Abstract
Ovarian carcinoma is one of the most common and lethal malignancies in the world. Replication factor C (RFC) plays an important role in DNA replication, DNA damage repair, and checkpoint control during cell cycle progression in all eukaryotes. Our previous study found that one unit of RFC complex, RFC3, is over-expressed in ovarian tumor tissues. However, its role in the development of ovarian carcinoma remains unclear. Western blot and real-time RT-PCR analysis were used to measure the expression of RFC3 in ovarian cancer cells. Lentivirus-mediated RFC3-specific shRNA was used to knock down RFC3 expression in ovarian cancer cells. Furthermore, the effect of RFC3 on tumor cellular proliferation and growth were examined, respectively. The expression level of RFC3 was remarkably up-regulated in ovarian cancer OVCAR-3 cells. With MTS and cell growth assays, the viability and proliferation of RFC3 knocking-down OVCAR-3 cell line were shown to be effectively restrained. Down-regulation of RFC3 expression arrested the cell cycle of OVCAR-3 cell in the S-phase and induced apoptosis. This study suggests that RFC3 may play an important role in the the process of ovarian carcinoma, and that it may be a potential biological treatment target in the future.
Keywords: Ovarian carcinoma, replication factor C (RFC), cell growth, cell proliferation, cell cycle arrest, apoptosis
Introduction
Ovarian carcinoma is the most frequent cause of cancer related death in women. Malignant ovarian lesions include primary lesions arising from normal structures within the ovary and secondary lesions from cancers arising elsewhere in the body. It was estimated that more than 100,000 new cases was reported every year all around the world [1]. Although progress has been made in the treatment of ovarian cancer by improved debulking surgery and the introduction of platinum-taxane chemotherapy, the over 5 year survival rate remains less than 50% [2-4]. Early-stage ovarian carcinoma is frequently asymptomatic and difficult to detect and thus, by the time of diagnosis, most women have advanced disease. Most of these patients, although initially responsive to platinum-taxane chemotherapy, eventually develop, succumb to drug resistant metastases and relapse.
Replication factor C (RFC) plays an important role in DNA replication, DNA damage repair, and checkpoint control during cell cycle progression in all eukaryotes [5-9]. RFC is comprised of 5 subunits, including a single 145-kDa subunit (RFC140/RFC1) and four 36- to 41-kDa subunits (RFC37/RFC2, RFC36/RFC3, RFC40/RFC4, and RFC38/RFC5) [10-14]. These subunits form RFC complex that acts as a clamp loader, enabling binding of the proliferating cell nuclear antigen (PCNA) clamp onto primed DNA in an ATP-dependent reaction [15,16]. Then, this DNA-RFC-PCNA complex recruits DNA polymerase to the site of DNA synthesis and initiates the DNA replication [17].
The characteristic that RFC plays an important role in DNA replication makes RFC identifying as one of most important cancer testis antigens. Indeed, many lines of evidence showed that over-expression of RFC subunits were associated with different kinds of malignancies. RFC2, RFC4 and RFC5 were up-regulated in nasopharyngeal carcinomas [18], hepatocellular carcinomas [19] and human papillomavirus-positive squamous cell carcinomas [20], respectively. Our previous study also showed that RCF3 was over-expressed in ovarian tumor issues and can be used as an independent biomarker for predicting ovarian cancer patient survival [21]. However, the role of RFC3 in the development of ovarian carcinoma remains unclear.
In this study, we found that RFC3 was remarkably over-expressed in ovarian cancer OVCAR-3 cells. Further investigation focused on the effect of knock-down of RFC3 on the development of ovarian tumor. Our findings provide a new insight into the role of RFC3 in the process of ovarian carcinoma.
Materials and methods
Cells
COV-3, ES-2, OVCAR-3, COV-54, CO-3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO, Scotland, UK), supplemented with 10% fetal calf serum (Biological industries, Beit Haemek, Israel), 100 IU/ml penicillin, 100 μg/ml streptomycin (Pen-Strep, Biological industries, Beit Haemek, Israel), and 2% L-glutamine (Biological industries, Beit Haemek, Israel) at 37°C and 5% CO2 in a humidified atmosphere.
