Abstract
Adenocarcinoma is the most common histologic type of non-small cell lung carcinomas. The existence of lung cancer stem cells (CSCs) and epithelial-mesenchymal transition (EMT) in human tissue is controversy. The aim of this study is to investigate the expression and clinical significance of CSCs and EMT markers and evaluate the correlation between the two in lung adenocarcinoma. A total of 97 cases comprise the tissue microarray from surgical resection for primary lung adenocarcinoma. Immunohistochemistry for ALDH1 and CD44 as CSC markers and E-cadherin, vimentin, fibronectin, SMA as EMT markers was performed. High ALDH1A1 expression was statistically associated with female gender (P=0.001), smoker (P=0.012), and high pT stages (P=0.046). High CD44 expression was statistically associated with female gender (P=0.008), non-smoker (P=0.000), and no pleural invasion (P=0.039). High expression of ALDH1 was associated with good overall survival (P=0.021). High expression of CD44 was correlated with both good overall survival (P=0.024) and disease-free survival (P=0.000). Vimentin expression was associated with pT stage (P=0.001) and pleural invasion (P=0.028). E-cadherin, fibronectin and SMA were not associated with clinicopathologic correlation and all EMT markers were not correlated with survival of lung adenocarcinoma. CSC markers expression was not related to EMT. Our results showed that the expression of CSCs was associated with a good prognosis in lung adenocarcinoma. The prognostic significance of EMT markers was skeptical in this study. There is a need for more research about CSC, EMT, and the relation between these two in human lung adenocarcinoma.
Keywords: Lung cancer, cancer stem cell, epithelial-mesenchymal transition, adenocarcinoma, prognosis, immunohistochemistry
Introduction
Lung cancer is the most lethal cancer in the world, and despite significant therapeutic improvements, its survival rate still remains low. Regardless of the advances in diagnostics and treatment that have been achieved in the last two decades, the overall high mortality rate has remained [1]. Adenocarcinoma of the lung is the most common histological type of non-small cell lung carcinomas, comprising about 60% of cases [2]. The incidence of adenocarcinoma of the lung has increased significantly in the past few decades. Despite continuous efforts to improve therapeutic outcomes with maintenance chemotherapy and targeted therapy with epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors, the overall five-year survival rate for lung cancers is still below 15%; therefore, improvements in both diagnostics and treatment are urgently needed [1,3].
Recently, epithelial-mesenchymal transition (EMT) has been reported to be associated with more aggressive tumor behavior and prognosis in malignant tumors [4-6]. EMT is characterized by a loss of cell adhesion and increased cell mobility due to cells gaining a mesenchymal phenotype. During the EMT process, tumor cells are expected to lose their epithelial phenotype and gradually and sequentially acquire a mesenchymal phenotype [5]. Although the concept of EMT in cancer is still controversial, EMT has been implicated in a number of epithelial cancers and has been shown to correlate with the metastatic potential of cancers [5,6]. Most studies about EMT in cancer have used in vitro systems that employ cell lines and focus on the detailed mechanism of EMT, identifying a number of the transcription factors and signaling pathways that are involved [5].
Ample studies have suggested that EMT may induce stemness properties in normal and malignant cells [7,8]. Also, activation of EMT has been associated with decreased drug sensitivity, and it has been found that it may even contribute to a decreased efficacy of therapy and resistance to tyrosine kinase inhibitors in EGFR mutated non-small cell lung carcinomas [9,10], apparently through the acquisition of stem cell-like properties by the tumor cells [11]. Therefore, cancer cells undergoing EMT may indeed become metastatic drug resistant cancer cell progenitors, or even metastatic cancer stem cells (CSCs) [12].
CSCs are a rare population of undifferentiated tumorigenic cells responsible for tumor initiation, maintenance, and spreading [13]. These cells display unlimited proliferation potential, the ability to self-renew, and the capacity to generate a progeny of differentiated cells that constitute the major tumor population [14]. According to the CSC hypothesis, most solid tumors contain a small subset of phenotypically distinct cells with the properties of unlimited self-renewal, innate chemoresistance, and enhanced clonogenic potential [15]. CSCs constitute a subpopulation of cells that are highly tumorigenic and that exhibit biological properties similar to those of normal tissue stem cells, including an unlimited self-renewal capacity, an extensive proliferative capacity, and a capacity to generate differentiated progeny [12].
CSCs express specific molecules, termed CSC markers. In vitro studies have confirmed that a fraction of cells expressing CD133, CD44, nuclear β-catenin, and/or ALDH1 are exclusively clonogenic and tumorigenic [16,17]. These molecules have been accepted to be representative markers for CSCs in different types of cancers [18,19]. Expression of ALDH1A1 (an isoform of ALDH1), CD133 and CD44, is known to be associated with poor survival in lung cancers in several studies [19-21].
The existence of human lung CSCs has not yet been reported actively; however, indirect evidence suggests the possible presence of CSCs in pulmonary tumors [14]. Stem-like cells have been identified in trials with mouse lungs, such as a cell population that is able to drive the malignant transformation in experimentally induced neoplasia [21]. Moreover, human lung tumors sometimes show phenotypic heterogeneity, suggesting that they may originate from a multipotent cell [22].
The aim of this study is to investigate the expression and clinical significance of CSC and EMT markers and evaluate the correlation between CSC and EMT in lung adenocarcinoma. In this study, we evaluate the association of prognostic significance with the expression pattern of various EMT and CSC related proteins in lung adenocarcinoma.
Material and methods
Tissue samples
We included a total of 97 formalin-fixed and paraffin-embedded tumor samples from patients who underwent surgical resection for primary lung adenocarcinoma. All patients gave written informed consent according to institutional guidelines. Clinical and pathological reports were reviewed to determine the status of age, gender, smoking history, tumor size (pT), nodal status (pN), distant metastasis (pM), stage, lymphovascular invasion, and pleural invasion. The pTNM classification and stage were applied according to guidelines from the 2010 American Joint Committee on Cancer staging manual.
