Abstract
It has been known that the occurrence of rheumatoid arthritis (RA) was closely correlated with DNA hypomethylation in CD4+ T cells, in which DNA methyltransferase plays a certain role. This study therefore investigated the effect of miR-126 on CD4+ T cell subgroup in RA patients and the alternation of DNA hypomethylation, in an attempt to provide new sights into the pathogenesis and treatment of RA. CD4+ T cells separated from RA patients were transfected with miRNA (miR)-126 expression vector or miR-126 inhibitor expression vector. The expression levels of CD11a, CD70 and DNMT1 mRNA were examined by real-time PCR. Protein levels of CD11a and CD70 were tested by flow cytometry while DNMT1 protein level was quantified by Western blotting. DNA was modified by sodium bisulfite and was sequenced for the methylation status of promoters of CD11a and CD70 genes. Both mRNA and protein expressions of CD11a and CD70 genes in CD4+ T cells were elevated by miR-126 transfection, along with decreased DNMT1 protein level but not mRNA level. The methylation degree of promoters of both CD11a and CD70 genes were significantly depressed after miR-126 transfection. The transfection by miR-126 inhibitor effectively reversed such effects. In RA patients, elevated miR-126 may promote the expression of CD11a and CD70 via the induction of hypomethylation of gene promoters by depressing DNMTI1 protein levels.
Keywords: Rheumatoid arthritis, miRNA-126, CD4+ T cells, DNA methylation
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that is commonly occurred and severely affected patients’ healthy. RA has the clinical feature as non-septic proliferative synovitis and may compromise the integrity of bone and cartilage tissues, thus causing articular dysfunctions [1]. Previous studies have established the role of epigenetics in the occurrence and development of various autoimmune diseases [2-4]. Classical epigenetic mechanisms include chromatin rearrangement, histone modification and DNA methylation [5,6]. Among all these regulatory mechanisms, DNA methylation is crucial for gene expressional regulation and is closely correlated with the occurrence and progression of various diseases.
Previous studies have reported the hypomethylation at whole-genomic level of T cells in autoimmune disease patients, and the negative relationship between DNA methylation level and the disease activity [7,8]. The application of DNA methylation inhibitor to induce the hypomethylation of T cell DNA in vitro and further introduction into host mice can lead to lupus-like autoimmune response or the occurrence of lupus [9-12], suggesting the important role of DNA methylation alternation of T cells in the pathogenesis of autoimmune diseases [9,11-14]. The suppression of DNA methylation level may also activate gene expression including CD11a, Perforin, CD70 and CD40 ligands [15-18]. Various factors including environment and age may all affect the occurrence of RA via regulating DNA methylation level [19]. All these abovementioned studies suggest the close relationship between DNA methylation and autoimmune diseases. As one of commonly occurred autoimmune diseases, RA is also correlated with DNA methylation. For example, the overexpression of interleukin-8 (IL-8) in CD4+ T cells in RA patients was dependent on the hypomethylation of gene promoter [20]. The methylation status in FOXP3 gene promoter region in CD4+/CD25+ T cell sub-populations is closely related with RA occurrence [21]. The molecular mechanism underlying pathologic hypomethylation of CD4+ T cells in RA patients, however, has not been fully illustrated yet.
MicroRNA (miRNA) is an endogenous small (21~25 nt) RNA molecule with hairpin-like structure, and can suppress the expression of target gene(s) via binding onto target mRNA, thus degrading mRNA and impeding further translation. Recent studies have shown the participation of miRNA in various immune responses [22] and its crucial role in the pathogenesis of autoimmune diseases [23]. In systemic lupus erythematosus (SLE) patients, the expression of miR-126 in CD4+ T cells showed negative relationship with protein expression level of DNMI1, and suppressing methylation level of CD70 and CD11a genes [24]. The abnormal expression of miR-126 also has been found in RA patients [25]. This study constructed miR-126 over-expression vectors and miR-126 inhibitor-expression vector, both of which were further transfected into CD4+ T cells of RA patients. The role of miR-126 on DNA methylation in RA was then determined by analyzing the expression of CD11a, CD70 and DNMT1 expression, along with DNA methylation level of CD11a and CD70 genes, in an attempt to provide new sights for RA pathogenesis and novel treatment strategy.