Western blot analysis
Cells were harvested and washed with phosphate-buffered saline (PBS), and then resuspended and lyzed in the buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, and 0.5% deoxycholate, 0.5 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin, and 2 μg/ml leupeptin). Proteins were quantified and boiled in electrophoresis SDS sample buffer. Then Samples were electrophoresed via 10% SDS-PAGE gel, and transferred to a polyvinylidine difluoride (PVDF) membrane (Millipore, USA). Membranes were blocked for 1 h in Tris-buffered saline with 0.05% Tween 20 (TBST) containing 5% (W/V) nonfat milk and incubated with RFC3 specific antibody (ab154899; Abcam, Cambridge, MA) for 2 h and washed twice with TBST for 15 min, then incubated with peroxidase-conjugated goat antibody to mouse IgG (1:5000; Amersham Pharmacia Biotech) for 30 min. Followed by washing three times, the membranes were visualized by an enhanced chemiluminescence system (ECL; Amersham Pharmacia Biotech).
RNA isolation and real-time RT-PCR
Total RNA were extracted from cells using Trizol (Invitrogen) according to the manufacturer’s protocol. qRT-PCR assays were performed to evaluated the expression of RFC3, and primers were designed as follows: RFC3-Fwd: 5’-TGATC-CCACCTATTCGTAGT-3’; RFC3-Rev: 5’-CAGTCTCCCTCAGATACACC-3’; GAPDH-Fwd: 5’-GAGTCAACGGATTTGGTCGT-3’, GAPDH-Rev: 5’-GACAAGCTTCCCGTTCTCAG-3’; The RFC3 mRNA expression levels were standardized to GAPDH mRNA by calculating ΔCt = Ct (RFC3)-Ct (GAPDH). All experiments were performed at least three times.
Construction of RFC3 shRNA lentiviruses
The shRNA target sequence (5’-AACTGCAGAGAAGTCTTGTAGAATTCAAGAGATTCTACAAGACTTCTCTGCTTTTTT C-3’) was designed for RFC3 gene and inserted into the lentiviral vector LV-008 (Forevergen Biosciences, China), which was used to express small hairpin RNAs and containing the GFP gene as a reporter. Lentiviral production was performed as mature method. Briefly, LV-008-shRFC3 plasmids and packaging vectors were cotransfected into HEK 293T cells to generate respective lentivirus. The supernatant containing infection lentiviruses was collected 72 h post-transfection, and the lentiviruses were concentrated by ultracentrifugation for 1.5 h at 25,000 rpm in a Beckman Instruments (Fullerton, CA, USA) SW28 rotor and resuspended in PBS. For lentiviruse infection, OVCAR-3 cells were cultured in six-well plate at a density of 50,000 cells per well and infected with lentiviruse in the presence of 5-10 μg/ml of polybrene. Cells were screened with 2 μg/ml puromycin for 10-15 days to get RFC3-knockdown cell.
MTS assay
The viability of cells were assessed by MTS [3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. Cells were collected in the logarithmic growth phase and plated at a density of 1 × 103 per well in 96-well plates in triplicate. Following treatment, every well was incubated with MTS reagent in complete medium with a ratio of 1:10 for 4 h. The absorbance value of each well was measured using a microplate reader (Diatek) at a wavelength of 490 nm.
Cell growth curves
Cells were digested into single cell suspension by 0.25% Trypsin-EDTA (1×) (GIBCO). The number of living cells was counted using Freshney counting plate, and then the cells were seeded in three wells of a 12-well plate with approximately 1 × 105 cells per milliliter. After digestion by trypsin, the living cells were counting at day 1, day 2, and day 3. The experiments were repeated three times and averages were used to plot the cell growth curves.
Cell cycle analysis
ShRFC3 cells and control cells were harvested and washed in PBS, and fixed in ice cold 70% ethanol for 1 h. Propidium iodide (PI) was added and staining the fixed cells in buffer [PBS containing RNase A (50 μg/ml), 0.1% sodium citrate and PI (50 μg/ml)] for 30 min at room-temperature. Cells were analyzed and recorded using FACS Calibur flow cytometer (BD Biosciences). Cell cycle analysis was performed with FlowJo software (TreeStar, Ashland, OR, USA).
Flow cytometry assay
Cell apoptosis was measured using an FITC annexin V apoptosis detection kit (BD PharMingen, 556547) according to its manufacturer’s instructions. Briefly, cells were seed in 6-wells plate with approximately 1 × 105 cells per milliliter. After incubation 24 h, cells were trypsinized digestion, harvested and washed in PBS twice. Followed by incubation with annexin V-FITC and PI for 15 min in the dark. Samples were immediately detected with flow cytometry. The percentage of apoptotic cells was determined on FACS Calibur flow cytometer (BD Biosciences) according to manufacturer’s guidelines.