Tissue microarray and immunohistochemistry
Hematoxylin and eosin (H&E) stained tissues slides were reviewed to confirm the histological diagnosis and to select representative areas for immunostaining. Two cylindrical cores (2 mm in diameter) in one case were obtained from formalin-fixed and paraffin-embedded tissue blocks corresponding to the H&E slides to construct the tissue microarray. Sectioning of microarray blocks produced 4 μm thick sections after completion of the tissue array. Microslide tissue sections were deparaffinized with xylene, hydrated using a diluted alcohol series, and immersed in 0.3% H2O2 in methanol to quench endogenous peroxidase activity. Sections were then microwaved for 15 min in 10 mM citrate buffer (pH 6.0) for antigen retrieval. Each section was blocked with 4% bovine serum albumin in PBS with 0.1% Tween 20 (PBST) for 30 min to reduce non-specific staining. Sections were then incubated with anti-fibronectin (dilution: 1:100, BD Biosciences, San Jose, CA, USA), anti-smooth muscle actin (1:400, Sigma-Aldrich, Saint Louis, MO, USA), anti-vimentin (dilution: 1:200, BD Biosciences), anti-E-cadherin (1:200, BD Biosciences), and anti-ALDH1A1 antibody (dilution: 1:50, Nobus Biologicals, Littleton, CO, USA) and anti-CD44 antibody (dilution: 1:500, Nobus Biologicals) in PBST containing 3 mg/ml goat globulin (Sigma) for 60 min at room temperature, followed by three successive washes with a buffer. The sections were then incubated with an anti-mouse/rabbit antibody (Envision plus, Dako, Carpinteria, CA, USA) for 30 min at room temperature. The chromogen used was 3,3’-diaminobenzidine (Dako). The sections were counterstained with Meyer’s hematoxylin. Omitting the primary antibody provided negative controls for immunostaining using normal mouse and rabbit serum.
Evaluation of immunohistochemical staining results and phenotyping of EMT
This study used two scoring methods. The first was created by Alamgeer et al. [20] for scoring of CSC expression. Scoring of ALDH1A1 and CD44 was performed according to the staining intensity at least 10% of tumor cells as flows: 0 = no staining, 1+ = weak staining, 2+ = moderate staining, and 3+ = strong staining. The score 0 and 1 were considered as negative, the score 2 and 3 were considered as positive. The second technique was Sinicrope et al.’s [23] scoring method for EMT markers, which was used to evaluate both immunohistochemical staining intensity and the proportion of stained epithelial cells. Staining intensity was further classified as follows: (1) 1, weak, (2) 2, moderate, or (3) 3, strong. Positive cells were quantified as a percentage of the total number of epithelial cells and assigned to one of the following five categories: (1) 0, < 5%, (2) 1, 5-25%, (3) 2, 26-50%, (4) 3, 51-75%, or (5) 4, > 75%. The percentages of epithelial cell positivity and staining intensity were multiplied to generate an immunoreactivity score for each case. The positive cutoff point was 4. For example, if the staining intensity was moderate (2 points) and the percentage of positive cells was 80% (4 points), then the immunoreactivity score was calculated as 2 × 4=8, and judged as positive. As a result, immunoreactivity score values ranged from 0 to 12. Two pathologists, who were blinded to patient outcomes, independently examined and scored each lesion. Differences in interpretation were resolved by consensual agreement.
We were divided into the following four phenotypes of EMT on the basis of the expression of EMT related markers proposed by Sung et al. [5]: (1) complete type, characterized by loss of the epithelial phenotype with acquisition of the mesenchymal phenotype; (2) incomplete type 1 (hybrid type), characterized by a tumor showing both mesenchymal and epithelial phenotypes; (3) incomplete type 2 (null type), defined by loss of the epithelial phenotype without acquisition of a mesenchymal phenotype; and (4) wild type, characterized by a tumor with no evidence of EMT.
Statistical analysis
A summary for the clinicopathologic factors was performed using descriptive analysis, the mean and standard deviation (SD) presented for quantitative variables, and the frequency and percentages for the qualitative variables. Comparisons of the clinicopathologic factors and each marker were analyzed using two sample t-tests for quantitative variables and a chi-square test for qualitative variables (Tables 1, 2 and 3). Comparisons of CSC expression and EMT markers were analyzed using a chi-square test (Table 4). Overall and disease-free survival curves were estimated using the Kaplan-Meier method, and the significance of differences between survival curves was determined using the log rank test (Figures 3 and 4). Comparison of the CSC markers and EMT phenotypes was analyzed using a chi-square test. All tests were two-sided, and a P-value of less than 0.05 indicated statistical significance. IBM SPSS 19.0 was used for analysis.
Table 1.