Materials and methods
CD4+ T cells separation and transfection
Peripheral blood samples (5 mL) were collected from RA patients and were separated for peripheral blood mononuclear cells (PBMCs) by density gradient centrifugation method. CD4+ T cell sub-population was selected by magnetic activated cell sorting (MACS) method. In brief, cell suspensions were firstly washed in PBS, followed by 300 g centrifugation for 10 min. 107 cells were mixed with 80 μL buffer and 20 μL MACS CD4+ beads, followed by 4°C incubation for 15 min. After gentle washing, cells were centrifuged again for re-suspension and were then removed for LS separation column in a Midi MACS apparatus (Miltenyi Biotec, Germany). CD4+ T cells were eluted by 5 mL elution buffer. Purity and viability of PBMCs were determined by flow cytometry and trypan blue staining, respectively. Cells were frozen at -80°C for further use.
CD4+ T cells were transfected with psilencer-miR-126, psilencer-negative (Affiliated Second people’s Hospital of Luzhou medical college, China), miR-126 inhibitor (5’-UCGUA CCGUG AGUAA UAAUG CG-3’) and scrambled oligonucleotide vectors (Qiagen, US) with the lep of Amaxa T transfection kit (Qiagen, US). In brief, CD4+ T cells were aliquot into four tubes (each containing 5×106 cells) for centrifugation (200 g, 10 min) and resuspension in 100 μL transfection buffer. After 20 min incubation at room temperature, four different vectors (psilencer-miR-126, psilencer-negative, miR-126 inhibitor and scrambled oligonucleotide) were separately added into these four tubes. After electroporation in the cube, cells were gently transferred into 12-well plate which contained 2 mL T cell culture medium (containing 10% fetal bovine serum and 5% penicillin-streptomycin compound). After incubation at 37°C for 6 hours, the plate was centrifuged at 140 g for 8 min, with re-suspension of T cells in fresh culture medium. After another 24-hour incubation, cell suspension samples were collected from pmaxGFP transfected cells for calculating transfection efficiency. In general, one fixed view was firstly counted for total cell number under a light-field inverted microscope. Fluorescent microscope was then used to count the number of fluorescent cells. Transfection efficiency was calculated by the ratio of fluorescent cells against total cells in the same field.
Real-time PCR
Real-time fluorescent quantitative PCR was used to quantify the mRNA levels of DNMT1, CD11a, CD70 and miR-126, along with housekeeping genes β-actin and 18s rRNA. The reaction system included 10 μL SYBR Green RT-PCR master mix (Invitrogen, US), forward and reverse primers (1 μL each), total RNA (3 μL) and enzyme-free water. The reaction parameters were: 50°C in vitro reverse transcription for 20 min, 95°C pre-denature for 15 min, followed by 40 cycles each containing 94°C denature for 15 sec, 55°C annealing for 30 sec and 72°C elongation for 30 sec.
Protein level assay
Flow cytometry was used to detect the protein expression level of CD70 and CD11a in CD4+ T cells. In brief, 45 hours after transfection, cells (2×106) along with blank controls were firstly centrifuged at 2 000 rpm for 10 min, rinsed in PBS, and added with FITC-CD11a or FITC-CD70 conjugated antibodies (eBioscience, US). After mixture and washing, cells were fixed by 0.5 mL paraformaldehyde and loaded on flow cytometry for detection.
Western blotting was used to detect the content of DNMT1 protein in CD4+ T cells. In brief, total proteins were firstly extracted from CD4+ T cells using total protein extraction kit (Qiagen, US) following manual instruction. Purified proteins were separated by SDS-PAGE and were transferred to nitrocellulose membrane, which was blocked in defatted mild powders by 1.5 hours at room temperature. After the incubation with mouse anti-human DNMT1 or anti-β-actin antibodies (1:200, eBioscience, US). Goat anti-mouse IgG antibody conjugated with horseradish peroxidase (HRP) was added for a further 2-hour incubation at room temperature. The chromogenic reaction was initiated by ECL reagent and was stopped by PBST buffer.