Statistical analyses
SPSS 18.0 statistical software was used for statistical analysis. Values are presented as mean ± SD. Statistical analysis was performed using the Student’s t-test or ANOVA. P < 0.05 was considered to indicate a statistically significant difference.
Results
RFC3 was abundantly over-expressed in ovarian cancer OVCAR-3 cell line
Our previous study showed that over-expression of RFC3 was associated with human ovarian tumorigenesis [21]. Here, we first sought to identify an RFC3 sensitive cell line. To this end, a panel of five ovarian cancer cell lines was used to examine the protein and mRNA accumulation of RFC3 by using Western blot and real-time RT-PCR, respectively. As shown in Figure 1, the protein accumulation of RFC3 in OVCAR-3 and COV-504 cells were relative higher than that in other cells, while the mRNA accumulation of RFC3 in OVCAR-3 cells was the highest. These results indicated that RFC3 was abundantly over-expressed in OVCAR-3 cells. And OVCAR-3 cells were used in the subsequent assays.
Figure 1.
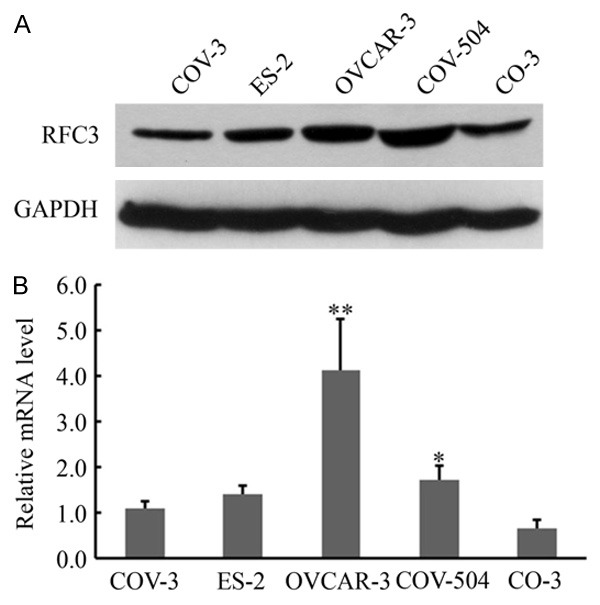
Expression of RFC3 proteins in ovarian cancer cell lines. A. RFC3 expressions were determined by Western blot analyses in six ovarian cancer cell lines as indicated. B. RFC3 mRNA expression levels in six ovarian cancer cell lines were examined by quantitative real-time RT-PCR analysis. GAPDH was used as an internal quantitative control. Three independent experiments were performed, and data were presented as means ± SD analyzed with AVOVA from three independent experiments. **: P < 0.01, *: P < 0.05.
Establishment of stable RFC3 knocking-down OVCAR-3 cells
To examine the role of RFC3 in ovarian cancer, a stable RFC3 knocking-down OVCAR-3 cell line was established using lentivirus-mediated RNA interference (RNAi) technology. Briefly, a fragment targeting RFC3 was designed and implanted to a lentiviral vector fused GFP as reporter, a non targeting fragment was used as negative control (NC). The lentivirus containing RFC3 and NC fragments were packaged and purified to infect OVCAR-3 cells. The efficiency of lentiviral infection was examined via detecting GFP expression, and showed that more than 90% of OVCAR-3 cells were infected (Figure 2A). Western blot analysis showed that the protein level of RFC3 was dramatically decreased in shRFC3 cells compared to that in NC cells (Figure 2B). Moreover, real-time RT-PCR further confirmed that the RFC3 mRNA was significantly suppressed in shRFC3 cells (Figure 2C).
Figure 2.