Association between ALDH1A1 and CD44 expression and clinicopathologic factors of lung adenocarcinoma patients
| Factors | Total No. (%) | ALDH1A1 No. (%) | CD44 No. (%) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| low | high | p-value | low | high | p-value | ||
| Age (years) | |||||||
| ≤ 65 | 56 (57.7) | 38 (60.3) | 18 (52.9) | .483 | 18 (60) | 38 (56.7) | .762 |
| > 65 | 41 (42.3) | 25 (39.7) | 16 (47.1) | 12 (40) | 29 (43.3) | ||
| Sex | |||||||
| Male | 42 (43.3) | 35 (55.6) | 7 (20.6) | .001 | 19 (63.3) | 23 (34.3) | .008 |
| Female | 55 (56.7) | 28 (44.4) | 27 (79.4) | 11 (36.7) | 44 (65.7) | ||
| Smoking | |||||||
| ≤ 10 PY | 59 (60.8) | 32 (53.3) | 27 (79.4) | .012 | 10 (34.5) | 49 (75.4) | .000 |
| > 10 PY | 37 (38.1) | 28 (46.7) | 7 (20.6) | 19 (65.5) | 16 (24.6) | ||
| pT | |||||||
| 1 | 42 (43.3) | 22 (34.9) | 20 (58.8) | .046 | 9 (30) | 33 (49.3) | .193 |
| 2 | 41 (42.3) | 31 (49.2) | 10 (29.4) | 14 (46.7) | 27 (40.3) | ||
| 3 | 9 (9.3) | 5 (7.9) | 4 (11.8) | 5 (16.7) | 4 (6) | ||
| 4 | 5 (5.2) | 5 (7.9) | 0 (0) | 2 (6.7) | 3 (4.5) | ||
| pN | |||||||
| 0 | 61 (62.9) | 36 (61) | 25 (83.3) | .177 | 16 (59.3) | 45 (72.6) | .086 |
| 1 | 8 (8.2) | 7 (11.9) | 1 (3.3) | 1 (3.7) | 7 (11.3) | ||
| 2 | 19 (19.6) | 15 (25.4) | 4 (13.3) | 10 (37) | 9 (14.5) | ||
| 3 | 1 (1.0) | 1 (1.7) | 0 (0) | 0 (0) | 1 (1.6) | ||
| pM | |||||||
| 0 | 88 (90.7) | 56 (88.9) | 32 (94.1) | .397 | 25 (83.3) | 63 (94) | .093 |
| 1 | 9 (9.3) | 7 (11.1) | 2 (5.9) | 5 (16.7) | 4 (6) | ||
| Stage | |||||||
| 1 | 54 (55.7) | 29 (46) | 25 (73.5) | .066 | 13 (43.3) | 41 (61.2) | .208 |
| 2 | 14 (14.4) | 12 19) | 2 (5.9) | 4 (13.3) | 10 (14.9) | ||
| 3 | 20 (20.6) | 15 (23.8) | 5( 14.7) | 8 (26.7) | 12 (17.9) | ||
| 4 | 9 (9.3) | 7 (11.1) | 2 (5.9) | 5 (16.7) | 4 (6) | ||
| Lymphovascular invasion | |||||||
| No | 39 (40.2) | 25 (39.7) | 14 (41.2) | .886 | 9 (30) | 30 (44.8) | .170 |
| Yes | 58 (59.8) | 38 (60.3) | 20 (58.8) | 21 (70) | 37 (55.2) | ||
| Pleural invasion | |||||||
| No | 57 (58.8) | 34 (54) | 23 (67.6) | .192 | 13 (43.3) | 44 (65.7) | .039 |
| Yes | 40 (41.2) | 29 (46) | 11 (32.4) | 17 (56.7) | 23 (34.3) | ||
PY, pack years.
Table 2.
Association between EMT markers and clinicopathologic factors of lung adenocarcinoma patients
| Factors preserve | E-cadherin No. (%) | vimentin No. (%) | fibronectin No. (%) | SMA No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| preserve | loss | p-value | low | high | p-value | low | high | p-value | low | high | p-value | ||
| Age (years) | ≤ 65 | 41 (57.7) | 15 (57.7) | .996 | 34 (55.7) | 22 (61.1) | .605 | 41 (60.3) | 15 (51.7) | .434 | 51 (62.2) | 5 (33.3) | .037 |
| > 65 | 30 (42.3) | 11 (42.3) | 27 (44.3) | 14 (38.9) | 27 (39.7) | 14 (48.3) | 31 (37.8) | 10 (66.7) | |||||
| Sex | Female | 39 (54.9) | 16 (61.5) | .561 | 38 (62.3) | 17 (47.2) | .148 | 37 (54.4) | 18 (62.1) | .486 | 48 (58.5) | 7 (46.7) | .394 |
| Male | 32 (45.1) | 10 (38.5) | 23 (37.7) | 19 (52.8) | 31 (45.6) | 11 (37.9) | 34 (41.5) | 8 (53.3) | |||||
| Smoking | ≤ 10 PY | 41 (60.3) | 18 (69.2) | .423 | 39 (66.1) | 20 (57.1) | .385 | 38 (57.6) | 21 (75) | .110 | 49 (62) | 10 (66.7) | .733 |
| > 10 PY | 27 (39.7) | 8 (30.8) | 20 (33.9) | 15 (42.9) | 28 (42.4) | 7 (25) | 30 (38) | 5 (33.3) | |||||
| pT | 1 | 31 (43.7) | 11 (42.3) | .857 | 35 (57.4) | 7 (19.4) | .001 | 31 (45.6) | 11 (37.9) | .771 | 33 (40.2) | 9 (60) | .361 |
| 2 | 31 (43.7) | 10 (38.5) | 17 (27.9) | 24 (66.7) | 27 (39.7) | 14 (48.3) | 36 (43.9) | 5 (33.3) | |||||
| 3 | 6 (8.5) | 3 (11.5) | 5 (8.2) | 4 (11.1) | 7 (10.3) | 2 (6.9) | 9 (11) | 0 (0) | |||||
| 4 | 3 (4.2) | 2 (7.7) | 4 (6.6) | 1 (2.8) | 7 (10.3) | 2 (6.9) | 4 (4.9) | 1 (6.7) | |||||