DNA methylation assay
Bisulfite modification and sequencing method was used to detect the DNA methylation level of CD11a and CD70 genes. Firstly, genomic DNA was extracted from human CD4+ T cells by DNA extraction kit (Boao Biotech, China). Total DNA was then modified by bisulfite using EpiTechR kit (Qiagen, US) following the manual instruction. The target DNA fragment was then amplified by nested PCR using primers as shown in Table 1. In 1st round, the reaction mixture included forward and reverse primers (10 ng/μL), dNTP (1.0 μL), Taq polymerase (0.2 μL) and DNA templates. The reaction began with 95°C pre-denature for 5 min, followed by 30 cycles each containing 95°C denature for 30 sec, 60°C annealing for 30 sec and 72°C elongation for 30 sec, and ended with 72°C elongation for 5 min. 2nd PCR reaction mixture consisted of forward and reverse primers (10 ng/μL), dNTP (1.0 μL), Taq polymerase (0.2 μL) and PCR products from 1st reaction (5.0 μL). The reaction began with 95°C pre-denature for 5 min, followed by 30 cycles each containing 95°C denature for 30 sec, 60°C annealing for 30 sec and 72°C elongation for 30 sec, and ended with 72°C elongation for 5 min.
Table 1.
Nested PCR primer sequence
| Target gene | Primer | Sequence (5’->3’) |
|---|---|---|
| CD11a (1st round) | Forward | GGTGAATTCTTAAGGTTAGGAGTTTAAGTTTAGT |
| Reverse | CAATCTAGAACTACAACTTAAAAAATTAAATTGC | |
| CD11a (2nd round) | Forward | GTTGAATTCGGTATATATGGTGAAATTTACTTTTAT |
| Reverse | CACTCIAGTACAACAAACATCCAAAAATATAAAAT | |
| CD70 (1st round) | Forward | GGTGAATTCTTAAAGGTTAGGAGTTCAAGTTTAGT |
| Reverse | CAACCTAGAACTACACATTCATTAAAAATTAAATT | |
| CD70 (2nd round) | Forward | GTTGAACTCGGTTAACTGGTGAAATTTTATTTTCAT |
| Reverse | CACTCTAGATACAACAAACATCCAAAAATTAAAAAT |
Nested PCR products were purified by Gel Extraction Kit (Qiagen, US), liaged with pGEMA_T Easy Vector, and were transfected into DH5α competent cells. After PCR identification, positive recombinant vectors were sequenced.
Statistical analysis
SPSS 15.0 software package was used to process all collected data, of which measurement data were presented as mean ± standard deviation (SD). The comparison of means between two independent samples was finished by student t-test. Whilst non-parametric data were compared by Mann-Whitney test. A statistical significance was defined when P<0.05.
Results
mRNA levels in CD4+ T cells after transfection
Using fluorescent quantitative PCR, we examined the mRNA levels of DNMT1, CD11a and CD70 in CD4+ T cells. As shown in Figure 1, miR-126 transfected CD4+ T cells had significantly elevated mRNA levels of CD11a and CD70 genes (P<0.05) but not DNMT1, when compared to the vector control group.
Figure 1.

mRNA levels of transfected cells.
Protein contents after transfection
We further utilized Western blotting method to detect the DNMT1 protein level in CD4+ T cells. Results (Figure 2) showed significantly decreased DNMT1 protein level in miR-126 transfected cells when compared to those were transfected with blank control vectors (P<0.05). Flow cytometry was further recruited to detect protein level of CD11a and CD70. As shown in Table 2, after miR126 transfection, CD4+ T cells had elevated percentages of CD11a and CD70-positive cells (P<0.05). The mean fluorescent intensity (MFI), however, had no significant difference across groups (P>0.05).
Figure 2.
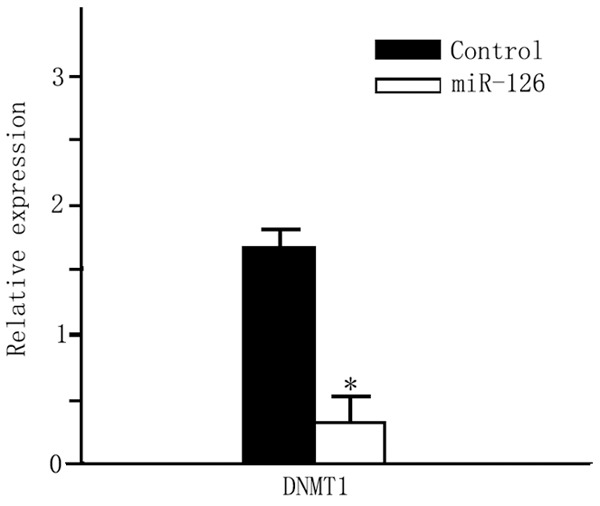
DNMT1 protein level. *P<0.05 compared to the control group.
