Abstract
Pentoxifylline (PTX) is a non-selective phosphodiesterase inhibitor with the effects of antioxidation, anti-inflammation and anti-fibrosis that has been shown to induce damage in liver. The purpose of this study is to investigate the effects and possible mechanisms of PTX on thioacetamide (TAA)-induced acute liver injury in rats. Male Sprague-Dawley (SD) rats were divided into four groups: control, PTX, TAA and PTX+TAA treated groups. Rats were administrated TAA together with or without PTX for a week and sacrificed 24 h after the last intragastric administration of PTX. Histopathological analysis was carried out. The liver function, the indices of oxidative stress including malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) in liver tissues, and pro-inflammatory cytokines expressions were examined. The mRNA level of NF-κB p65 in liver was also determined. PTX significantly attenuated TAA-induced liver injury. The serum transaminase and MDA levels were reduced while the levels of SOD and GSH were increased, as compared with the TAA-treated group. PTX also remarkably suppressed the secretions of pro-inflammatory cytokines and the nuclear factor-κB (NF-κB) activation induced by TAA. In addition, the histopathological analysis showed that the range and degree of liver tissue lesions were improved obviously in PTX treated group. Pentoxifylline could ameliorate the effects of thioacetamide-induced acute liver injury in rats by inhibiting oxidative stress, expressions of pro-inflammatory cytokines and NF-κB activation.
Keywords: Acute liver injury, pentoxifylline, oxidative stress, pro-inflammatory cytokines, NF-κB
Introduction
The liver may be considered as one of the most important organs which regulate the balance of metabolism [1]. Acute liver injury caused by a variety of reasons induces inflammation, oxidative stress, and necrosis of liver cells [2]. Thioacetamide (TAA) is widely used to induce acute-toxic liver injury, and specially causes membrane damage, oxidative stress and accumulation of lipid droplets in the hepatocyte cytoplasm to enhance inflammation and liver injury [3]. Hepatitis usually occurs within few hours after TAA treatment.
The correlation between inflammation and oxidative stress in the course of liver injury is indisputable. The systemic inflammatory response is mediated by activated pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukins (IL-1β and IL-6) and oxygen radicals which may sensitize hepatocytes to the toxicity and damage [4,5]. In addition, the nuclear factor-κB (NF-κB) is known as a cardinal regulator of inflammatory res-ponse. Multiple studies have shown that it can modulate the transcription activations of pro-inflammatory cytokines [6].
Pentoxifylline (PTX), 1-[5-oxohexyl]-3, 7-dimethylxanthine] is a non-specific phosphodiesterase inhibitor that has been routinely employed for circulatory diseases for over 20 years [7]. Recent researches have implied therapeutic effects of PTX on alcoholic hepatitis, non-alcoholic fatty liver and liver fibrosis, but its effect on acute liver injury remains unclear [8-10]. Therefore, these studies prompted us to observe whether PTX might play a protective role against acute liver injury induced by TAA in rats and to investigate its possible mechanisms.
Methods
Animal
Male Sprague-Dawley (SD) rats purchased from the Animal Centre of Xi’an Jiaotong University (Shaanxi, China) weighing 180-200 g were housed in a standard animal laboratory with free access to food and water in this study. The rats were adapted in a standard temperature at 20-25°C, 45-55% relative humidity and a 12-h light/dark cycle, and acclimatized for 1 week to use. This experiment was carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University (Permit Number: SCXK2007-001). All efforts were made to minimize animal suffering and the number of animals used.
