Abstract
Epidermal growth factor receptor (EGFR) mutations occur mostly in patients with lung adenocarcinoma; such patients are also more likely to express cyclooxygenase-2 (COX-2), indicating a possible relationship between EGFR mutation and COX-2. The COX-2 and EGFR pathways mutually enhance their procarcinogenic effects in different tumor types. Therefore, simultaneous EGFR and COX-2 inhibition may be a promising therapeutic approach for patients with lung adenocarcinoma. We obtained tissue and serum samples from patients with non-small cell lung cancer (NSCLC) to detect the relationship between EGFR mutation and serum COX-2 level. Subsequently, gefitinib was combined with celecoxib to investigate the efficacy of inhibition in vitro in two NSCLC cell lines: HCC827 (del E746-A750) and A549 (wild-type EGFR). The cells were treated with gefitinib or celecoxib alone or with gefitinib plus celecoxib. Cell proliferation and apoptosis were assessed and correlated with expression of COX-2 and phosphorylated (p)-EGFR. The EGFR mutation rate of the high-COX-2 patients was significantly higher than that in the low-COX-2 patients. Multivariate analysis showed that high COX-2 levels were independently associated with EGFR mutation. Celecoxib and gefitinib inhibited cell growth in both cell lines. At sufficiently high concentrations, celecoxib plus gefitinib significantly mutually enhanced their anti-proliferative and apoptotic effects in both cell lines. At low concentrations, the combination had no additional effects on A549 cells. There was increased down regulation of COX-2 and p-EGFR when both cell lines were treated with high-concentration celecoxib plus gefitinib compared to either agent alone. This study demonstrates that high serum COX-2 levels may indicate EGFR mutations and that the efficacy of combined celecoxib and gefitinib is significantly greater in NSCLC cells with EGFR mutations; at high concentrations, the combination is efficacious in wild-type NSCLC cells.
Keywords: Non-small cell lung cancer, epidermal growth factor receptor mutation, cyclooxygenase-2, EGFR
Introduction
Lung cancer is one of the leading causes of cancer-related mortality worldwide, and most patients have advanced disease at diagnosis [1]. Even with the best available chemotherapy strategies, the 5-year survival rate for most patients with progressive non-small cell lung cancer (NSCLC) is disappointing [2]. Studies purporting to improve the survival of patients with NSCLC now mostly focus on new targeted molecular therapies against key signaling pathways, in particular, epidermal growth factor receptor (EGFR)-targeted therapy.
EGFR tyrosine kinase inhibitors (EGFR-TKIs) have proven efficacious for treating patients harboring sensitive EGFR mutations [3-7]. EGFR mutation detection has become a routine molecular test with significant implications for prognosis and therapeutic options. However, acquiring sufficient amounts of tissue for analyzing EGFR mutations is not often feasible, and more than 70% of patients with sensitive EGFR mutations do not benefit from EGFR-TKI treatment [8-10]. Therefore, the efficacy of EGFR-TKI is limited.
Cyclooxygenase (COX) is a rate-limiting enzyme that converts arachidonic acid to prostaglandin (PG) [11]; there are two isozymes: COX-1 and COX-2. COX-1 is constitutively expressed in many normal tissue types. COX-2 is inducible and is overexpressed in inflammatory and many neoplastic tissues [12-18]. COX-2 appears to participate in various aspects of carcinogenesis, primarily through PG synthesis [19-22]. In vitro studies have suggested that selective COX-2 inhibitors inhibit cancer cell growth and induce apoptosis in lung, digestive tract, and breast cancer [23-28].
Multitargeted therapy is likely to come to the fore as far as future therapeutic approaches for cancer are concerned. As both COX-2 and EGFR appear to be involved in many aspects of carcinogenesis, there has been great interest in evaluating the simultaneous inhibition of both pathways. Preclinical studies on colon, head and neck, and breast cancer reported a synergistic effect when COX-2 inhibitors were combined with EGFR-TKI [29-32]. Although a previous study showed that combined celecoxib and gefitinib did not benefit patients with NSCLC, there was prolonged disease control for one non-smoker female patient with adenocarcinoma who received the therapy for > 3 years [33-35]. Molecular analyses revealed that her tumor harbored an EGFR mutation. It is unknown whether EGFR mutation status is associated with the efficacy of combined celecoxib and gefitinib. Therefore, we performed this study to investigate the relationship between serum COX-2 and tissue EGFR mutation status and the effect of combining the EGFR-TKI gefitinib and the selective COX-2 inhibitor celecoxib in NSCLC cell lines expressing wild-type or mutant EGFR.