| pN | 0 | 41 (64.1) | 20 (80) | .240 | 40 (71.4) | 21 (63.6) | .227 | 45 (71.4) | 16 (61.5) | .349 | 53 (70.7) | 8 (57.1) | .538 |
| 1 | 8 (12.5) | 0 (0) | 3 (5.4) | 5 (15.2) | 6 (9.5) | 2 (7.7) | 7 (9.3) | 1 (7.1) | |||||
| 2 | 14 (21.9) | 5 (20) | 13 (23.2) | 6 (18.2) | 12 (19) | 7 (26.9) | 14 (18.7) | 5 (35.7) | |||||
| 3 | 1 (1.6) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (3.8) | 1 (1.3) | 0 (0) | |||||
| pM | 0 | 62 (87.3) | 26 (100) | .057 | 56 (91.8) | 32 (88.9) | .633 | 62 (91.2) | 26 (89.7) | .813 | 73 (89) | 15 (100) | .178 |
| 1 | 9 (12.7) | 0 (0) | 5 (8.2) | 4 (11.1) | 6 (8.8) | 3 (10.3) | 9 (11) | 0 (0) | |||||
| Stage | 1 | 37 (52.1) | 17 (65.4) | .127 | 37 (60.7) | 17 (47.2) | .336 | 40 (58.8) | 14 (48.3) | .696 | 46 (56.1) | 8 (53.3) | .131 |
| 2 | 12 (16.9) | 2 (7.7) | 6 (9.8) | 8 (22.2) | 10 (14.7) | 4 (13.8) | 13 (15.9) | 1 (6.7) | |||||
| 3 | 13 (18.3) | 7 (26.9) | 13 (21.3) | 7 (19.4) | 12 (17.6) | 8 (27.6) | 14 (17.1) | 6 (40) | |||||
| 4 | 9 (12.7) | 0 (0) | 5 (8.2) | 4 (11.1) | 6 (8.8) | 3 (10.3) | 9 (11) | 0 (0) | |||||
| Lymphovascular invasion | No | 30 (42.3) | 9 (34.6) | .497 | 27 (44.3) | 12 (33.3) | .289 | 30 (44.1) | 9 (31) | .229 | 32 (39) | 7 (46.7) | .579 |
| Yes | 41 (57.7) | 17 (65.4) | 34 (55.7) | 24 (66.7) | 38 (55.9) | 20 (69) | 50 (61) | 8 (53.3) | |||||
| Pleural invasion | |||||||||||||
| No | 45 (63.4) | 12 (46.2) | .127 | 41 (67.2) | 16 (44.4) | .028 | 39 (57.4) | 18 (62.1) | .666 | 46 (56.1) | 11 (73.3) | .212 | |
| Yes | 26 (36.6) | 14 (53.8) | 20 (32.8) | 20 (55.6) | 29 (42.6) | 11 (37.9) | 36 (43.9) | 4 (26.7) | |||||
PY, pack years.
Table 3.
Relationships between EMT phenotype and clinicopathologic factors
| Factors | Total No. (%) | Complete No. (%) | Hybrid No. (%) | Null No. (%) | Wild No. (%) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| ≤ 65 | 56 (57.7) | 5 (55.6) | 24 (57.1) | 10 (58.8) | 17 (58.6) | .998 |
| > 65 | 41 (42.3) | 4 (44.4) | 18 (42.9) | 7 (41.2) | 1 (41.4) | |
| Sex | . | |||||
| Male | 42 (43.3) | 7 (77.8) | 20 (47.6) | 9 (52.9) | 19 (65.5) | 256 |
| Female | 55 (56.7) | 2 (22.2) | 22 (52.4) | 8 (47.1) | 10 (34.5) | |
| Smoking | ||||||
| ≤ 10 PY | 59 (60.8) | 8 (88.9) | 24 (58.5) | 10 (58.8) | 17 (63) | .383 |
| > 10 PY | 37 (38.1) | 1 (11.1) | 17 (41.5) | 7 (41.2) | 10 (37) | |
| pT | ||||||
| 1 | 42 (43.3) | 3 (33.3) | 12 (28.6) | 8 (47.1) | 19 (65.5) | .148 |
| 2 | 41 (42.3) | 5 (55.6) | 23 (54.8) | 5 (29.4) | 8 (27.6) | |
| 3 | 9 (9.3) | 1 (11.1) | 5 (11.9) | 2 (11.8) | 1 (3.4) | |
| 4 | 5 (5.2) | 0 (0) | 2 (4.8) | 2 (11.8) | 1 (3.4) | |
| pN | ||||||
| 0 | 61 (62.9) | 7 (77.8) | 22 (59.5) | 13 (81.3) | 19 (70.4) | .649 |
| 1 | 8 (8.2) | 0 (0) | 6 (16.2) | 0 (0) | 2 (7.4) | |
| 2 | 19 (19.6) | 2 (22.2) | 8 (21.6) | 3 (18.8) | 6 (22.2) | |
| 3 | 1 (1.0) | 0 (0) | 1 (2.7) | 0 (0) | 0 (0) | |
| pM | ||||||
| 0 | 88 (90.7) | 9 (100) | 36 (85.7) | 17 (100) | 26 (89.7) | .267 |
| 1 | 9 (9.3) | 0 (0) | 6 (14.3) | 0 (0) | 3 (10.3) | |
| Stage | ||||||
| 1 | 54 (55.7) | 6 (66.7) | 18 (42.9) | 11 (64.7) | 19 (65.5) | .369 |
| 2 | 14 (14.4) | 1 (11.1) | 9 (21.4) | 1 (5.9) | 3 (10.3) | |
| 3 | 20 (20.6) | 2 (22.2) | 9 (21.4) | 5 (29.4) | 4 (13.8) | |
| 4 | 9 (9.3) | 0 (0) | 6 (14.3) | 0 (0) | 3 (10.3) | |
| Lymphovascular invasion | ||||||
| No | 39 (40.2) | 2 (22.2) | 16 (38.1) | 7 (41.2) | 14 (48.3) | .556 |
| Yes | 58 (59.8) | 7 (77.8) | 26 (61.9) | 10 (58.8) | 15 (51.7) | |
| Pleural invasion | ||||||
| No | 57 (58.8) | 4 (44.4) | 22 (52.4) | 8 (47.1) | 23 (79.3) | .058 |
| Yes | 40 (41.2) | 5 (55.6) | 20 (47.6) | 9 (52.9) | 6 (20.7) |
PY, pack years.
Table 4.