Experimental design
Forty male SD rats were randomly divided into four groups (n=10 per group): (1) control group: treated with appropriate vehicles (distilled water); (2) PTX group: treated with saline via intraperitoneal injection on the first day and PTX (100 mg/kg dissolved in distilled water, St Louis, MO, USA) by intragastric administration daily for a week [11]; (3) TAA group: treated with TAA (300 mg/kg dissolved in distilled water, St Louis, MO, USA) via intraperitoneal injection on the first day and appropriate vehicles (distilled water) by intragastric administration daily for a week [12]; (4) TAA+PTX group: treated with TAA (300 mg/kg) on the first day and PTX (100 mg/kg) daily respectively (the oral administration of PTX was given 2 h after TAA treatment). No animals died during the experiment. At the end of the week and 24 h after the last intragastric administration of PTX, animals were anesthetized with chloral hydrate. Inferior caval vein blood was collected and centrifuged at 4,000 rpm for 15 min. The serum was stored at -80°C for biochemical estimation and ELISA analysis. Liver was rapidly excised and cut into two specimens. One was fixed in 4% paraformaldehyde for histological examinations, and another was stored in liquid nitrogen until analysis.
Serum liver functional assay
The activities of alanine transaminase (ALT) and aspartate transaminase (AST) were measured in all groups using a biochemistry autoanalyzer (Olympus AU2700, Japan).
Assessment of oxidative stress
Liver samples which collected from each group were homogenized with cold phosphate buffer (pH 7.4) and centrifuged at 4,000 rpm for 30 min at 4°C. The supernatant was used to assay malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) activities. The levels of MDA, SOD and GSH were measured using a MDA, SOD, GSH assay kit (Boshide of Biological Science, Wuhan, China) respectively following the manufacturer’s instructions.
Determination of inflammatory cytokines
Serum was collected from each group as above. The levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) were measured by Enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) respectively according to the manufacturer’s instructions.
Histological examination
The liver tissues of each group fixed in 4% paraformaldehyde and processed by routine histological procedures, were embedded in paraffin and 5 μm sections were cut from the blocks. The paraffin-embedded liver sections were stained with hematoxylin-eosin (H&E) for histopathological examination to evaluate the degree of inflammation in the liver parenchyma. Each sample was examined in a blinded manner with light microscopy (Leica TCS SP, German) and observed at 200× magnification.
Quantitative real-time polymerase chain reaction (PCR)
Total liver RNA was extracted by TRIzol kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s suggested protocol. The RNA was then reverse transcribed using a RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Lithuania). The mRNA expression level of nuclear factor (NF)-κB was measured by Quantitative RT-PCR. The primer sequences used were as follows: NF-κB p65, 5’-TCCCCTGTACGATAGTCGGCTC-3’ (forward) and 5’-GAGCGTTGCTTTGGATCAAGG-3’ (reverse); and β-actin, 5’-CCTCATGCCATCCTGCGTCTG-3’ (forward) and 5’-TTGCTCGAAGTCTAGGGCAACATAG-3’ (reverse). The expression level of the transcript was normalized to the mean β-actin mRNA expression in the same tissue sample.
Statistical analysis
The experimental data were expressed as the mean ± SD and analyzed using IBM SPSS 17.0 statistics software (IBM, Armonk, NY, USA). Statistical significance among the groups was analyzed using one-way analysis of variance with the Student-IVewman-Keuls test. P<0.05 was considered statistically significant.
Results
Effects of PTX on liver function
For assessment of the liver damage, the serum was obtained to measure transaminase (ALT and AST) activities. Statistics showed that there was no difference between PTX group and control group. In TAA group, serum levels of liver enzyme activities were significantly increased compared with controls (P<0.01), whereas the levels in PTX and TAA co-treatment group were much lower (P<0.01) (Figure 1). Thus, these statistics suggested that PTX could ameliorate the TAA-induced liver injury.
Figure 1.
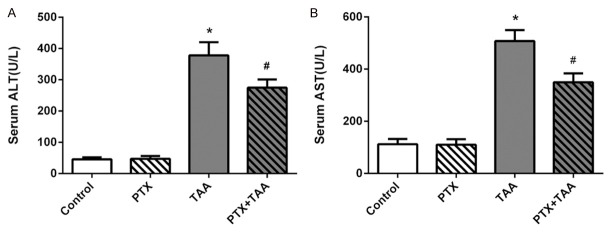
Changes in liver function in each group. The serum levels of ALT and AST are displayed on different groups exposed to PTX and/or TAA administration. Control rats receiving normal diet and water. All values refer to mean ± standard deviation (SD). A. ALT. B. AST. *P<0.01 as compared with control group. #P<0.01 as compared with TAA group.