Table 2.
Expression of CD70 and CD11a in CD4+ T cells
| Cell percentage | Mean fluorescent intensity (MFI) | |||
|---|---|---|---|---|
|
|
||||
| Control | miR-126 | Control | miR-126 | |
| CD11a | 48.33±1.04 | 58.83±1.15* | 7.6±2.1 | 8.34±2.5 |
| CD70 | 3.85±0.8 | 12.06±1.20* | 17.77±2.44 | 18.44±2.75 |
P<0.05 compared to the control group.
Methylation status of gene promoter regions
Using bisulfite modification and sequencing method, we examined the methylation condition of CD70 (TNFSF7) and CD11a (ITGAL) promoter regions. The methylation percentage of CD70 in controlled group was about 48.59%±3.58%. After miR-126 transfection, the methylation level was decreased to 30.42%±2.97% (Figure 3A) CD11a has methylation percentage at 50.19%±4.12% in control ones, and only 31.56%±3.68%, suggesting the significant suppressed methylation of CD11a gene after miR-126 transfection (Figure 3B).
Figure 3.
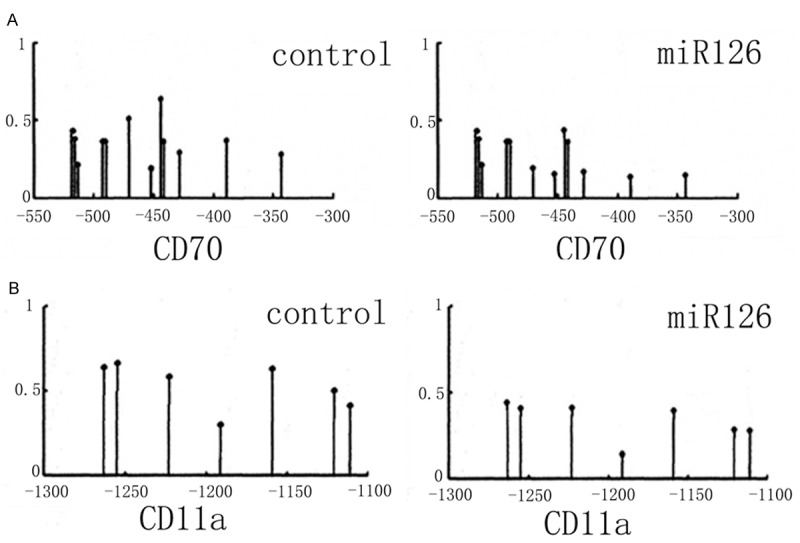
Gene promoter region methylation level. A. CD70 gene; B. CD11a gene.
mRNA level after miR-126 inhibitor transfection
Compared to those transfected with scrambled oligonucleotide, CD4+ T cells transfected with miR-126 inhibitor showed significantly suppressed CD11a and CD70 mRNA levels (Figure 4, P<0.05).
Figure 4.
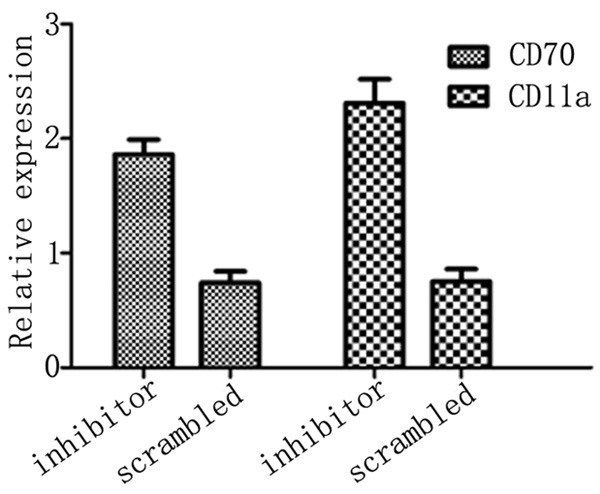
CD70 and CD11a mRNA level after inhibitor transfection.
Protein level after miR-126 inhibitor transfection
The protein expression level of DNMT1 was significantly elevated after transfection with miR-126 inhibitor in CD4+ T cells from RA patients, when compare to those transfected with scrambled oligonucleotide (Figure 5, P<0.05). The percentage of CD11a and CD70 positive cells in CD4+ subpopulation of T cells was significantly decreased after miR-126 inhibitor transfection (P<0.05) while the mean fluorescent intensity (MFI) remained unchanged (Table 3, P>0.05).