Association between CSCs expression and EMT markers
| low | ALDH1A1 No. (%) | CD44 No. (%) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| low | high | p-value | low | high | p-value | |
| E-cadherin | ||||||
| preserve | 48 (67.6) | 23 (32.4) | .365 | 22 (31) | 49 (69) | .984 |
| loss | 15 (57.7) | 11 (42.3) | 8 (30.8) | 18 (69.2) | ||
| vimentin | ||||||
| low | 35 (57.4) | 26 (42.6) | .042 | 17 (27.9) | 44 (72.1) | .396 |
| high | 28 (77.8) | 8 (22.2) | 13 (36.1) | 23 (63.9) | ||
| fibronectin | ||||||
| low | 44 (64.7) | 24 (35.3) | .939 | 23 (33.8) | 45 (66.2) | .345 |
| high | 19 (65.5) | 10 (34.5) | 7 (24.1) | 22 (75.9) | ||
| SMA | ||||||
| low | 54 (65.9) | 28 (34.1) | .662 | 27 (32.9) | 55 (67.1) | .319 |
| high | 9 (60) | 6 (40) | 3 (20) | 12 (80) | ||
Figure 3.
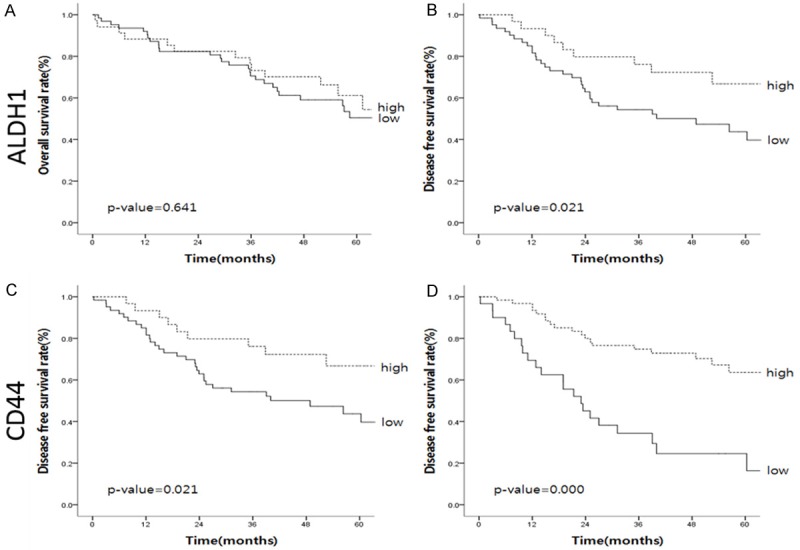
Survival curves of ALDH1 and CD44. High expression of ALDH1A1 was associated with good disease-free survival but not associated with overall survival (A and B). High expression of CD44 was correlated with both good overall survival and disease-free survival (C and D).
Figure 4.
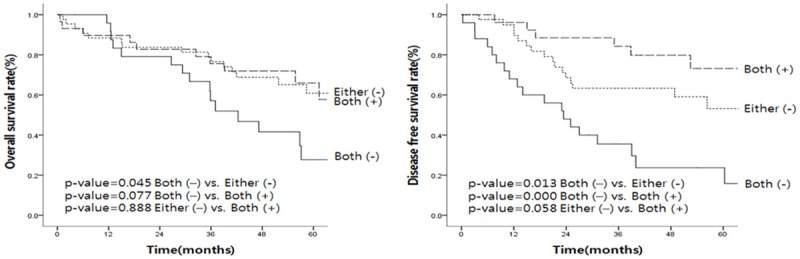
Association of CSC markers and survival. Overall survival and disease-free survival rates were worst in both negative cases, and best in both positive cases.
Results
Expression of the CSC markers and association of clinicopathologic factors
The expression levels of ALDH1A1 and CD44 were subdivided in four different score (Figure 1). Zero and 1+ were categorized as the low expression group (negative), and 3+ and 4+ were categorized as the high expression group (positive). A few cases also showed ALDH1A1 staining on normal bronchial epithelia at variable intensity (data not shown). ALDH1A1 was expressed in cytoplasm of tumor cells. CD44 was expressed in a small proportion of bronchial epithelia, but not in alveolar cells; neoplastic cells expressed it at various levels. Obvious CD44 expression was defined as basolateral membranous staining with a clear outline. Faint expression was defined as less signal intensity with an intermittent outline.
Figure 1.
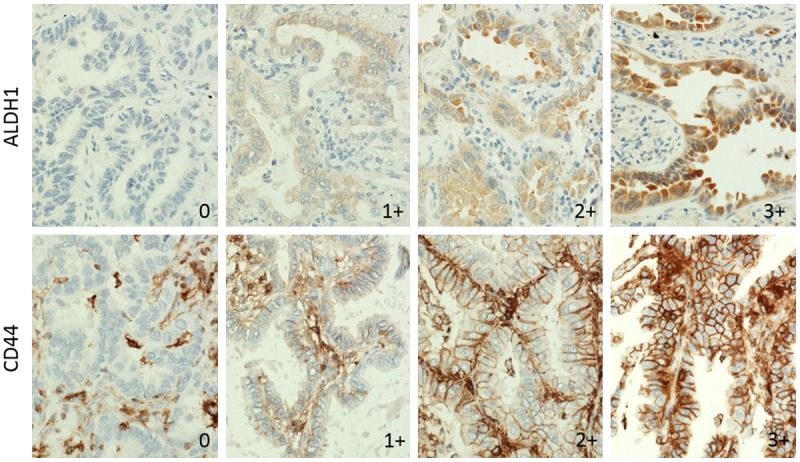
Expression feature of ALDH1 and CD44. The expression levels are subdivided into four categories: 0 and 1+ are categorized as the low expression group (negative) and 3+ and 4+ are categorized as the high expression group (positive). ALDH1 is expressed in the cytoplasm and CD44 is expressed in the membrane.
Clinicopathologic characteristics according to ALDH1A1 and CD44 are summarized in Table 1. Thirty-four patients (35.1%) were high ALDH1A1 expressers, and 63 patients (64.9%) were low ALDH1A1 expressers. High ALDH1A1 expression was statistically associated with female gender (P=0.001), non- or light smoker (P=0.012), and pT1 than higher pT stages (P=0.046). Sixty-seven patients (69.1%) were high CD44 expressers, and 30 (30.9%) were low CD44 expressers. High CD44 expression was statistically associated with female gender (P=0.008), non- or light smoker (P=0.000), and no pleural invasion (P=0.039) (Table 1).