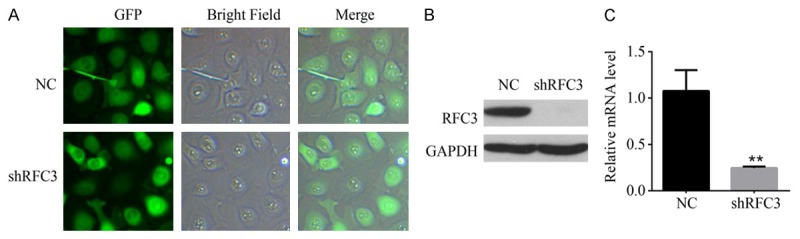
Lentivirus-mediated shRNA down-regulated RFC3 expression in OVCAR-3 cells. A. Representative images of OVCAR-3 cells showed the lentiviral infection efficiency that OVCAR cells were infected with shRNA-mediated RFC3 interfered lentivirus and NC lentivirus as indicated. B. RFC3 protein expression levels in shRFC3 cells and NC cells were detected by Western blot analyses. GAPDH is shown as loading control. C. mRNA expression levels of RFC3 in RFC3-interfered cells and control cells were detected by qRT-PCR analyses. GAPDH was used as an internal quantitative control. Data were presented data were presented as means ± SD analyzed with AVOVA from three independent experiments. **: P < 0.01.
Knock-down of RFC3 alleviated the viability and proliferation of OVCAR-3 cells
We sought to explorer the effect of RFC3 knocking-down on ovarian tumor cells. To this end, we examined the viability and proliferation of RFC3 knocking-down OVCAR-3 cells, respectively. Briefly, RFC3 knocking-down OVCAR-3 and NC cells at different time points (Day 1, Day 2 and Day 3, respectively) were examined by MTS assays. Our findings showed that knock-down of RFC3 decreased cell viability compared to NC (Figure 3A). Moreover, the cell growth was also examined by digesting cells at different time points into a single cell suspension and counting the living cells. As shown in Figure 3B, knock-down of RFC3 notably decreased the number of living cells. Taken together, these results indicated that knock-down of RFC3 was detrimental to the viability and proliferation of ovarian cancer cells.
Figure 3.
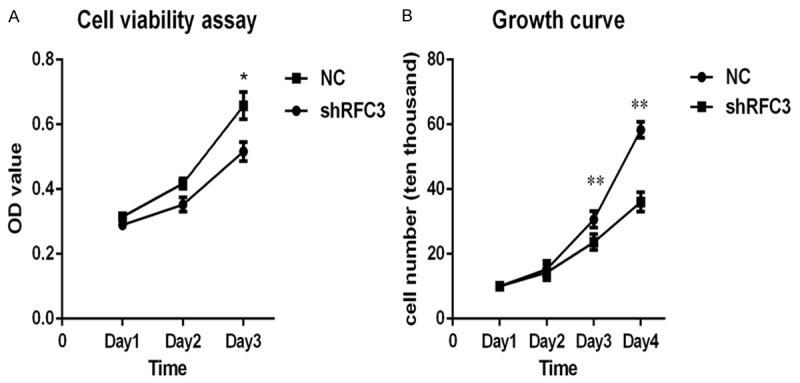
Knock-down of RFC3 alleviated cell viability and proliferation in OVCAR-3 cells. A. MTS assays showed that knock-down of RFC3 suppressed cell viability in ovarian cancer cells. B. The effect of RFC3-interfering on ovarian cancer cell proliferation, and cell proliferation were evaluated via cell growth curve assays. Data were presented data were presented as means ± SD analyzed with AVOVA from three independent experiments. **: P < 0.01, *: P < 0.05.
Knock-down of RFC3 arrested cell cycle in the S-phase
Because that abnormal cell proliferation is highly correlated with dysregulation of cell cycle, we examined whether knock-down of RFC3 affected cell cycle. Fluorescence activated cell sortor (FACS) assays showed that the ratio of cell numbers in the S phase was significantly increased from 18.96% in the NC cells to 34.96% in the RFC3 knocking-down cells (Figure 4), indicating that cell cycle of S-phase was arrested. These results suggested that suppression of ovarian cancer cells growth by knock-down of RFC3 may due to disturbing cell cycle.
Figure 4.
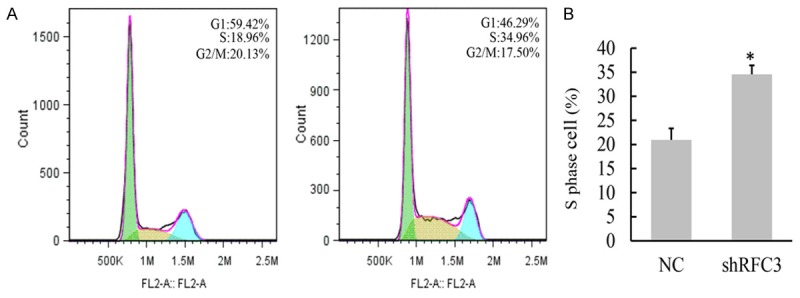
Knock-down of RFC3 arrested cell cycle in the S-phase. A. Cell cycle distribution in OVCAR-3 cells (left) and shRFC3 OVCAR-3 cells (right) were shown as analyzed by FACS. B. The percentage of OVCAR-3 cells in S phase was significantly higher in shRFC3 cells than that in control cells, indicating a block of DNA replication. Data were presented data were presented as means ± SD analyzed with AVOVA from three independent experiments. *: P < 0.05.