Materials and methods
Drugs and reagents
Gefitinib was provided by AstraZeneca UK Limited, celecoxib was provided by Pfizer (Groton, CT, USA). Both drugs were dissolved in 100 mmol/L dimethyl sulfoxide (DMSO) and stored at -20°C until used. Tetrazolium (MTT) was purchased from Amresco. RPMI 1640, 0.25% trypsin-0.02% ethylenediamine tetraacetic acid (EDTA), and fetal bovine serum were purchased from Gibco (Grand Island, NY, USA). The Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) Apoptosis Detection Kit was purchased from BioVision. The enzyme-linked immunosorbent assay (ELISA) kit for COX-2 was purchased from USCN Life Science. Primary antibodies for COX-2 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies for phosphorylated (p)-EGFR were purchased from Abcam Technology.
Patients and serum samples
From December 2012 to February 2014, 44 patients with newly diagnosed lung adenocarcinoma were enrolled in this study. Forty-four samples were collected from the primary site. All samples were examined histologically to confirm the diagnosis of adenocarcinoma. The tumor tissue samples were fixed in formalin and paraffin-embedded.
To obtain the serum, 5 mL peripheral blood was collected in coagulation-promoting tubes prior to the initiation of therapy, and subsequently centrifuged for 30 min to separate the serum. The tissue EGFR mutation status was assessed using the Amplified Refractory Mutation System. Serum COX-2 levels were determined using ELISA.
The Ethics Committee of the First Affiliated Hospital, Shihezi University School of Medicine (Xinjiang, China), approved this study. Eligible patients were provided a written informed consent form; those who signed the form were recruited into the study.
Cell lines and culture
Two NSCLC cell lines with different EGFR mutation status and EGFR-TKI sensitivity were used: A549 (wild-type) and HCC827 (mutant, del E746-A750). The cell lines were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Both cell lines were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 µg/mL streptomycin in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Analysis of cell growth inhibition
Cells (6000-8000/well) were seeded in a 96-well plate and incubated overnight at 37°C before continuous 48-h exposure to gefitinib or celecoxib individually (5, 10, 20, 40, 80, 160 μmol/L) or in combination (gefitinib: 5, 10, 20, 40, 80, 160 μmol/L; celecoxib: 5, 10, 20, 40, 80, 160 μmol/L). Subsequently, MTT (0.5 mg/mL) was added to each well. After 4 h, the medium was aspirated and DMSO was added to each well. Color intensity at 490 nm was measured using a Tecan microplate fluorometer (Research Triangle Park, NC, USA). All experiments were replicated three times.
The formula devised by Jin (q = Ea + b/Ea + Eb-Ea × Eb), which can be used to measure the inhibitory effect of two drugs in combination, was used to analyze the effect of the gefitinib and celecoxib combination. Ea and Eb are the inhibitory effects of drug a or b, respectively. Q-values that are > 1.15, 0.85-1.15, or < 0.85 indicate synergistic, additive, or antagonistic effects, respectively.
Quantification of apoptosis by flow cytometry
The pro-apoptotic effects of gefitinib and celecoxib on the NSCLC cell lines were analyzed by flow cytometry. Cells were seeded in 6-well plates and incubated for 48 h with gefitinib (40 μmol/L), celecoxib (80 μmol/L), or gefitinib (40 μmol/L) and celecoxib (80 μmol/L). The cells were stained with annexin V-FITC (0.5 µg/mL) and PI (1 µg/mL) for flow cytometry analysis. All experiments were performed three times.
Protein extraction and western blotting
A549 and HCC827 cells were treated for 48 h with gefitinib (40 μmol/L), celecoxib (80 μmol/L), and gefitinib (40 μmol/L) and celecoxib (80 μmol/L) and the effects on COX-2 and p-EGFR expression relative to β-actin expression were evaluated. Cells were lysed for 30 min on ice in buffer consisting of 250 nM NaCl, 5 mM EDTA, 50 mM Tris buffer (pH 7.5), 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM sodium orthovanadate, 0.5% sodium deoxycholate, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 1 µg/mL pepstatin. Protein concentrations were measured using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). The samples were loaded on an SDS-polyacrylamide gel, and then electrophoretically transferred to a nitrocellulose membrane. The membranes were incubated with the appropriate primary antibodies. Signal intensity was measured using a chemiluminescence detection system (Pierce). Autoradiograms of the western blots were scanned, and the bands were quantified using AlphaEaseFC software (Alpha Innotech).
Statistical analysis
The data are expressed as the mean ± standard deviation. The χ2 test or Fisher’s exact test was used to assess the association between EGFR mutation and each clinicopathologic parameter. Logistic regression models were used to assess the association between individual factors and incidence of EGFR mutation. Variance analysis was used to determine the remaining level of significance. Statistical analyses were conducted using SPSS version 18.0 (IBM, New York, NY, USA). A P-value of < 0.05 was considered statistically significant.
