Abstract
Midkine (MK) is a heparin-binding growth factor involved in growth, survival, migration, and differentiation of various target cells and dysregulation of MK signaling is implicated in a variety of inflammatory diseases and cancers. Although MK has been reported to act on endothelial cells and to have proangiogenic effects, the exact role of MK in angiogenesis is poorly defined. Progranulin (PGRN) is a secreted glycoprotein that functions as an important regulator of development, cell cycle progression, cell motility, tumorigenesis, angiogenesis. We screened the PGRN from the hepatic cancer cell cDNA library which was interacted with MK, and confirmed the binding by co-immunoprecipitation and co-localization. During our study, the interaction between MK and PGRN had the important role on the HUVECs proliferation, migration, and tubulogenesis, which indicated the interaction may regulate the angiogenesis, also the in vivo angiogenesis model CAM showed the promotion effect stimulated by MK and PGRN. These findings provide the first evidence linking the association of MK and PGRN and may identify the mechanism of MK during the hepatocellular carcinoma angiogenesis.
Keywords: Midkine, progranulin, protein-protein interaction, hepatocellular carcinoma, angiogenesis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common human tumors worldwide [1]. It is a popular malignancy with more angiogenesis, rapid growth, early metastasis and high mortality. Curative options for HCC include resection and liver transplantation, but prognosis after resection remains unsatisfactory due to a high incidence of postoperative recurrence. HCC is insensitive to chemotherapeutic effects are not satisfactory [2]. Therefore, new therapies with high efficacy, low toxicity, and better specificity for HCC should be considered.
Midkine (MK) is a member of the heparin-binding growth factor family and has been identified as the product of a retinoic acid-responsive gene [3], it is overexpressed in hepatocellular carcinoma and can promote HCC cells’ proliferation and invasion. It also involved in the angiogenesis and tumorigenesis of hepatocellular carcinoma [4], during the previous studied, we found the antisense oligonucleotide (ASODN) that targets MK can suppress the growth of hepatic cancer cells and angiogenesis in nude mice [5,6]. Additionally, siRNA or an ASODN that targets MK inhibits neointima formation [7] and renal injury after ischemia [8], but the cellular signaling receptors during hepatocarcinoma angiogenesis for MK have not yet been identified and characterized. Study of the molecular basis of MK signal transduction pathways in hepatic cancer cells would further enhance the understanding of their roles in the development and growth of hepatocellular cacinoma.
Methods and materials
Cell lines and culture
Adherent 293T cells (ATCC: CRL-11268, USA) and HepG2 cells (ATCC: HB-8065, USA) were maintained in Dulbecco’s modified Eagle’s medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA). Human Umbilical Vein Endothelial cells (HUVECs) (Cascade Biologics: C-003-5C) were cultured in M200 medium supplemented with Low Serum Growth Supplement (LSGS) (Cascade Biologics, USA).
Plasmid constructs
Yeast expression vectors pDBLeu and pEXP-AD502 (both from Invitrogen, USA) are fusion vectors for the linkage of proteins to the Gal4 DNA binding domain and to the Gal4 transactivation domain, respectively. The fragment encoding the full length of MK was amplified by polymerase chain reaction (PCR) and cloned in frame into the SalI/EcoRI sites of pDBLeu (pDBLeu-MK) to serve as bait in the screening assay.
The mammalian expression vector pcDNA3 (Invitrogen, USA) was used to produce the MK and PGRN proteins. The cDNA fragments encoding human full length MK and PGRN was amplified by PCR and subcloned in frame into the HindIII/BamHI sites of pcDNA3 to produce plasmids pcDNA3-MK and pcDNA3-PGRN, which express the proteins of MK and PGRN in the 293T cells.
The recombinant proteins YFP-PGRN and RFP-MK were produced by pEYFP-N1 (Invitrogen, USA) and pDsRed-Monomer-C1 (BD Biosciences Clontech, UK) vectors. The PCR products were subcloned in frame into the XhoI/HindIII sites of pEYFP-N1 and SalI/BamHI sites of pDsRed-Monomer-C1, and the fusion proteins were expressed in the HepG2 cells.
All constructs were verified by nucleic acid sequencing, subsequent analysis was performed using BLAST software (available on the World Wide Web at ncbi.nlm.nih.gov/blast/).
Yeast two-hybrid (Y2H) library screen
Plasmid pDBLeu-MK was used as bait to screen the Y2H human hepatic cancer cells cDNA library according to the PROQUEST™ Two-Hybrid System manufacturer’s protocol (Invitrogen, USA). Briefly, the cDNA library which was inserted into pEXP-AD502 vector and the pDBLeu-MK vector, co-transformated into the yeast MAV203 strain. Yeast cells which contained pDBLeu-MK and pEXP-AD-X cDNA library were laid on SC-Leu-Trp-His+25 mM 3-AT plates. After cultivated at 30°C for 60-72 h, the obvious positive colonies were acquired. Replica plating were performed by gently pressing the autoclaved filter discs onto SC-Leu-Trp-His plates to transfer the colonies to YPAD plates and selection plates (SC-Leu-Trp plates and SC-Leu-Trp-Ura plates), the former were incubated for X-Gal assay to screen positive colonies, and the latter were for further screening through lacking off Uracil. The fresh colonies of 5 yeast control strains from the glycerol stocks onto SC-Leu-Trp plates were prepared as parallel controls. Finally, the selected plasmids of positive colonies were amplied in E.coli DH5α cells, then plasmids were digested by SalI and NcoI, and the insertion fragmentations were recorded. The positive colonies were sequenced, and the genes were classified according to sequence homology analysis in GenBank (http://blast.ncbi.nlm.nih.gov/). All of the candidate protein coding plasmids and pDBLeu empty vector were co-transformated into yeast cells to identify the self-activity and to re-identify the activity.