Expression of EMT markers and association of clinicopathologic factors
Representative cases of each EMT marker (e.g., E-cadherin, vimentin, fibronectin, and SMA) expression are presented in Figure 2. Clinicopathologic characteristics according to each EMT marker are summarized in Table 2. Loss of E-cadherin expression was present in 26 patients (26.8%). There was no statistically associated significance with any clinicopathologic factors. Positive vimentin expression was observed in 36 of 97 cases (37.1%). Vimentin expression was associated with pT stage (P=0.001) and pleural invasion (P=0.028). The high expression of other mesenchymal markers-fibronectin and SMA-was revealed in 19 (19.6%) and 15 (15.5%) patients, respectively. SMA expression was statistically associated with only age. Fibronectin did not correlate with clinicopathologic factors.
Figure 2.
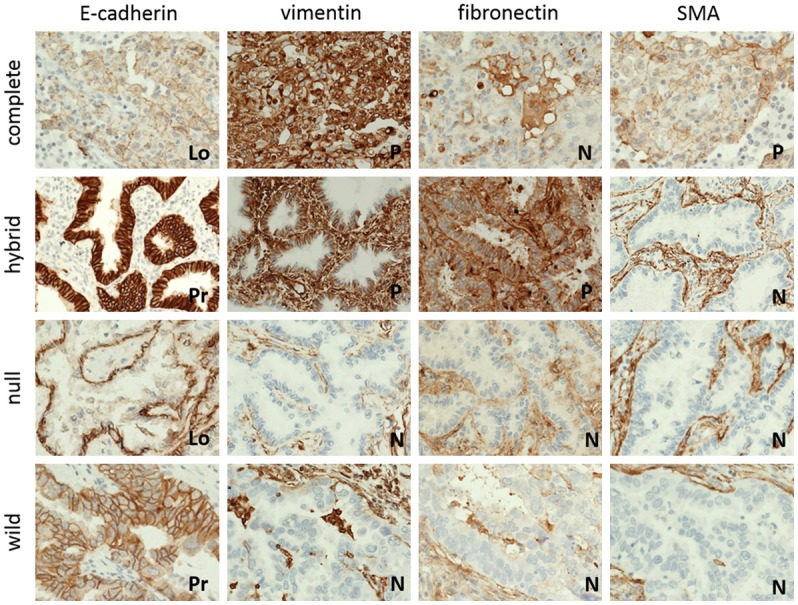
Representative cases of EMT phenotype according to expression of EMT markers. The cases are subdivided into four phenotypes: complete, hybrid, null and wild (Lo, loss; Pr, preserve; P, positive; N, negative).
Classification of EMT phenotype and association with clinicopathologic factors and survival
The cases were subdivided into four phenotypes according to expression of EMT markers: complete, hybrid, null, and wild (Figure 2). Clinicopathologic characteristics according to EMT phenotypes are summarized in Table 3. Of the 97 cases, we identified 9 (9.3%) cases of complete type, 42 (43.3%) cases of hybrid type, 17 (17.5%) cases of null type, and 29 (29.9%) cases of wild type. EMT phenotypes did not correlate with patients’ clinicopathologic characteristics, including age, gender, smoking history, tumor size, histological tumor type, stage, lymphovascular invasion, and pleural invasion (Table 3).
Association of CSC expression and EMT
High ALDH1A1 expression was significantly correlated with low expression of vimentin (P=0.42) (Table 4). However, an association between ALDH1A1 expression and E-cadherin, fibronectin, and SMA expression was not identified. CD44 expression was not associated with E-cadherin, vimentin, fibronectin, and SMA. There was no statistical significance between ALDH1A1, CD44, and EMT phenotypes (data not shown).
Impact of CSC expression on survival
Overall survival was not statistically related to ALDH1A1 expression (P=0.641) (Figure 3A). High expression of ALDH1A1 was associated with good disease-free survival (P=0.021) (Figure 3B). High expression of CD44 was correlated with both good overall survival (P=0.024) and disease-free survival (P=0.000) (Figure 3C and 3D).
To further analyze the prognostic value of CSC expression in lung adenocarcinoma, overall survival and disease-free survival rates were worst in both negative cases, and best in both positive cases (Figure 4). The overall survival curve for both positive cases was similar to that of either one of the positive cases, whereas disease-free survival for both positive cases was better than that of either one positive case, although the difference was not statistically significant (P=0.058). The overall survival and disease-free survival rates were worse in cases of both negative CSC markers (Figure 4). EMT phenotypes were not associated with patients’ five-year overall survival and disease-free survival (data not shown).
Discussion
EMT is an embryonic key developmental program that is often activated during cancer invasion and metastasis [24]. It is a process in which cells undergo a morphological switch from the epithelial polarized phenotype to the mesenchymal fibroblastic phenotype [25]. As a result of EMT, epithelial cells lose their defined cell-cell/cell-substratum contacts and their structural/functional polarity and become spindle-shaped and morphologically similar to activated fibroblasts [26]. EMT has been documented in a large number of cancers. Most studies have used in vitro systems that employ cell lines and focus on the detailed mechanism of epithelial-mesenchymal transition, identifying a number of the transcription factors and signaling pathways that are involved [5].
At the molecular level, EMT is defined by the loss of cell-cell adhesion molecules; the down-regulation of epithelial differentiation markers, including cytokeratins and E-cadherin; and the transcriptional induction of mesenchymal markers such as vimentin, fibronectin, and N-cadherin with a nuclear localization of β-catenin [27]. Nuclear β-catenin induces a gene expression pattern favoring tumor invasion, and mounting evidence indicates multiple reciprocal interactions of E-cadherin and β-catenin with EMT-inducing transcriptional repressors to stabilize an invasive mesenchymal phenotype of epithelial tumor cells [28]. We used E-cadherin as an epithelial marker and vimentin, fibronectin, and SMA as a mesenchymal marker to detect EMT in the human tissue of resected lung adenocarcinoma. Loss of E-cadherin expression is a well-documented condition for invasiveness [29]; however, the results are debatable [30]. Our work demonstrated that the loss of E-cadherin itself may not be associated with invasive behavior, whereas tumors with vimentin expression, regardless of E-cadherin expression, showed aggressive behavior, such as the tendency of high pT stage and pleural invasion.