Figure 5.
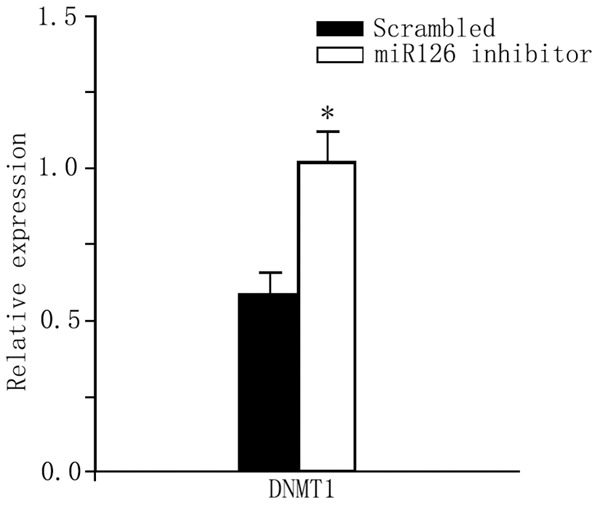
DNMT1 protein expression level. *P<0.05 compared to the scrambled oligonucleotide control group.
Table 3.
CD70 and CD11a expression in transfected CD4+ T cells
| Cell percentage | Mean fluorescent intensity (MFI) | |||
|---|---|---|---|---|
|
|
||||
| Scrambled control | MiR126 inhibitor transfection | Scrambled control | MiR126 inhibitor transfection | |
| CD11a+ | 53.33±0.57 | 48.23±1.15* | 17.6±2.1 | 14.34±2.5 |
| CD70+ | 14.97±2.65 | 6.06±2.20* | 10.77±2.46 | 7.44±2.75 |
P<0.05 compared to the scrambled control group.
Gene promoter methylation level after miR-126 inhibitor transfection
In scrambled control cells, the methylation of CD70 gene was 44.86%±3.42%. After miR-126 inhibitor transfection, the methylation level was significantly elevated to 52.32%±3.58% (Figure 6A, P<0.05). CD11a gene methylation level showed similar patterns, as the increase from 48.16%±3.96% to 55.98%±4.23% (Figure 6B, P<0.05).
Figure 6.
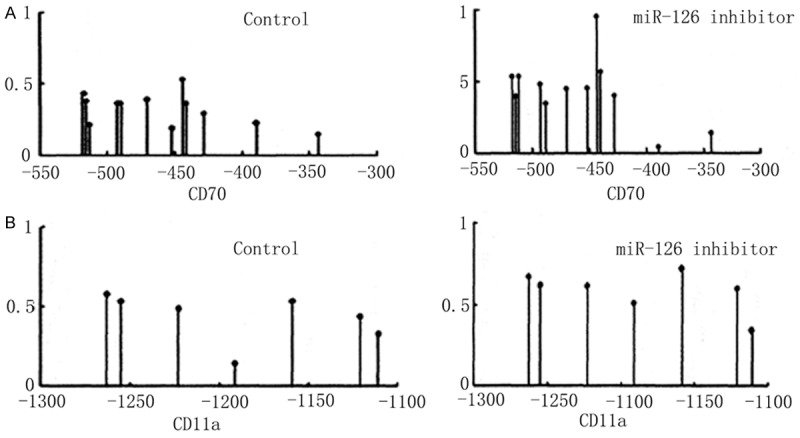
DNA methylation level of CD70 (A) and CD11a (B) genes.
Discussion
With the advancement of epigenetics study, DNA methylation has been found to be closely correlated with autoimmune diseases. Currently more than 100 DNA methylation sensitive genes have been identified by oligonucleotide sequencing analysis, including CD11a, CD70, CD10L/IgEFcRYI and perforin [15-18]. Those abovementioned methylation sensitive genes were found to be over-expressed in CD4+ T cells from SLE patients, with suppressed DNA methylation status at the gene promoter region [13-15,26]. MiRNA is one important factor regulating DNA methylation level, and has been found to be correlated with the occurrence of RA [20]. This study thus detected both gene expression and DNA methylation levels of CD11a and CD70 genes by over-expression and silencing of miRNA-126. Our results showed significantly elevated mRNA and protein expression levels of both CD11a and CD70 in RA patients derived CD4+ T cells tranfected with miR-126 plasmids, along with depressed DNMI1 protein but not mRNA. The introduction of miR-126 inhibitor reversed such effects, suggesting the potency of elevated miR-126 expression in inducing hypomethylation of CD11a and CD70 genes, possibly via depressing DNMT1 protein levels, thus causing over-expression of CD11a and CD70, leading to the occurrence and progression of RA.