Assay of protein-protein interactions using the Y2H system
The full-length MK and PGRN gene were cloned in frame into pDBLeu and pEXP-AD502 respectively. The pDBLeu-MK and pEXP-AD502-PGRN were co-transformated in to MAV203 yeast strains, and then laid on SC-Leu-Trp plates. The co-transformated colonies were then identified on the SC-Leu-Trp-His+25 mM 3-AT plates and analyzed the X-gal activity respectively. The pDBLeu vector+pEXP-AD502-PGRN and the pDBLeu-MK+pEXP-AD502 vector combinations were co-transformated as negative control, while the fresh colonies of 5 yeast control strains were prepared as positive controls.
Assay of protein-protein interactions using the co-immunoprecipitation system
Human 293T cells (5 × 106 cells) were co-transfected with 10 μg of total plasmid DNA using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Cells were harvested at 24 h post-transfection and lysed in 1 ml of immunoprecipitation lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA) containing 0.1 mM phenylmethylsulfonyl fluoride, 1 μg/ml pepstain, 1 μg/ml leupeptin, and 1 μg/ml antipain. Extracts were incubated on the ice for 30 min, and the supernatants were collected after 30 min centrifugation at 4°C (Therom). Antibody (1 μg) was bound to protein A-Sepharose (Santa Cruz, California, CA) by incubation at 4°C for 2 h with rocking. The conjugates were mixed with cell lysis (500 μg of total protein) and incubated overnight at 4°C with rocking. Unbound proteins were removed by extensive washing with lysis buffer. Protein complexes bound to the conjugated antibody were separated by denaturing polyacrylamide gel electrophoresis and subjected to immunoblotting. Anti-MK (N-17) polyclonal antibody (Santa Cruz, CA) and anti-acrogranin (N-19) polyclonal antibody (Santa Cruz, CA) were used for immunoprecipitation, anti-MK monoclonal antibody (Abcam, Cambridge, UK), anti-progranulin monoclonal antibody (R&D system, Minneapolis, MN, CA), anti-rabbit IgG (H+L) HRP antibody (Southern Biotech, CA) and anti-mouse IgG HRP antibody (R&D system, Minneapolis, MN) were used for immunoblotting. Detection was by enhanced chemiluminescence (ECL, Bio-Rad, US).
Co-localization assays of midkine and progranulin
Intracellular co-localization experiments were carried out in HepG2 cells. Cells were grown on collagen-coated cover slips in tissue culture plates to a confluency of 30-40% and were transfected with the plasmids indicated using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. The expression, localization, and co-localization of the fluorescence-tagged proteins were directly analyzed using the OLYMPUS BX51 fluorescence microscope. Digital images were processed using Adobe Photoshop.
Cell proliferation assay
Human umbilical vein endothelial cells (HUVECs) were plated at a density of 5 × 104/ml into the 96-well culture plates in M200 medium supplement containing 2% (v/v) fetal bovine serum, 1 μg/ml hydrocortisone, 10 ng/ml human epidermal growth factor, 3 ng/ml basic fibroblast growth factor, 10 μg/ml heparin, incubated for 24 h, and subsequently serum-starved for 24 h in serum free M200 medium. HUVECs were either left untreated or challenged with MK, PGRN and MK+PGRN at different concentration respectively (100 ng/ml and 500 ng/ml). As a positive control, 10 ng/ml VEGF-A was added. Cells were incubated for 48 h and 72 h, 10 μl MTT ((3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (5 mg/ml) reagent was added to the wells and assay was performed after 4 h at 37°C. The absorbance of this colored solution can be quantified by measuring at 490 nm by a spectrophotometer.
Cell migration assay
The migration analysis was performed according to the QCM™ 24-Well Colorimetric Cell Migration Assay manufacturer’s procedure (CHEMICON, MA, USA). HUVECs were serum starved for 24 h. Cells (5 × 105 cells/ml) were then seeded in Boyden chambers (upper chamber), 300 μl per chamber. Lower chambers contained 500 μl of serum free medium or MK and PGRN treatment respectively or co-treatment (500 ng/ml). After 24 h, the cells in the upper chambers were removed, whereas the cells that migrated to the lower chamber were counted after fixation and staining in cell stain solution for 20 min, dipped the insert into a breaker of water several times to rinse, removed non-migratory cells layer from the interior of the insert, lysed the migrated cells in 200 μl of extraction buffer for 15 min at room temperature. Transfer 100 μl of the dye mixture to a 96-cell microtiter plate suitable for colorimetric measurement at 560 nm.
Wound-healing migration assay
Fibrin gel in vitro angiogenesis assay. HUVECs were seeded in the 96-well plates (1 × 104 cells/100 μl), and staved in SFM for 12 h, then the plates were scratched with a thin disposable tip to generate a wound in the cell monolayer. The cells were incubated for additional 24 h in SFM without or with MK and PGRN (20 ng/ml, 100 ng/ml and 500 ng/ml). Cells were analyzed and photographed with a Leica inverted microscope at different time points (0 h, 6 h, 12 h, 24 h).