Some studies have shown that centrally located tumor cells stained positively for epithelial markers, but this was absent at the invasive front of the tumor in lung cancer [31]. So, the expression of EMT-related proteins that are related to metastasis may better reflect and predict the prognosis and survival in patients with non-small cell lung carcinoma. Shi et al.’s [32] study demonstrated that the expression of various EMT-related proteins is associated with a poor prognosis in lung adenocarcinoma. However, in our study, neither the expression of EMT-related markers nor EMT phenotypes correlated with the factors associated with tumor invasion and metastasis, such as lymph node metastasis, lymphovascular invasion or distant metastasis, and survival. The literature reports various results regarding EMT of human cancers; it is difficult to conclude the role of EMT in cancer as associated with clinicopathologic features and survival from this study. Further studies or evaluations about the validation of EMT-related markers, tissue microenvironment, scoring system, and phenotyping in connection with survival or predictive factors are needed.
CSCs have been defined as “a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor” [33]. CSCs have been identified in a variety of solid tumors, including glioblastomas [34], breast cancer [35], and lung cancer [14]. The immunohistochemical expression of potential CSC markers was investigated in a series of lung adenocarcinomas [36]. Among them, CD133, CD44, ALDH1, and combinations thereof had independent prognostic. Reviewing results of studies investigating CSC markers, one finds several incomprehensible issues [36]. Many in vitro studies have found that the proportion of CSCs is usually very small (0.01 to 1% of the population of cancer cell lines) [37]. In contrast, immunohistochemical studies examining various types of primary cancers have reported remarkably high fractions of positive cells [19,38].
ALDH1 is a cytosolic isoenzyme, a member of the aldehyde dehydrogenase family responsible for the oxidization of intracellular aldehydes to carboxylic acids [20]. Increased ALDH1 activity has been found in hematopoietic stem cells [24,39] and has been reported as a surrogate marker of CSCs in several malignancies [40]. In vitro experiments suggest that isolated lung cancer cells with high ALDH1 activity are associated with CSC characteristics, including capacities of proliferation, self-renewal, and resistance to chemotherapy [41]. Jiang et al. [41] showed that ALDH1 overexpression is associated with poor prognosis in stage 1 non-small cell lung carcinoma and that ALDH1 expression overlapped with CD133 in a small subset of patients. Similarly, Sullivan et al. [42] reported that only ALDH1A1, but not CD133, is a marker of poor prognosis in stage 1 non-small cell lung carcinoma [20].
CD44 has been identified as a specific marker of CSCs. In addition, CD44 plays an important role in tumor cells that are undergoing an EMT-like process and is associated with cancer progression [43]. There were many previous reports that indicated that the expression of various EMT-related molecules was associated with neoplastic progression and poor survival in some malignancies [32]. Okudela et al. [36] demonstrated the independent prognostic value of CD133, CD44, and ALDH1 in lung adenocarcinomas. Studies have demonstrated a prognostic value for these molecules in a variety of cancers [19,44,45]. On the other hand, some have demonstrated the opposite results that CD133 had no prognostic value in non-small cell lung carcinomas [46].
In our study, the expression of ALDH1A1 and CD44 was statistically associated with gender, smoking history, pT stage, and pleural invasion, but it was not associated with lymph node metastasis, distant metastasis, lymphovascular invasion, and stage. At the survival curves of our study, high ALDH1A1 expression showed good diffuse-free survival, and high CD44 expression was associated with good overall and diffuse-free survival. Tumors expressing both ALDH1A1 and CD44 were associated with the best disease-free survival. One or more positive patients of ALDH1A1 and CD44 expression showed better overall survival than both negative patients. Unlike many previous studies showing that CSC expression was associated with poor clinicopathologic features and survival, our study revealed that it was related to a good prognosis. It is needed in order to evaluate the factors influencing CSC expression, such as experimental environment and cancer microenvironment. In addition, it should be reconsidered regarding the definition and presence of CSC.
Studies have suggested that CD133 and CD44 could participate in EMT, a key biological event in the invasion process [47,48]. Regardless of their specificity as CSC markers, these molecules seem to have biological activities that promote malignant expansion [36]. We evaluated the association between EMT and CSC. There was no significant correlation between ALDH1A1, CD44, and EMT markers such as E-cadherin, fibronectin, and SMA exception. Expression of vimentin was associated with low ALDH1A1 expression.
In summary, we have used immunohistochemical staining of CSC and EMT markers on human lung adenocarcinoma tissue to explore ALDH1 and CD44 as CSC markers and E-cadherin, vimentin, fibronectin, and SMA as EMT markers. We studied whether these markers were correlated with clinicopathologic factors and survival at the tissue microarray of lung cancer. Our results showed that the expressions of CSC markers were correlated with female gender, less smoking history, low pT, and no pleural invasion. Increased expression of ALDH1A1 and CD44 resulted in good overall survival and disease-free survival. These results showed that most of the EMT markers were not associated with clinicopathologic correlation or survival of lung adenocarcinoma. Only vimentin expression was associated with high pT stage and pleural invasion. We strongly suggest that CSC marker expression is not related to EMT in lung adenocarcinoma. There is a need for more research about CSC, EMT, and the relation between the two in human lung cancer.
Acknowledgements
This paper was supported by Bumsuk Academic Research Fund in 2013.
Disclosure of conflict of interest
None.