Knock-down of RFC3 induced cell apoptosis
Cell cycle arrest always caused cell apoptosis, which is a genetically determined process of cell self-destruction [22]. Therefore, we sought to examine whether knock-down of RFC3 was implicated in cell apoptosis by flow cytometric apoptosis assay. As shown in Figure 5, knock-down of RFC3 significantly induced a decrease of living cells from 89.53% to 80.69% comparing NC cells, while early apoptotic cells and necrotic cells were increased from 9.40% in the control cells to 15.67% in the shRFC3 cells. These findings were consistent with the observations that suppression of RFC3 inhibited cells growth (Figure 3). Taken together, these results suggested that knock-down of RFC3 significantly alleviated ovarian cancer cell proliferation by inducing cell cycle arrest and apoptosis.
Figure 5.
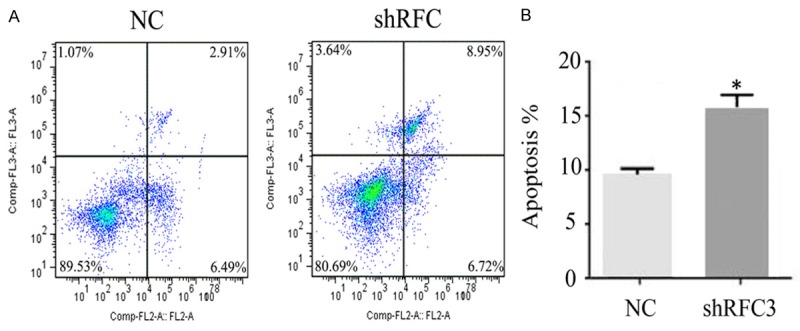
Knock-down of RFC3 induced ovarian cancer cell apoptosis. Knockdown of RFC3 decreased the persentage of survival cells in ovarian cancer cells (89.53% to 80.69%), and increased the proportion of early apoptotic cells and neurotic cells (9.40% to 15.67%).
Discussion
Ovarian cancer is one of the most common and lethal malignancies in the world [23]. The identification of more therapeutic targets that contribute to the development and progression of ovarian cancer is obviously desirable to combat this deadly disease. RFC is identified as one of most important cancer testis antigens due to its indispensable role in DNA replication [24]. Our previous study showed that over-expression of RCF3 was tightly associated with ovarian cancer, implying its potential role in ovarian tumor development [21]. Thus, in the present study, we aimed to investigate the biological functions of RFC3 in the ovarian tumor cells.
After identifying that RCF3 was associated with ovarian cancer, we focused on whether down-regulation of RFC3 would affect the development ovarian cancer OVCAR-3 cells. Thus, Lentivirus-mediated RNAi methods provide an attractive approach to efficiently suppress gene expression of RFC3. In this study, we monitored infection efficiency of lentivirus by fluorescence microscope, and confirmed knock-down of target gene by western blot and real-time RT-PCR, which gave a basis for the continued observation of RFC3’s role in OVCAR-3 cells. As with any potential therapeutic model, delivery is the rate limiting step. Due to the knock-down of target genes in a manner of sequence specific, the application of RNAi has been becoming one of the most widely used gene silencing technologies. These characteristic make RNAi has opened the door to the possibility of using this technique in a model of cancer therapeutics [25,26].
The RNAi knock-down assays showed that suppression of RFC3 in OVCAR-3 cells led to remarkable suppression of tumor cell viability and proliferation proliferation. This suppression may partly due to inducing tumor cell cycle arrest and apoptosis. Our findings were in agreement with the previous study that RFC4 up-regulation was discovered in hepatocellular carcinoma (HCC) tissues relative to non-malignant liver tissue, and knock-down of endogenous RFC4 decreased cellular proliferation, increased apoptosis, and enhanced the chemo-sensitivity of the HCC cell line HepG2 [27]. We noticed that knock-down of RFC3 resulted in cell cycle arrest in the S-phase. It is known that the S-phase checkpoint is activated at replication complex coordinates DNA replication when complex block because of DNA damage [28,29]. Given that RFC3 is one of the key components of DNA replication complex, down-regulation of RFC3 resulted in the block of DNA replication complex formation and eventually suppressed the DNA replication.