Effects of PTX on oxidative stress
In order to evaluate liver oxidative stress, we measured the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH) in liver tissues. The level of MDA was significantly elevated in TAA-treated group while contents of SOD and GSH were decreased compared with the control group (P<0.01). In the group receiving PTX and TAA, SOD and GSH were increased almost at a normal level while the level of MAD was preserved (P<0.05) (Figure 2), which suggested PTX could inhibit the activation of oxidative stress.
Figure 2.
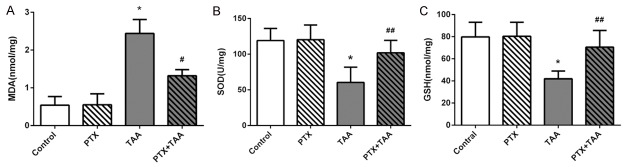
Levels of MDA, SOD, and GSH in liver tissues in different groups. Levels are displayed on different groups exposed to PTX and/or TAA administration. Control rats receiving normal diet and water. All values refer to mean ± standard deviation (SD). A. MDA. B. SOD. C. GSH. *P<0.01 as compared with control group. #P<0.01 as compared with TAA group. ##P<0.05 as compared with TAA group.
Effects of PTX on pro-inflammatory cytokine response
To determine whether PTX suppressed inflammation caused by PTX, we examined the serum TNF-α, IL-1β and IL-6 levels. As shown in results, the levels of TNF-α, IL-1β and IL-6 in serum were significantly elevated in TAA rats as compared to that of the control group (P<0.01). In contrast, PTX treatment attenuated these pro-inflammatory cytokines levels, which suggested that PTX inhibited the inflammatory response induced by TAA administration. However, PTX per se had no effect on pro-inflammatory cytokines levels in serum (Figure 3).
Figure 3.
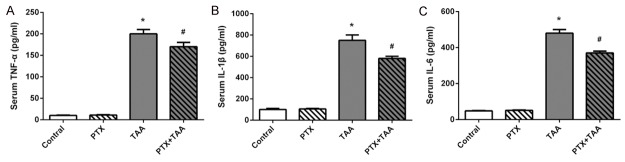
Serum levels of TNF-α, IL-1β, and IL-6 in different groups. Levels are displayed on different groups exposed to PTX and/or TAA administration. Control rats receiving normal diet and water. All values refer to mean ± standard deviation (SD). A. TNF-α. B. IL-1β. C. IL-6. *P<0.01 as compared with control group. #P<0.01 as compared with TAA group.
Effects of PTX on liver histopathological changes
To evaluate the effects of PTX on hepatocellular inflammation, histological changes in liver were observed by hematoxylin-eosin (H&E) staining. Liver sections from the control and PTX control groups, demonstrated a normal lobular architecture with radiating hepatic cords and clear central veins, without inflammation or necrosis (Figure 4A and 4B). In contrast, TAA-treated group showed significant changes in liver structure with severe infiltration of inflammatory cells around the central vein and centrilobular regions (Figure 4C). However, treatment with PTX and TAA demonstrated only moderate inflammatory cell infiltration involving the liver interstitial areas and maintained a rather normal morphology (Figure 4D). These data clearly indicated that PTX significantly diminished the degree of liver injury.
Figure 4.