References
- 1.Cufer T, Ovcaricek T, O’Brien ME. Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer. 2013;49:1216–1225. doi: 10.1016/j.ejca.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;335:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Usami Y, Satake S, Nakayama F, Matsumoto M, Ohnuma K, Komori T, Semba S, Ito A, Yokozaki H. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol. 2008;215:330–339. doi: 10.1002/path.2365. [DOI] [PubMed] [Google Scholar]
- 5.Sung CO, Park CK, Kim SH. Classification of epithelial-mesenchymal transition phenotypes in esophageal squamous cell carcinoma is strongly associated with patient prognosis. Mod Pathol. 2011;24:1060–1068. doi: 10.1038/modpathol.2011.59. [DOI] [PubMed] [Google Scholar]
- 6.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelialmesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 7.Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, Wang RY, Brisken C, Guerra R, Andreeff M, Mani SA. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, Kim CH, Lee JC. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer. 2009;63:219–226. doi: 10.1016/j.lungcan.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Nurwidya F, Takahashi F, Murakami A, Takahashi K. Epithelial mesenchymal transition in drug resistance and metastasis of lung cancer. Cancer Res Treat. 2012;44:151–156. doi: 10.4143/crt.2012.44.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shien K, Toyooka S, Yamamoto H, Soh J, Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, Tsukuda K, Takigawa N, Kiura K, Gazdar AF, Lam WL, Miyoshi S. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. 2013;73:3051–3061. doi: 10.1158/0008-5472.CAN-12-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koren A, Motaln H, Cufer T. Lung cancer stem cells: a biological and clinical perspective. Cell Oncol (Dordr) 2013;36:265–275. doi: 10.1007/s13402-013-0141-9. [DOI] [PubMed] [Google Scholar]
- 13.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 14.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 15.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 16.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, Clarke MF, Hoey T, Lewicki J, Gurney AL. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura H, Okudela K, Yazawa T, Shimoyamada H, Sato H. Cancer stem cell: Implication in cancer biology and therapy with special reference to lung cancer. Lung Cancer. 2009;66:275–281. doi: 10.1016/j.lungcan.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Yang YM, Chang JW. Current status and issues in cancer stem cell study. Cancer Invest. 2008;26:741–755. doi: 10.1080/07357900801901856. [DOI] [PubMed] [Google Scholar]
- 19.Woo T, Okudela K, Mitsui H, Yazawa T, Ogawa N, Tajiri M, Yamamoto T, Rino Y, Kitamura H, Masuda M. Prognostic value of CD133 expression in stage I lung adenocarcinomas. Int J Clin Exp Pathol. 2010;4:32–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Alamgeer M, Ganju V, Szczepny A, Russell PA, Prodanovic Z, Kumar B, Wainer Z, Brown T, Schneider-Kolsky M, Conron M, Wright G, Watkins DN. The prognostic significance of aldehyde dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage non-small cell lung cancer. Thorax. 2013;68:1095–1104. doi: 10.1136/thoraxjnl-2012-203021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Berns A. Stem cells for lung cancer? Cell. 2005;121:811–813. doi: 10.1016/j.cell.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 24.Thiery JP, Chua K, Sim WJ, Huang R. Epithelial mesenchymal transition during development in fibrosis and in the progression of carcinoma. Bull Cancer. 2010;97:1285–1295. doi: 10.1684/bdc.2010.1206. [DOI] [PubMed] [Google Scholar]
- 25.Pirozzi G, Tirino V, Camerlingo R, Franco R, La Rocca A, Liguori E, Martucci N, Paino F, Normanno N, Rocco G. Epithelial to mesenchymal transition by TGFβ-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One. 2011;6:e21548. doi: 10.1371/journal.pone.0021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 27.Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 2011;71:245–254. doi: 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L. IGF-II induces rapid beta-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 29.Nair KS, Naidoo R, Chetty R. Expression of cell adhesion molecules in oesophageal carcinoma and its prognostic value. J Clin Pathol. 2005;58:343–351. doi: 10.1136/jcp.2004.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren K, Nordenskjold B, Landberg G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer. 2009;101:1769–1781. doi: 10.1038/sj.bjc.6605369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial- mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Wu H, Zhang M, Ding L, Meng F, Fan X. Expression of the epithelial-mesenchymal transition-related proteins and their clinical significance in lung adenocarcinoma. Diagn Pathol. 2013;8:89. doi: 10.1186/1746-1596-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 35.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 36.Okudela K, Woo T, Mitsui H, Tajiri M, Masuda M, Ohashi K. Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and β-catenin, in primary lung adenocarcinoma--their prognostic significance. Pathol Int. 2012;62:792–801. doi: 10.1111/pin.12019. [DOI] [PubMed] [Google Scholar]
- 37.Ahn SM, Goode RJ, Simpson RJ. Stem cell markers: Insights from membrane proteomics? Proteomics. 2008;8:4946–4957. doi: 10.1002/pmic.200800312. [DOI] [PubMed] [Google Scholar]
- 38.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 39.Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, McDonnell DP. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, Honorio S, Xie Y, Scaglioni PP, DiMaio JM, Gazdar AF, Shay JW, Wistuba II, Minna JD. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai Y, Sagara Y, Sagara Y, Sagara Y, Tanimoto A. Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology. 2011;59:776–780. doi: 10.1111/j.1365-2559.2011.03884.x. [DOI] [PubMed] [Google Scholar]
- 45.Shien K, Toyooka S, Ichimura K, Soh J, Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, Tsukuda K, Yamane M, Oto T, Kiura K, Miyoshi S. Prognostic impact of cancer stem cell-related markers in non-small cell lung cancer patients treated with induction chemoradiotherapy. Lung Cancer. 2012;77:162–167. doi: 10.1016/j.lungcan.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126:950–958. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- 47.Chen YS, Wu MJ, Huang CY, Lin SC, Chuang TH, Yu CC, Lo JF. CD133/Src axis mediates tumor initiating property and epithelial-mesenchymal transition of head and neck cancer. PLoS One. 2011;6:e28053. doi: 10.1371/journal.pone.0028053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–1074. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]