During cell division, epigenetically defined chromatin structure is often propagated with high fidelity through replication-coupled chromatin assembly. Failure to transmit epigenetic modifications such as histone modifications and DNA methylations would lead to changes of gene expression patterns in the progeny cells. Because of the importance of RFC3 in the formation of DNA replication complex, over-expression of RFC3 may be responsible for DNA replication and repair and inducing tumor formation. Indeed, previous pathway analysis revealed that an enrichment of genes in the Wnt/β-catenin signaling pathway was associated with RFC3 expression [30]. These genes stimulate the transcription of proliferation stimulating genes involving in cellular growth, proliferation and DNA replication, recombination and repair [24]. These results suggest RFC3 could have an integral role in driving cell proliferation in cells and could explain the anti proliferative knock-down effect we observed in ovarian cancer cells.
In summary, this report showed that RFC3 was remarkably up-regulated in ovarian cancer OVCAR-3 cells. Knock-down of RFC3 suppressed cell viability and proliferation. This suppression may partly due to inducing tumor cell cycle arrest and apoptosis. These results indicated that RFC3 may play an important role in the the process of ovarian carcinoma. Therefore, the specific enzymatic inhibitor to RFC3 may have therapeutic significance to treat ovarian cancer.
Acknowledgements
This study was supported by Natural Science Foundation of Guangdong Province (No. S2012010006150; No. S2012040006148) and Science and Technology Planning Project of Guangdong Province, China (No. 2011B031800056; No. 2011B031800276).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013;415:341–5. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 3.Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, Marks J, Godwin A, Gorelik E, Jacobs I, Menon U, Lu K, Badgwell D, Bast RJ, Lokshin AE. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28:2159–66. doi: 10.1200/JCO.2008.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Culligan KM, Hays JB. DNA mismatch repair in plants. An Arabidopsis thaliana gene that predicts a protein belonging to the MSH2 subfamily of eukaryotic MutS homologs. Plant PHYSIOL. 1997;115:833–9. doi: 10.1104/pp.115.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada M, Okuzaki D, Tanaka S, Tougan T, Tamai KK, Shimoda C, Nojima H. Replication factor C3 of Schizosaccharomyces pombe, a small subunit of replication factor C complex, plays a role in both replication and damage checkpoints. Mol Biol Cell. 1999;10:3991–4003. doi: 10.1091/mbc.10.12.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascucci B, Stucki M, Jonsson ZO, Dogliotti E, Hubscher U. Long patch base excision repair with purified human proteins. DNA ligase I as patch size mediator for DNA polymerases delta and epsilon. J Biol Chem. 1999;274:33696–702. doi: 10.1074/jbc.274.47.33696. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Brill SJ. Rfc4 interacts with Rpa1 and is required for both DNA replication and DNA damage checkpoints in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:3725–37. doi: 10.1128/MCB.21.11.3725-3737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia ST, Xiao LT, Bi DL, Zhu ZH. Arabidopsis replication factor C subunit 1 plays an important role in embryogenesis. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2007;33:179–87. [PubMed] [Google Scholar]
- 10.Furukawa T, Ishibashi T, Kimura S, Tanaka H, Hashimoto J, Sakaguchi K. Characterization of all the subunits of replication factor C from a higher plant, rice (Oryza sativa L. ), and their relation to development. Plant Mol Biol. 2003;53:15–25. doi: 10.1023/B:PLAN.0000009258.04711.62. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Burgers PM. Molecular cloning and expression of the Saccharomyces cerevisiae RFC3 gene, an essential component of replication factor C. Proc Natl Acad Sci U S A. 1994;91:868–72. doi: 10.1073/pnas.91.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luckow B, Bunz F, Stillman B, Lichter P, Schutz G. Cloning, expression, and chromosomal localization of the 140-kilodalton subunit of replication factor C from mice and humans. Mol Cell Biol. 1994;14:1626–34. doi: 10.1128/mcb.14.3.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullmann G, Fien K, Kobayashi R, Stillman B. Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:4661–71. doi: 10.1128/mcb.15.9.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray FC, MacNeill SA. The Schizosaccharomyces pombe rfc3+ gene encodes a homologue of the human hRFC36 and Saccharomyces cerevisiae Rfc3 subunits of replication factor C. Curr Genet. 2000;37:159–67. doi: 10.1007/s002940050514. [DOI] [PubMed] [Google Scholar]