Tubulogenesis by fibrin gel in vitro angiogenesis assay
The tubulogenesis analysis was performed according to the fibrin gel in vitro angiogenesis assay manufacturer’s procedure (CHEMICON, MA, USA). HUVECs were harvested and counted by standard methods, centrifuged and responded at a final concentration of 5 × 104 cells/ml in SFM without or with MK and PGRN protein (500 ng/ml). Dispensed 30 μl fibrinogen solution into the 96-well plate, gently shook, then added 20 µl per well thrombin to the fibrinogen solution, shaked the plate gently immediately, incubated the plate at 37°C for 30 min to polymerize. The prepared cells were seeded onto the fibrin gel (100 μl/well), incubated at 37°C overnight, removed the culture medium completely, added 30 μl per well fibrinogen in each medium to indicated wells of 96-well plat, shook the plate gently, then added 20 μl per well thrombin to the fibrinogen solution, shook the plate gently immediately, and incubated the plate at 37°C for 5 min, added 100 μl per well of SFM without or with treatment. Images of tubular structures were taken after 24 h incubation, using a Leica inverted microscope.
Angiogenesis by chick chorioallantoic membrane (CAM) assay
Angiogenesis analysis in chick chorioallantoic membranes (CAM) was performed as previously described [9]. The fertilized white leghorn chicken eggs were purchased from Wuhu avian breeding center (Huzhou, China), they were incubated under routine conditions (37°C and 70% humidity). On day 6 of development, a square window was opened in the egg shell, after removal of 3.5 ml of albumen allowing detachment of the embryo from the eggshell. The window was sealed with tape, and the eggs were returned to the incubator for 24 h. On day 7 of development, gelatin sponges were cut into a size of 1 mm3 and placed on the top of the CAM under sterile conditions. The sponges were then absorbed with 10 μl of PBS without or with MK and PGRN protein (100 ng/egg), the VEGF (50 ng/egg) was used as the positive control, each group had 8 eggs. CAMs were examined daily until day 10 of development, and then they were fixed in 4% formaldehyde and photographed with a stereomicroscope equipped with a CCD (Pixera EL600, USA). Only CAMs still alive at day 10 were included in this analysis, and then they were all sacrificed by fixing in 4% formaldehyde solution. All of the experiments were carried out according to the standards of animal care as outlined in the Huzhou Central Hospital’s guide for the Care and Use of Laboratory Animals.
Results
Isolation of PGRN as a MK binding partner
To better understand the biological functions of MK, we performed a yeast two hybrid screen. Briefly, we linked the MK to the Gal4 DNA-binding domain in the plasmid pDBLeu. We used the respective constructs as bait to screen a library of human hepatic cancer cells cDNA expressed as fusion proteins to the Gal4 activation domain in the vector pEXP-AD502. A yeast two hybrid human hepatic cancer cells cDNA library was screened with the construct encoding MK. We screened ~1.0 millioon colonies and identified 43 colonies that activated with MK gene. Further tests involved the retransformation of yeast with the purified target plasmids and bait. Only 6 of the original 43 yeast clones expressed hybrid proteins that still interacted with the MK bait (Data not show here). One of the positive colonies encoded the growth factor progranulin (accession number NM_002087.2).
Confirmation of the interaction between PGRN and MK in yeast
The yeast two hybrid assay was repeated to verify the interaction between the MK and the PGRN. The plasmid encoding the MK linked to the Gal4 DNA-binding domain and the plasmid encoding PGRN fused to the Gal4 activation domain were used to co-transformate into the yeast. Like the yeast control strain A, B, C, D, E as the positive control, our assays indicated that MK interacted with PGRN in yeast, based on the activation of the LacZ reporter gene and the growth phenotypes on SD-Leu-Trp-His+ 25 mM 3-AT plates (Figure 1).
Figure 1.
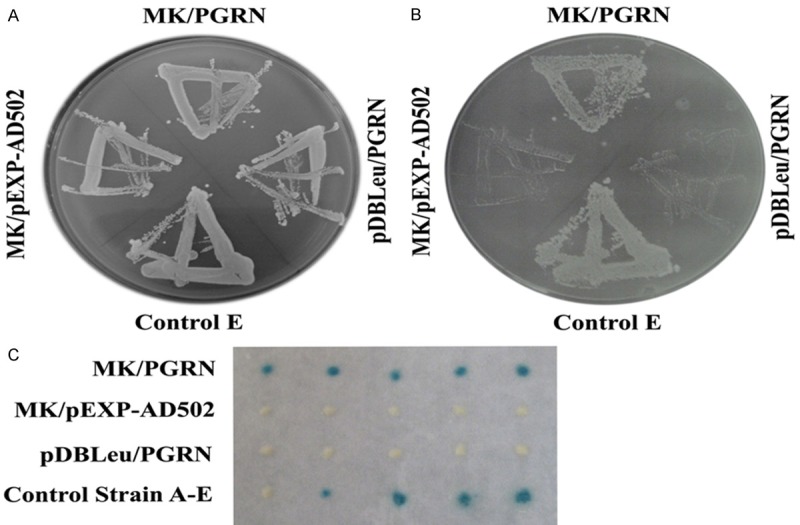
Binding of MK and PGRN in yeast. Show is the yeast two-hybrid assay to test the interaction of PGRN which was fused to the Gal4 activation domain and the MK which was fused to the Gal4 DNA-binding domain. Each pair of plasmids, as indicated, were co-transformated into yeast strain MAV203. Yeast transformation were selected on SD-Leu-Trp plates, all of the pairs were growth well on the SD-Leu-Trp plates (A), then tested for growth inhibition on plates containing 25 mM 3-AT (B), the MK/PGRN group and the positive control (strain control E) grow well while the negative control (MK/pEXP-AD502 and pBDLeu/PGRN) did not grow (B). The X-gal activity also have been tested, the MK+PGRN group show the blue colour while the negative control(MK/pEXP-AD502 and pBDLeu/PGRN) did not show blue color (C), the strain A-E were performed as positive control.