CD11a is one subunit of lymphocyte related antigen-1 and is expressed on the surface of lymphocytes, macrophage and mononuclear cells. It is known to play a critical role in the activation and proliferation of T cells, as well as in the inflammation and body immune response. Studies have reported the induction of autoimmune disease by over-expression of CD11a in animal models [27]. DNA methylation is one typical epigenetic mechanism regulating gene expression. It has been reported that CD11a hypomethylation was correlated with over-expression of CD4+ T cells in SLE patients [14]. The over-expression of cytokines may stimulate B cells to produce large amounts of auto antibodies, further causing the occurrence of autoimmune diseases. CD70, as one B cell co-stimulator molecule, can facilitate the production of immunoglobulin (Ig) by the synergistic effects with CD27. The over-expression of CD70 also leads to over-production of auto antibodies, further causing tissue damage [28]. The hypomethylation level of CD70 gene has been correlated with over-expression of CD4+ T cells in SLE patients [15]. Based on all those previous studies and our results, the over-expression of miR-126 may suppress DNMT1 expression, causing depressed DNA methylation level of CD11a and CD70 genes, thus elevating the gene expression level and leading to RA occurrence.
In summary, this study for the first time found the role of miR-126 in depressing DNA methylation level of CD4+ T cell-related autoimmune genes, thus activating gene expression and causing RA pathogenesis. This study provides new insights for the etiology of RA and indicate novel treatment strategy.
Acknowledgements
This study was supported in part by the Sichuan Provincial Department of Science and technology of China (grant No. 2015010293).
Disclosure of conflict of interest
None.
References
- 1.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Y, Sawalha AH. Epigenetic regulation and the pathogenesis of systemic lupus erythematosus. Transl Res. 2009;153:4–10. doi: 10.1016/j.trsl.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Zhao S, Long H, Lu Q. Epigenetic perspectives in systemic lupus erythematosus: pathogenesis, biomarkers, and therapeutic potentials. Clin Rev Allergy Immunol. 2010;39:3–9. doi: 10.1007/s12016-009-8165-7. [DOI] [PubMed] [Google Scholar]
- 4.Strickland FM, Richardson BC. Epigenetics in human autoimmunity. Epigenetics in autoimmunity-DNA methylation in systemic lupus erythematosus and beyond. Autoimmunity. 2008;41:278–86. doi: 10.1080/08916930802024616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286:481–6. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 6.Richardson B. Primer: epigenetics of autoimmunity. Nat Clin Pract Rheumatol. 2007;3:521–7. doi: 10.1038/ncprheum0573. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Lu Q. DNA methylation in T cells from idiopathic lupus and drug-induced lupus patients. Autoimmun Rev. 2008;7:376–83. doi: 10.1016/j.autrev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J, Berdasco M, Fraga MF, O’Hanlon TP, Rider LG, Jacinto FV, Lopez-Longo FJ, Dopazo J, Forn M, Peinado MA, Carreño L, Sawalha AH, Harley JB, Siebert R, Esteller M, Miller FW, Ballestar E. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20:170–9. doi: 10.1101/gr.100289.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quddus J, Johnson KJ, Gavalchin J, Amento EP, Chrisp CE, Yung RL, Richardson BC. Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J Clin Invest. 1993;92:38–53. doi: 10.1172/JCI116576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yung RL, Quddus J, Chrisp CE, Johnson KJ, Richardson BC. Mechanism of drug-induced lupus. I. Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025–35. [PubMed] [Google Scholar]
- 11.Richardson B, Sawalha AH, Ray D, Yung R. Murine models of lupus induced by hypomethylated T cells (DNA hypomethylation and lupus) Methods Mol Biol. 2012;900:169–80. doi: 10.1007/978-1-60761-720-4_8. [DOI] [PubMed] [Google Scholar]