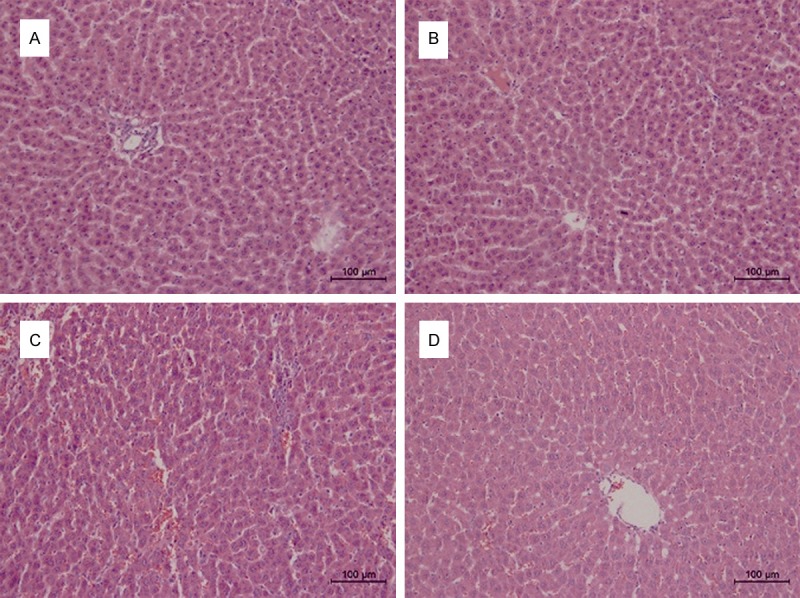
Effect of PTX on the histologic changes in liver. Liver sections are displayed on different groups exposed to PTX and/or TAA administration by H&E staining (histopathology; original magnification 200×). A. Control group: normal cell structure and lobular architecture. B. PTX control group: normal cell structure and lobular architecture. C. TAA-treated group: significant hepatocellular damage with severs inflammatory cell infiltration. D. PTX and TAA co-treated group: mild inflammation.
Effects of PTX on activation of NF-κB
It has been demonstrated that NF-κB is a key player in the progression of liver inflammation and its activation is essential for pro-inflammatory cytokine production. To elucidate whether PTX could modulate NF-κB, we detected the expression of NF-κB subunit p65 mRNA level. Compared to the control group, the mRNA level of NF-κB was significantly increased after TAA treatment. In the PTX and TAA co-treated group, the expression of NF-κB revealed an effective decrease at the mRNA level (Figure 5). All of these results suggested that PTX was able to inhibit NF-κB in acute liver-injured rats induced by TAA administration.
Figure 5.
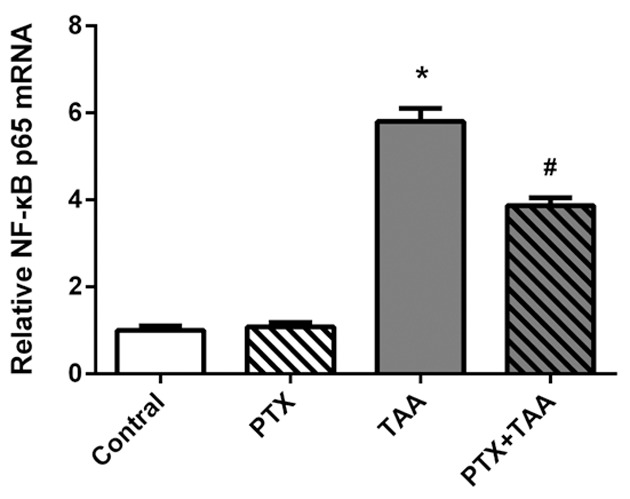
The expressions of mRNA for NF-κB p65 in liver tissues by quantitative RT-PCR analysis. Levels are displayed on different groups exposed to PTX and/or TAA administration. Control rats receiving normal diet and water. The mRNA levels were normalized to β-actin. Mean values ± SD are provided. *P<0.01 as compared with control group. #P<0.01 as compared with TAA group.