Binding of PGRN to MK
The in vivo interaction between MK and PGRN was verified using a co-immunoprecipitation assay in order to determine whether these two proteins are bound in native human 293 T cells. For the co-immunoprecipitaion assays, the cell extracts were incubated with either anti-MK antibody or control IgG, and the immunoprecipitated complexes were subjected to a reducing SDS-PAGE and detected with anti-PGRN antibody. A specific PGRN band was present in the immunoprecipitated complexes brought down by anti-MK antibody but not control IgG antibody, demonstrating that PGRN specifically binds to the MK in vivo (Figure 2A). The binding analysis also performed by anti-PGRN antibody to precipitate the immunoprecipitated complexes, and detected by anti-MK antibody, the specific MK band was also present in the immunoprecipitated complexes brought down by anti-PGRN antibody but not control IgG antibody (Figure 2B).
Figure 2.
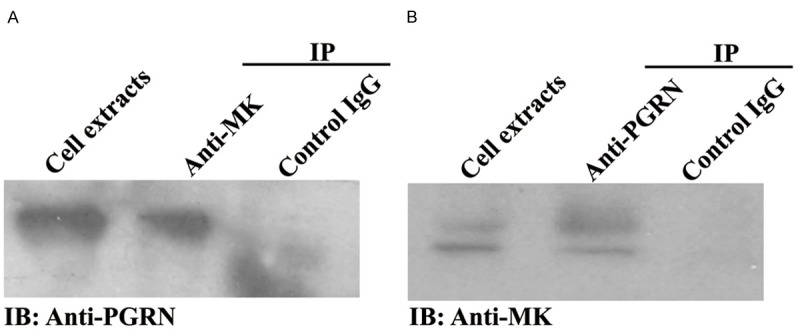
Confirmation of interaction between MK and PGRN by co-immunoprecipitation. The 293T cells were co-transfected with MK-pcDNA3 and PGRN-pcDNA3 plasimids, 24 h later, the cells were lysed with lysis buffer for co-immunoprecipitation, collected the supernatant, the PGRN was pulled down with anti-MK antibody, and detected with anti-PGRN antibody (A), while the MK was pulled down with anti-PGRN antibody, and detected with anti-MK antibody (B), both MK and PGRN had the specific band, and the cell extracts was loaded as the positive control, the control IgG was used as the negative control for the co-immunoprecipitaion.
PGRN and MK co-localized in the HepG2 cells
Next, we examined the subcellular localization of MK and PGRN to determine whether these two proteins overlap in the same cell. We first co-transfected human HepG2 cells with plasmids encoding GFP-linked PGRN and RFP-fused MK. As revealed in Figure 3, in the living human HepG2 cells, PGRN is clearly expressed and overlapped with MK. These findings are in agreement with the physical interactions detected in the yeast two-hybrid system and also confirmed by co-immunoprecipitaion assays, and suggest that in HepG2 cells, the growth factor MK may be mediated, at least in part, by the PGRN protein.
Figure 3.
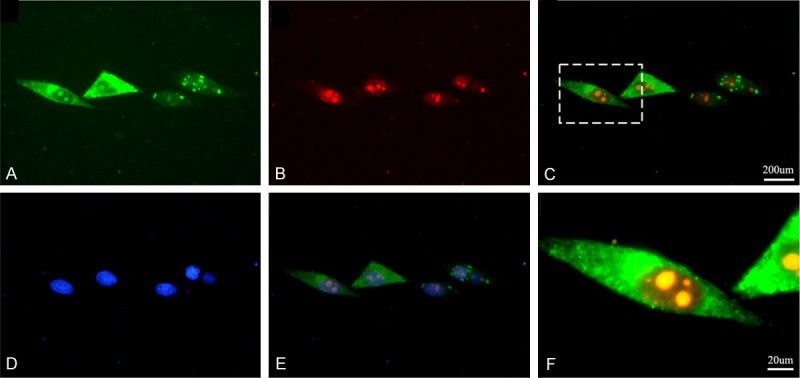
MK and PGRN co-localize in the HepG2 cells in vivo. The MK-RFP and the PGRN-GFP fusion protein expression plasmids were co-transfected into the HepG2 cells, the expression of PGRN-GFP (A) and MK-RFP (B) was detected by fluorescence microscopy respectively. The PGRN-GFP and MK-RFP were overlapped in the HepG2 cells (C), the yellow show the overlap areas, the (F) shows the high magnification of overlapped areas. The cells also stained with Hochest33258 to show the nuclear (D, E).
Interaction of PGRN and MK stimulates the proliferation, migration and tubulogenesis of HUVECs
To elucidate the physiological effect of MK and PGRN interaction in angiogenesis, we used HUVECs as a model system. We initially performed an in vitro proliferation assay on the 96-well plates. Cells were serum-starved of 24 h and either left untreated or challenged with MK, PGRN, or both growth factors together. As a positive control, cells were stimulated with VEGF (10 ng/ml). We observed almost a three-fold growth induction of HUVECs in the presence of MK and PGRN, respectively.