- 12.Richardson B, Kahn L, Lovett EJ, Hudson J. Effect of an inhibitor of DNA methylation on T cells. I. 5-Azacytidine induces T4 expression on T8+ T cells. J Immunol. 1986;137:35–9. [PubMed] [Google Scholar]
- 13.Richardson BC, Strahler JR, Pivirotto TS, Quddus J, Bayliss GE, Gross LA, O’Rourke KS, Powers D, Hanash SM, Johnson MA. Phenotypic and functional similarities between 5-azacytidine-treated T cells and a T cell subset in patients with active systemic lupus erythematosus. Arthritis Rheum. 1992;35:647–62. doi: 10.1002/art.1780350608. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Kaplan M, Ray D, Ray D, Zacharek S, Gutsch D, Richardson B. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum. 2002;46:1282–91. doi: 10.1002/art.10234. [DOI] [PubMed] [Google Scholar]
- 15.Lu Q, Wu A, Richardson BC. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. J Immunol. 2005;174:6212–9. doi: 10.4049/jimmunol.174.10.6212. [DOI] [PubMed] [Google Scholar]
- 16.Oelke K, Lu Q, Richardson D, Wu A, Deng C, Hanash S, Richardson B. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004;50:1850–60. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Sun Y, Gao F, Wu X, Tang J, Yin H, Luo Y, Richardson B, Lu Q. Epigenetics and SLE: RFX1 downregulation causes CD11a and CD70 overexpression by altering epigenetic modifications in lupus CD4+ T cells. J Autoimmun. 2010;35:58–69. doi: 10.1016/j.jaut.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan MJ, Lu Q, Wu A, Attwood J, Richardson B. Demethylation of promoter regulatory elements contributes to perforin overexpression in CD4+ lupus T cells. J Immunol. 2004;172:3652–61. doi: 10.4049/jimmunol.172.6.3652. [DOI] [PubMed] [Google Scholar]
- 19.Liu CC, Fang TJ, Ou TT, Wu CC, Li RN, Lin YC, Lin CH, Tsai WC, Liu HW, Yen JH. Global DNA methylation, DNMT1, and MBD2 in patients with rheumatoid arthritis. Immunol Lett. 2011;135:96–9. doi: 10.1016/j.imlet.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2010;184:6773–81. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- 21.Qin H, Zhu X, Liang J, Wu J, Yang Y, Wang S, Shi W, Xu J. MicroRNA-29b contributes to DNA hypomethylation of CD4+ T cells in systemic lupus erythematosus by indirectly targeting DNA methyltransferase 1. J Dermatol Sci. 2013;69:61–7. doi: 10.1016/j.jdermsci.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–91. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–9. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 24.Zhao S, Wang Y, Liang Y, Zhao M, Long H, Ding S, Yin H, Lu Q. MicroRNA-126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum. 2011;63:1376–86. doi: 10.1002/art.30196. [DOI] [PubMed] [Google Scholar]
- 25.Castro-Villegas C, Pérez-Sánchez C, Escudero A, Filipescu I, Verdu M, Ruiz-Limón P, Aguirre MA, Jiménez-Gomez Y, Font P, Rodriguez-Ariza A, Peinado JR, Collantes-Estévez E, González-Conejero R, Martinez C, Barbarroja N, López-Pedrera C. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFalpha. Arthritis Res Ther. 2015;17:49. doi: 10.1186/s13075-015-0555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng C, Kaplan MJ, Yang J, Ray D, Zhang Z, McCune WJ, Hanash SM, Richardson BC. Decreased Ras-mitogen-activated protein kinase signaling may cause DNA hypomethylation in T lymphocytes from lupus patients. Arthritis Rheum. 2001;44:397–407. doi: 10.1002/1529-0131(200102)44:2<397::AID-ANR59>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Yung R, Powers D, Johnson K, Amento E, Carr D, Laing T, Yang J, Chang S, Hemati N, Richardson B. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866–71. doi: 10.1172/JCI118743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin H, Zhao M, Wu X, Gao F, Luo Y, Ma L, Liu S, Zhang G, Chen J, Li F, Zuo X, Lu Q. Hypomethylation and overexpression of CD70 (TNFSF7) in CD4+ T cells of patients with primary Sjogren’s syndrome. J Dermatol Sci. 2010;59:198–203. doi: 10.1016/j.jdermsci.2010.06.011. [DOI] [PubMed] [Google Scholar]