Discussion
The present study showed that PTX effectively attenuated TAA-induced histological changes and the serum transaminase levels were improved. Moreover, the oxidative stress activation and NF-κB with pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) regulated by this factor were inhibited. Taken together, our study indicated that PTX could protect against the acute liver injury in rat model induced by TAA.
TAA proves highly useful as an experimental liver injury model. It has been used for years because the lesions caused by this hepatotoxic drug replicate these seen in most cases of liver disease in human, which makes it be a nice model to study the mechanism in vivo. In previous researches, the pathological roles of high-does TAA is mainly restricted to acute liver injury instead of direct damage to other organs, and is known to induce oxidative stress, lipid peroxidation [13], as well as to cause a decrease in antioxidant status [14].
Pentoxifylline (PTX) is a phosphodiesterase inhibitor that is widely used in peripheral vascular disease treatment, and for which a wide range of reported immunomodulatory, antioxidant and anti-inflammation activities have been described [15]. Recently, the protective effect of PTX in rat liver injury was demonstrated where PTX administration inhibited serum aminotransferase activities, including ALT and AST [16]. These enzymes which released from damaged hepatocytes into the blood, have been utilized as very important indicators to judge the severity of hepatic injury [17]. In our study, data revealed that oral administration of PTX attenuated the elevations of ALT and AST in serum induced by TAA. Meanwhile, we used histologic methods to reveal the cell inflammation as supportive means also indicated that PTX could diminish the degree of liver injury.
The level of MDA which is a product of lipoperoxidative processes is known to be an important indicator in terms of oxidative stress [18], however, SOD and GSH as primary defenses can reduce the oxidative stress and inhibit the activation of inflammatory mediators. This study showed that the oxygen-derived free radicals major role in the pathophysiology of TAA-induced acute liver injury in rats was possible. In rats with TAA-treated, decrease in liver SOD, GSH and increase in MDA liver level were determined. By PTX administration, these levels were concluded to be normalized as control groups. Therefore, these results suggested that PTX could prevent oxidative stress induced by TAA.
On the other hand, the liver resembles a central organ of cytokines activity; the production of cytokines in liver often depends upon the initial induction of early-response pro-inflammatory cytokines released from tissue-resident macrophages (Kupffer cells) [2,19]. Hepatotoxins such as TAA could quickly induce pro-inflammatory cytokines by Kupffer cells. TNF-α, IL-1β and IL-6 are considered to be such inflammatory biomarkers [20,21]. These cytokines which usually lead to tissue destruction, play an important role in inflammatory conditions. Furthermore, they have been shown to antagonize hepatocyte proliferation in previous studies [22,23]. Our results obtained that PTX administrated rats demonstrated lower serum levels of TNF-α, IL-1β and IL-6 compared to the TAA control group. In this study, PTX has been shown to possess strong beneficial effects against inflammation in acute liver injury.
NF-κB plays a critical role in the transcriptional control of expressions of pro-inflammatory genes in various cells [24]. Several lines of evidence suggest that the induction of NF-κB dependent gene expression in Kupffer cells contributes to TAA-induced liver injury [25]. Although PTX is known to suppress the activation of NF-κB and inhibit inflammation [26,27], it has been the first time to show that PTX prevents activation of NF-κB on acute liver injury. In the current work we observed that compared with the control group, TAA led to increased NF-κB activity, however, PTX could inhibit the activation of NF-κB in TAA-treated rats. Consequently, this research suggested that the protective effects of PTX were mediated through the suppression of the NF-κB system in acute liver-injured rats.
In conclusion, the present study demonstrates that administration of PTX can reduce liver injury in rats induced by TAA. The protective effect of PTX may be due to the block in oxidative stress, the expression of pro-inflammatory cytokines, and the activation of NF-κB. Our studies suggest that antioxidant and anti-inflammatory action of PTX are possible mechanisms to improve liver injury. Since the pro-inflammatory cytokines exists in Kupffer cell, which is the target of PTX is still unclear; study in vitro is needed in the future.