To investigate whether the interaction of MK and PGRN plays any role in migration of HUVECs, we performed the in vitro “wounding healing” motility assay. The HUVECs were plated at high density in complete medium, after 24 h starvation in serum free medium, confluent HUVECs were wounded and incubated for additional 24 h in serum free medium without or with MK and PGRN protein treatment. In contrast to control, progranulin and midkine both evoked a substantial migration of the cells into the denuded area, and the co-treatment of MK and PGRN indicated a significant promotion of migration, that may suggest the interaction of MK and PGRN have the effectively promote migration of HUVECs. Also, we performed the trans-well migration analysis, the results was consistent with the wound healing.
For the in vitro angiogenesis assay, we cultured the HUVECs under the 3D fibrin matrix, after 24 h incubation in serum free medium without or with MK and PGRN protein treatment, we found the MK and PGRN can promote the tubulogenesis, and the co-treatment of MK and PGRN have the cooperation effectivity.
Interaction of PGRN and MK promotes the angiogenesis in CAM
The chick chorioallantoic membrane (CAM) assay is a widely used in vivo model that recapitulates the multiple physiological, biochemical and cellular processes that occur during the angiogenic cascade and is relatively rapid for systematically evaluating antiangiogenic agents. Incubation of MK and PGRN on chorioallantoic membranes of 7-day-old chick embryos stimulated an angiogenic response that could be readily photographed after 72 h by stereomicroscopy. MK and PGRN were applied at the dose of 100 ng per egg, the MK and PGRN could stimulate the angiogenesis respectively, but the angiogenesis effect co-induced by MK and PGRN was increased (Figure 7), contrast by the MK and PGRN stimulation respectively, the VEGF was setup as the positive control while the PBS was set as the negative control.
Figure 7.
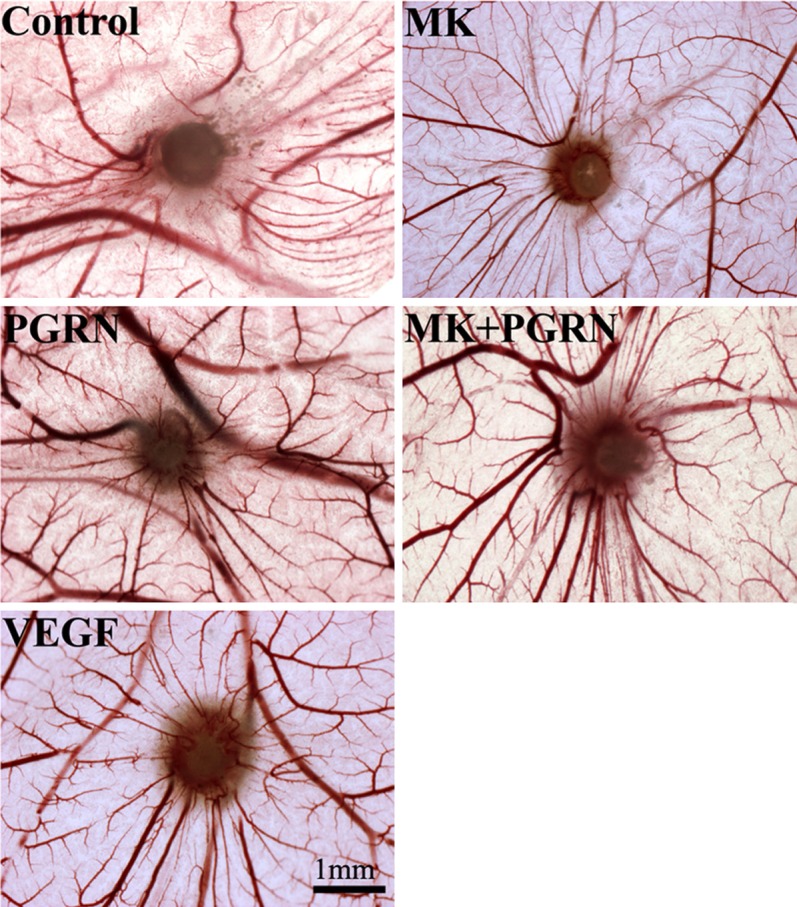
CAM model to investigate the angiogenesis stimulated by MK and PGRN. For angiogenesis analysis, eggs were treated with MK, PGRN, and MK/PGRN proteins (100 ng), the VEGF was used as positive control, while the PBS was used as the negative control.
Discussion
Angiogenesis is the process by which new blood vessels are formed [10]. It is a complex multistep process involving extracellular matrix remodeling, endothelial cell (EC) migration and proliferation, capillary differentiation, and anastomosis [11]. Angiogenesis is observed only transiently during particular circumstances such as reproduction, development, and wound healing. Sustained angiogenesis is characteristic of several pathological conditions including tumor growth [12-15]. Indeed, angiogenesis is essential for tumor growth and it is the interaction of neoplastic with this neovasculature that results in dissemination and metastasis. Endothelial cell proliferation and directed migration are regulated by ploypeptide growth factors and cytokines produced by both the tumor and the host responses to the tumor [16-19]. The variable expression of integrins modulates both the adherence of endothelial cells to components of the basement membrane and the three-dimensional assembly of capillary microvessels [20,21].