- 15.Sakato M, O’Donnell M, Hingorani MM. A central swivel point in the RFC clamp loader controls PCNA opening and loading on DNA. J Mol Biol. 2012;416:163–75. doi: 10.1016/j.jmb.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Levin MK, Sakato M, Zhou Y, Hingorani MM. Mechanism of ATP-driven PCNA clamp loading by S. cerevisiae RFC. J Mol Biol. 2009;388:431–42. doi: 10.1016/j.jmb.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mossi R, Hubscher U. Clamping down on clamps and clamp loaders--the eukaryotic replication factor C. Eur J Biochem. 1998;254:209–16. [PubMed] [Google Scholar]
- 18.Li L, Tao Q, Jin H, van Hasselt A, Poon FF, Wang X, Zeng MS, Jia WH, Zeng YX, Chan AT, Cao Y. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma. Clin Cancer Res. 2010;16:2949–58. doi: 10.1158/1078-0432.CCR-09-3178. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Tao Q, Cheung KF, Jin H, Poon FF, Wang X, Li H, Cheng YY, Rocken C, Ebert MP, Chan AT, Sung JJ. Epigenetic identification of ubiquitin carboxyl-terminal hydrolase L1 as a functional tumor suppressor and biomarker for hepatocellular carcinoma and other digestive tumors. Hepatology. 2008;48:508–18. doi: 10.1002/hep.22343. [DOI] [PubMed] [Google Scholar]
- 20.Martinez I, Wang J, Hobson KF, Ferris RL, Khan SA. Identification of differentially expressed genes in HPV-positive and HPV-negative oropharyngeal squamous cell carcinomas. Eur J Cancer. 2007;43:415–32. doi: 10.1016/j.ejca.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen H, Cai M, Zhao S, Wang H, Li M, Yao S, Jiang N. Overexpression of RFC3 is correlated with ovarian tumor development and poor prognosis. Tumour Biol. 2014;35:10259–66. doi: 10.1007/s13277-014-2216-2. [DOI] [PubMed] [Google Scholar]
- 22.Pucci B, Kasten M, Giordano A. Cell cycle and apoptosis. Neoplasia. 2000;2:291–9. doi: 10.1038/sj.neo.7900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozols RF. Progress in ovarian cancer: an overview and perspective. Eur J Cancer Suppl. 2003;1:43–55. [Google Scholar]
- 24.Lockwood WW, Thu KL, Lin L, Pikor LA, Chari R, Lam WL, Beer DG. Integrative genomics identified RFC3 as an amplified candidate oncogene in esophageal adenocarcinoma. Clin Cancer Res. 2012;18:1936–46. doi: 10.1158/1078-0432.CCR-11-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu SY, Lopez-Berestein G, Calin GA, Sood AK. RNAi therapies: drugging the undruggable. Sci Transl Med. 2014;6:240p–247p. doi: 10.1126/scitranslmed.3008362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita Y, Kuwano K, Ochiya T. Development of Small RNA Delivery Systems for Lung Cancer Therapy. Int J Mol Sci. 2015;16:5254–70. doi: 10.3390/ijms16035254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arai M, Kondoh N, Imazeki N, Hada A, Hatsuse K, Matsubara O, Yamamoto M. The knockdown of endogenous replication factor C4 decreases the growth and enhances the chemosensitivity of hepatocellular carcinoma cells. Liver Int. 2009;29:55–62. doi: 10.1111/j.1478-3231.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 28.Arima Y, Hirota T, Bronner C, Mousli M, Fujiwara T, Niwa S, Ishikawa H, Saya H. Down-regulation of nuclear protein ICBP90 by p53/p21Cip1/WAF1-dependent DNA-damage checkpoint signals contributes to cell cycle arrest at G1/S transition. Genes Cells. 2004;9:131–42. doi: 10.1111/j.1356-9597.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim HS, Brill SJ. Rfc4 interacts with Rpa1 and is required for both DNA replication and DNA damage checkpoints in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:3725–37. doi: 10.1128/MCB.21.11.3725-3737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–56. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]