Acknowledgements
This work was supported by the National Spark Program Project (No. 2011 GAB50001). We also wish to acknowledge the Animal Centre of Xi’an Jiaotong University.
Disclosure of conflict of interest
None.
References
- 1.Koblihová E, Lukšan O, Mrázová I, Ryska M, Červenka L. Hepatocyte transplantation attenuates the course of acute liver failure induced by thioacetamide in Lewis rats. Physiol Res. 2015 doi: 10.33549/physiolres.932914. PMID: 25804092. [DOI] [PubMed] [Google Scholar]
- 2.Muriel P. Cytokines in liver diseases. In: Sahu S, editor. Hepatotoxicity: from genomics to in vitro and in vivo models. West Sussex, UK: John Wiley and Sons LTD; 2008. pp. 371–390. [Google Scholar]
- 3.Koen YM, Sarma D, Hajovsky H, Galeva NA, Williams TD, Staudinger JL, Hanzlik RP. Protein targets of thioacetamide metabolites in rat hepatocytes. Chem Res Toxicol. 2013;26:564–574. doi: 10.1021/tx400001x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun S, Zhang H, Xue B, Wu Y, Wang J, Yin Z, Luo L. Protective effect of glutathione against lipopolysaccharide-induced inflammation and mortality in rats. Inflamm Res. 2006;55:504–510. doi: 10.1007/s00011-006-6037-7. [DOI] [PubMed] [Google Scholar]
- 5.Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goraca A. Influence of block of NF-kappa B signaling pathway on oxidative stress in the liver homogenates. Oxid Med Cell Longev. 2013;2013:308358. doi: 10.1155/2013/308358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Flores G, Ortiz-Lazareno PC, Lerma-Diaz JM, Dominguez-Rodriguez JR, Jave-Suarez LF, Aguilar-Lemarroy Adel C, de Celis-Carrillo R, del Toro-Arreola S, Castellanos-Esparza YC, Bravo-Cuellar A. Pentoxifylline sensitizes human cervical tumor cells to cisplatin-induced apoptosis by suppressing NF-kappa B and decreased cell senescence. BMC Cancer. 2011;11:483. doi: 10.1186/1471-2407-11-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker R, Armstrong MJ, Corbett C, Rowe IA, Houlihan DD. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;37:845–854. doi: 10.1111/apt.12279. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2011;10:49–59. doi: 10.1186/1476-511X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Movassaghi S, Sharifi ZN, Mohammadzadeh F, Soleimani M. Pentoxifylline protects the rat liver against fibrosis and apoptosis induced by acute administration of 3, 4-Methylenedioxymethamphetamine (MDMA or Ecstasy) Iran J Basic Med Sci. 2013;16:922–927. [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed AF, El-Maraghy NN, Ghaney RH, Elshazly SM. Therapeutic effect of captopril, pentoxifylline, and cordycepssinensis in pre-hepatic portal hypertensive rats. Saudi J Gastroenterol. 2012;18:182. doi: 10.4103/1319-3767.96451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salam OM, Mohammed NA, Sleem AA, Farrag AR. The effect of antidepressant drugs on thioacetamide-induced oxidative stress. Eur Rev Med Pharmacol Sci. 2013;17:735–744. [PubMed] [Google Scholar]
- 13.Miiller D, Sommer M, Kretzschmar M, Zimmermann T, Buko VU, Lukivskaya O, Dargel R. Lipid peroxidation in thioacetamide-induced macronodular rat liver cirrhosis. Arch Toxicol. 1991;65:199–203. doi: 10.1007/BF02307309. [DOI] [PubMed] [Google Scholar]
- 14.Abul H, Mathew TC, Dashti HM, Al-Bader A. Level of super-oxide dismutase, glutathione peroxidase and uric acid in thioacetamide-induced cirrhotic rats. Anat Histol Embryol. 2002;31:66–71. doi: 10.1046/j.1439-0264.2002.00359.x. [DOI] [PubMed] [Google Scholar]