Growth factors play pivotal roles in intercellular communication, and eventually in tissue building and remodeling. The growth factor midkine (MK), also known as neurite growth-promoting factor 2, promoting the growth, survival, migration and differentiation of various cell types [22,23]. Several reports suggest that MK is a ligand for symdecan-3 [24], anaplastic lymphoma kinase [25], low-density lipoprotein receptor-related protein [26], α4β1- and α6β1-integrin [22], and the receptor-like protein-tyrosine phosphatase-zeta [27]. Clinical reports indicate that MK is overexpressed in a variety of human carcinomas and that high levels of MK in patient serum correlate with poor clinical outcome [28]. Furthermore, high MK expression levels in primary human tumors were shown to correlate with increased tumor angiogenesis [29]. It has also been reported that in xenografts from two cancer cell lines of epithelial origin, overexpression of the MK gene can cause increased angiogenicity, resulting in enhanced malignant proliferation of cancer cells [30]. Taken together, an increasing number of studies suggest that MK is a candidate molecular target for the therapy for carcinomas. However, the MK’s biologic significance in angiogenesis in general and its mechanism of action in ECs in particular still remains to be clarified.
During our experiments, we use yeast two-hybrid screening to identify protein interaction partners of MK, screening the yeast expression cDNA library using the MK as bait and identified the PGRN, a growth factor that has been previously described in various tumor cells as a direct binding protein of MK.
PGRN is a pluripotent growth factor that mediates cell cycle progression and cell motility, it is a secreted growth factor with high molecular weight that is involved in various biological and pathological processes, including mesothelial differentiation [31], sexual differentiation of the brain [32], macrophage development [33], rheumatoid arthritis and osteoarthritis [34], and wound response and tissue repair [35]. During wound repair it promotes granulation and neovascularization. It is also highly expressed in aggressive cancer cell lines and clinical specimens including breast, ovarian, and renal cancers as well as gliomas [36]. In experimental systems it confers an aggressive phenotype on poorly tumorigenic epithelial cancer cells [37]. Given its actions in wound repair and tumorigenesis PGRN may prove a useful clinical target, both for prognosis and for therapy.
We found that MK and PGRN co-localized in the human Hela cells (Figure 3), and the MK and PGRN complexes could be precipitate by MK or PGRN antibody respectively (Figure 2). These results suggested an in vivo association between MK and PGRN. In line with the previous findings, PGRN interacted with MK in the hepatic cancer cells which was detected by Yeast Two Hybrid system (Figure 1).
Introduced by the the MK alone showed promotion effect on tumor cell growth, while the PGRN also could significantly stimulate tumor cells proliferation. The previously studies found MK and PGRN also can stimulate the endothelial cells proliferation. Our findings show the significant proliferation and migration effect while the endothelial cells were stimulated by MK and PGRN simultaneously (Figures 4, 5). The MK and PGRN also could effect the tubulogenesis, the co-stimulation effect was significantly increased (Figure 6). The in vivo CAM model show the angiogenesis effect was induced by MK and PGRN co-stimulation (Figure 7). These finding suggests that the MK/PGRN complex may play important role during the angiogenesis process. Although the molecular mechanisms underlying the role of MK/PGRN interaction in the angiogenesis remain unclear, it is speculated that PGRN may acts as the co-factor of the MK receptor and may present PGRN to its receptor, followed by the activations of MK-mediated signal transduction and gene regulation pathways.
Figure 4.
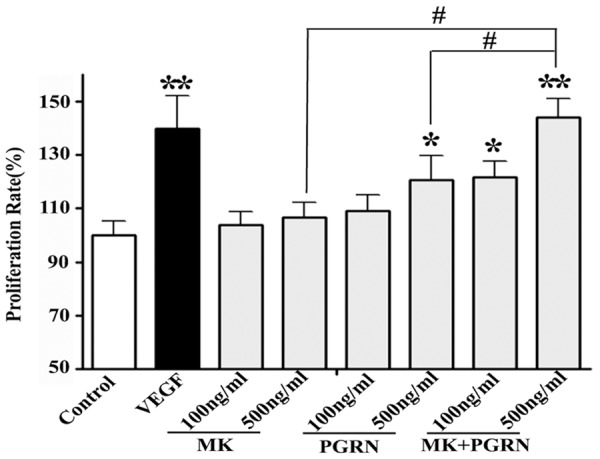
The interaction of MK and PGRN promote the HUVECs proliferation. For in vitro cell growth assay, HUVECs were incubated in SFM and SFM with MK, PGRN, and MK/PGRN proteins (50 ng/ml and 100 ng/ml), the VEGF was used as positive control. The quantification of MTT assay was analyzed at the 24 h, 48 hours and 72 h.
Figure 5.

The interaction of MK and PGRN stimulate the HUVECs migration. (A) For in vitro wound-healing motility assay, HUVECs were incubated in SFM and SFM with MK, PGRN, and MK/PGRN proteins (20 ng/ml, 100 ng/ml, and 500 ng/ml), the VEGF was used as positive control. The quantification was analyzed with professional software after 6 h incubation (B), contrast with the SFM, the proliferation rate of MK, PGRN, and MK/PGRN were significantly difference, P < 0.05, and the MK/PGRN treatment group had the significant difference contrast with MK and PGRN treatment respectively, P < 0.05. (C) The migration assay was confirmed by the trans-well kit, the HUVECs were treated with MK, PGRN, and MK/PGRN (100 ng/ml), the cell lysate was measured at the 560 nm by the colorimetric measurement, the proliferation rate was quantified (D), and the result was consistent with the wound healing experiment, all the treatment groups had the significant difference contrast with the control, while the MK/PGRN had the significant difference contrast with MK or PGRN treated group respectively. Control, serum free medium, scale bar = 100 um, *P < 0.05, **P < 0.01, #P < 0.05.
