmacol Sci. 2004;96:276–9. doi: 10.1254/jphs.fmj04004x7. [DOI] [PubMed] [Google Scholar]