- 15.Shaw SM, Shah MK, Williams SG, Fildes JE. Immunological mechanisms of pentoxifylline in chronic heart failure. Eur J Heart Fail. 2009;11:113–118. doi: 10.1093/eurjhf/hfn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Movassaghi S, Sharifi ZN, Mohammadzadeh F, Soleimani M. Pentoxifylline Protects the Rat Liver Against Fibrosis and Apoptosis Induced by Acute Administration of 3, 4-Methylenedioxymethamphetamine (MDMA or Ecstasy) Iran J Basic Med Sci. 2013;16:922. [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaya İ, Karapehlivan M, Yilmaz M. Investigation of effects on plasma nitric oxide, malondialdehyde and total sialic acid levels of glyphosate in Kars creek transcaucasian barb (Capoeta capoeta [Guldenstaedt, 1773] ) in Turkey. Fresenius Environ Bull. 2012;21:123–126. [Google Scholar]
- 19.Simpson KJ, Lukacs NW, Colletti L, Strieter RM, Kunkel SL. Cytokines and the liver. J Hepatol. 1997;27:1120–1132. doi: 10.1016/s0168-8278(97)80160-2. [DOI] [PubMed] [Google Scholar]
- 20.Wu YL, Lian LH, Wan Y, Nan JX. Baicalein inhibits nuclear factor-κB and apoptosis via c-FLIP and MAPK in D-GalN/LPS induced acute liver failure in murine models. Chem Biol Interact. 2010;188:526–534. doi: 10.1016/j.cbi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Diao Y, Zhao XF, Lin JS, Wang QZ, Xu RA. Protection of the liver against CCl4-induced injury by intramuscular electrotransfer of a kallistatin-encoding plasmid. World J Gastroenterol. 2011;17:111. doi: 10.3748/wjg.v17.i1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Wang M, Carr BI. The inhibitory effect of interleukin 1β on rat hepatocyte DNA synthesis is mediated by nitric oxide. Hepatology. 1998;28:430–435. doi: 10.1002/hep.510280221. [DOI] [PubMed] [Google Scholar]
- 23.Ogiso T, Nagaki M, Takai S, Tsukada Y, Mukai T, Kimura K, Moriwaki H. Granulocyte colony-stimulating factor impairs liver regeneration in mice through the up-regulation of interleukin-1β. J Hepatol. 2007;47:816–825. doi: 10.1016/j.jhep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-κB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta. 2007;1770:989–996. doi: 10.1016/j.bbagen.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Demirel U, Yalnız M, Aygün C, Orhan C, Tuzcu M, Sahin K, Ozercan IH, Bahçecioğlu IH. Allopurinol ameliorates thioacetamide-induced acute liver failure by regulating cellular redox-sensitive transcription factors in rats. Inflammation. 2012;35:1549–1557. doi: 10.1007/s10753-012-9470-5. [DOI] [PubMed] [Google Scholar]
- 26.Lerma-Díaz J M, Hernández-Flores G, Domínguez-Rodríguez JR, Ortíz-Lazareno PC, Gómez-Contreras P, Cervantes-Munguía R, Scott-Algara D, Aguilar-Lemarroy A, Jave-Suárez LF, Bravo-Cuellar A. In vivo and in vitro sensitization of leukemic cells to adriamycin-induced apoptosis by pentoxifylline: Involvement of caspase cascades and IκBα phosphorylation. Immunol Lett. 2006;103:149–158. doi: 10.1016/j.imlet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Hepgül G, Tanrıkulu S, Ünalp HR, Akguner T, Erbil Y, Olgaç V, Ademoğlu E. Preventive effect of pentoxifylline on acute radiation damage via antioxidant and anti-inflammatory pathways. Dig Dis Sci. 2010;55:617–625. doi: 10.1007/s10620-009-0780-x. [DOI] [PubMed] [Google Scholar]