Figure 6.
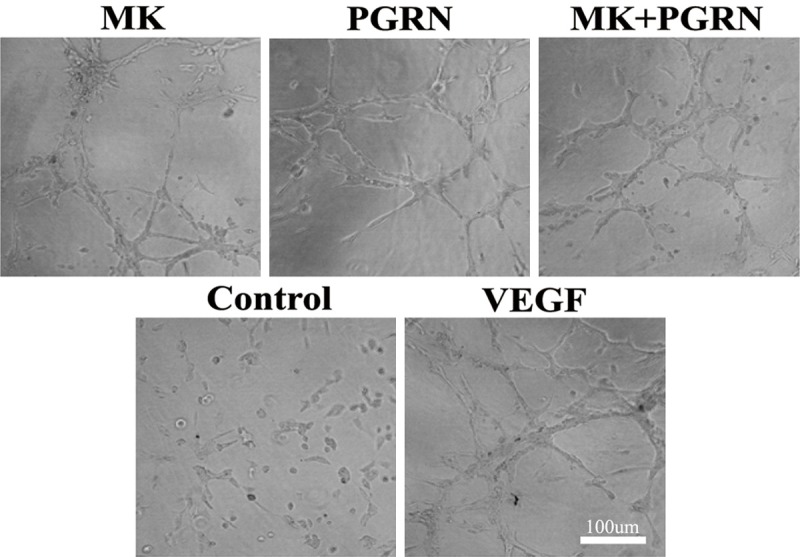
The interaction of MK and PGRN effect the tubulogenesis in HUVECs model. For the in vitro angiogenesis analysis, HUVECs were incubated with MK, PGRN, and MK/PGRN proteins (500 ng/ml) according with the Fibrin Gel In Vitro Angiogenesis Assay manufacturer’s procedure (CHEMICON). Images of tubular structures were taken after 24 h incubation, using a Leica inverted microscope.
In conclusion, we have first identified PGRN by yeast two hybrid as a MK-binding protein, and subsequent characterization of this novel association as well as the functional assay showing that the stimulation of endothelial cell proliferation and migration by PGRN growth factors is mediated by MK extend our understanding of the actions of growth factors in angiogenesis and also provide us a potential target for developing and optimizing the therapeutic in tumor angiogenesis and would repair.
Acknowledgements
The work was supported by grant from the National Natural Science Foundation of China (No. 30772534), The Huzhou Natural Science Foundation (No. 2010YS05), and The Natural Science Foundation of Zhejiang (No. Y2111181 and No. LQ13H160019).
Disclosure of conflict of interest
None.
References
- 1.Hao H, Liu M, Wu P, Cai L, Tang K, Yi P, Li Y, Chen Y, Ye D. Lipoxin A4 and its analog suppress hepatocellular carcinoma via remodeling tumor microenvironment. Cancer Lett. 2011;309:85–94. doi: 10.1016/j.canlet.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Hessheimer AJ, Forner A, Boix L, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene. 2006;25:3848–3856. doi: 10.1038/sj.onc.1209548. [DOI] [PubMed] [Google Scholar]
- 3.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 4.Muramatsu T. Midkine: a promising molecule for drug development to treat diseases of the central nervous system. Curr Pharm Des. 2011;17:410–423. doi: 10.2174/138161211795164167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takei Y, Kadomatsu K, Matsuo S, Itoh H, Nakazawa K, Kubota S, Muramatsu T. Antisense oligodeoxynucleotide targeted to Midkine, a heparin- binding growth factor, suppresses tumorigenicity of mouse rectal carcinoma cells. Cancer Res. 2001;61:8486–8491. [PubMed] [Google Scholar]
- 6.Takei Y, Kadomatsu K, Goto T, Muramatsu T. Combinational antitumor effect of siRNA against midkine and paclitaxel on growth of human prostate cancer xenografts. Cancer. 2006;107:864–873. doi: 10.1002/cncr.22068. [DOI] [PubMed] [Google Scholar]
- 7.Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5:145–159. doi: 10.1016/s1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 8.Katsuno M, Adachi H, Minamiyama M, Waza M, Tokui K, Banno H, Suzuki K, Onoda Y, Tanaka F, Doyu M, Sobue G. Reversible disruption of dynactin 1-mediated retrograde axonal transport in polyglutamine-induced motor neuron degeneration. J Neurosci. 2006;26:12106–12117. doi: 10.1523/JNEUROSCI.3032-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins-Green M, Feugate JE. The 9E3/CEF4 gene product is a chemotactic and angiogenic factor that can initiate the wound-healing cascade in vivo. Cytokine. 1998;10:522–535. doi: 10.1006/cyto.1997.0311. [DOI] [PubMed] [Google Scholar]
- 10.Swerlick RA. Angiogenesis. J Dermatol. 1995;22:845–852. doi: 10.1111/j.1346-8138.1995.tb03934.x. [DOI] [PubMed] [Google Scholar]
- 11.Paweletz N, Knierim M. Tumor-related angiogenesis. Crit Rev Oncol Hematol. 1989;9:197–242. doi: 10.1016/s1040-8428(89)80002-2. [DOI] [PubMed] [Google Scholar]
- 12.Blood CH, Zetter BR. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990;1032:89–118. doi: 10.1016/0304-419x(90)90014-r. [DOI] [PubMed] [Google Scholar]
- 13.Bicknell R, Harris AL. Novel growth regulatory factors and tumour angiogenesis. Eur J Cancer. 1991;27:781–785. doi: 10.1016/0277-5379(91)90189-k. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 15.Killion JJ, Fidler IJ. The biology of tumor metastasis. Semin Oncol. 1989;16:106–115. [PubMed] [Google Scholar]
- 16.Fràter-Schröder M, Risau W, Hallmann R, Gautschi P, Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84:5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlodavsky I, Korner G, Ishai-Michaeli R, Bashkin P, Bar-Shavit R, Fuks Z. Extracellular matrix-resident growth factors and enzymes: possible involvement in tumor metastasis and angiogenesis. Cancer Metastasis Rev. 1990;9:203–226. doi: 10.1007/BF00046361. [DOI] [PubMed] [Google Scholar]
- 18.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 20.Warren BA, Greenblatt M, Kommineni VR. Tumour angiogenesis: ultrastructure of endothelial cells in mitosis. Br J Exp Pathol. 1972;53:216–224. [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallo T, Sade R, Folkman J, Cotran RS. Ultrastructural autoradiographic studies of the early vasoproliferative response in tumor angiogenesis. Am J Pathol. 1973;70:345–362. [PMC free article] [PubMed] [Google Scholar]
- 22.Muramatsu H, Zou P, Suzuki H, Oda Y, Chen GY, Sakaguchi N, Sakuma S, Maeda N, Noda M, Takada Y, Muramatsu T. alpha4beta1- and alpha6beta1- integrins are functional receptors for midkine, a heparin-binding growth factor. J Cell Sci. 2004;117:5405–5415. doi: 10.1242/jcs.01423. [DOI] [PubMed] [Google Scholar]
- 23.Herradon G, Ezquerra L, Nguyen T, Silos-Santiago I, Deuel TF. Midkine regulates pleiotrophin organ-specific gene expression: evidence for transcriptional regulation and functional redundancy within the pleiotrophin/midkine developmental gene family. Biochem Biophys Res Commun. 2005;333:714–721. doi: 10.1016/j.bbrc.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi T, Kadomatsu K, Okamoto T, Ichihara-Tanaka K, Kojima T, Saito H, Tomoda Y, Muramatsu T. Expression of syndecan-1 and -3 during embryogenesis of the central nervous system in relation to binding with midkine. J Biochem. 1997;121:197–205. [PubMed] [Google Scholar]
- 25.Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, Wellstein A. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 26.Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S, Muramatsu T. LDL receptor-related protein as a component of the midkine receptor. Biochem Biophys Res Commun. 2000;270:936–941. doi: 10.1006/bbrc.2000.2549. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi N, Muramatsu H, Ichihara-Tanaka K, Maeda N, Noda M, Yamamoto T, Michikawa M, Ikematsu S, Sakuma S, Muramatsu T. Receptor-type protein tyrosine phosphatase zeta as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci Res. 2003;45:219–224. doi: 10.1016/s0168-0102(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 28.Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, Okamoto K, Oda M, Sakuma S, Aikou T, Muramatsu H, Kadomatsu K, Muramatsu T. Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer. 2000;83:701–706. doi: 10.1054/bjoc.2000.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan M, Ji T, Wu Z, Zhou J, Zhang C. Evaluation of expression of midkine in oral squamous cell carcinoma and its correlation with tumour angiogenesis. Int J Oral Maxillofac Surg. 2007;36:159–164. doi: 10.1016/j.ijom.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Muramaki M, Miyake H, Hara I, Kamidono S. Introduction of midkine gene into human bladder cancer cells enhances their malignant phenotype but increases their sensitivity to antiangiogenic therapy. Clin Cancer Res. 2003;9:5152–5160. [PubMed] [Google Scholar]
- 31.Sun X, Gulyás M, Hjerpe A. Mesothelial differentiation as reflected by differential gene expression. Am J Respir Cell Mol Bio. 2004;130:510–518. doi: 10.1165/rcmb.2003-0266OC. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Nishiahara M. Granulin precursor gene: a sex steroid-inducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab. 2002;75:31–37. doi: 10.1006/mgme.2001.3274. [DOI] [PubMed] [Google Scholar]
- 33.Barreda DR, Hanington PC, Walsh CK, Wong P, Belosevic M. Differentially expressed genes that encode potential markers of goldfish macrophage development in vitro. Dev Comp Immunol. 2004;28:727–746. doi: 10.1016/j.dci.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Justen HP, Grunewald E, Totzke G, Gouni-Berthold I, Sachinidis A, Wessinghage D, Vetter H, Schulze-Osthoff K, Ko Y. Differential gene expression in synovium of rheumatoid arthritis and osteoarthritis. Mol Cell Biol Res Commun. 2000;3:165–172. doi: 10.1006/mcbr.2000.0211. [DOI] [PubMed] [Google Scholar]
- 35.He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–612. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- 36.Ong CH, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histol Histopathol. 2003;18:1275–1288. doi: 10.14670/HH-18.1275. [DOI] [PubMed] [Google Scholar]
- 37.He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer Res. 1999;59:3222–3229. [PubMed] [Google Scholar]