Results
Correlation between serum COX-2 levels and EGFR mutation
We analyzed the correlation between tissue EGFR mutations and clinical characteristics, and found that serum COX-2 levels were the only correlative factor (Table 1). Mutations at EGFR gene were found in 29 of the 44 patients. In 29 cases (65.91%) were observed EGFR gene mutations, including 12 cases of L858R mutation, 15 cases of exon 19 mutation, and 2 cases of exon 18 mutation. The EGFR mutation rates of the patients with high serum COX-2 levels (≥ 100 ng/mL) were significantly higher than that for the patients with low COX-2 levels (< 100 ng/mL) (92.9% vs. 53.3%, P = 0.025). Multivariate analysis also showed that high levels of COX-2 were independently associated with EGFR gene mutations (Table 2, P = 0.033, odds ratio [OR] = 12.385, 95% confidence interval [CI] = 1.231-124.567). We analyzed the potential for predicting mutations based on high COX-2 levels. The sensitivity, specificity, positive predictive value, and negative predictive value of high COX-2 levels for predicting EGFR mutations were 44.8%, 93.3%, 92.9%, and 46.7%, respectively.
Table 1.
Clinicopathologic features of patients with NSCLC with mutant or wild-type EGFR
| Variable | Mutant EGFR (n = 29) | Wild-type EGFR (n = 15) | χ2 | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 14 | 12 | 2.908 | 0.088 |
| Female | 15 | 3 | ||
| Age, y | ||||
| < 60 | 12 | 6 | 0.008 | 0.930 |
| ≥ 60 | 17 | 9 | ||
| Smoking status | ||||
| Never-smoker | 14 | 4 | 1.120 | 0.290 |
| Current/former smoker | 15 | 11 | ||
| Stage | ||||
| IIIB | 8 | 5 | 0.157 | 0.692 |
| IV | 21 | 10 | ||
| ECOG score | ||||
| 0~1 | 19 | 8 | 0.619 | 0.431 |
| 2~3 | 10 | 7 | ||
| Serum COX-2 level | ||||
| < 100 ng/mL | 16 | 14 | 4.994 | 0.025 |
| ≥ 100 ng/mL | 13 | 1 |
Table 2.
Multivariable analysis of predictive factors for incidence of EGFR mutation
| Variable | Coefficient | SE | OR | 95% CI | P |
|---|---|---|---|---|---|
| Gender | -1.855 | 1.328 | 0.156 | 0.012-2.112 | 0.162 |
| Age, y | 0.022 | 0.030 | 1.023 | 0.965-1.084 | 0.452 |
| Stage | -0.189 | 0.853 | 0.828 | 0.156-4.411 | 0.825 |
| Smoking status | -1.076 | 1.308 | 0.341 | 0.026-4.431 | 0.411 |
| ECOG score | -1.292 | 0.842 | 0.275 | 0.053-1.432 | 0.125 |
| Serum COX-2 level | 2.516 | 1.178 | 12.385 | 1.231-124.567 | 0.033 |
COX-2 and p-EGFR expression in NSCLC cells
Figure 1 depicts the baseline expression of COX-2 and p-EGFR in the two cell lines. Both cell lines expressed COX-2 and p-EGFR proteins; however, COX-2 and p-EGFR expression in HCC827 cells was higher than that in A549 cells.
Figure 1.
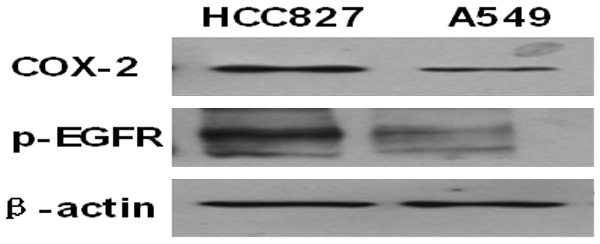
Western blot analysis of COX-2 and p-EGFR expression in A549 and HCC827 cells.
Gefitinib and celecoxib induction of growth inhibition
Cell growth inhibition of the A549 and HCC827 cells was measured by detecting cell density following the addition of gefitinib and celecoxib. The median inhibitory concentration (IC50) of celecoxib, gefitinib, and celecoxib plus gefitinib in the A549 and HCC827 cells were 163.4 μmol/L, 105.6 μmol/L, and 43.68 μmol/L, respectively, and 69.2 μmol/L, 14.9 μmol/L, and 7.9 μmol/L, respectively (Figure 2). High-dose celecoxib enhanced the growth inhibitory effect of gefitinib in both cell lines, but no enhancement of inhibition was observed in the A549 cell line when low-dose celecoxib were used. The q-value indicated the same synergistic therapeutic effect (Table 3; Figure 2).
Figure 2.
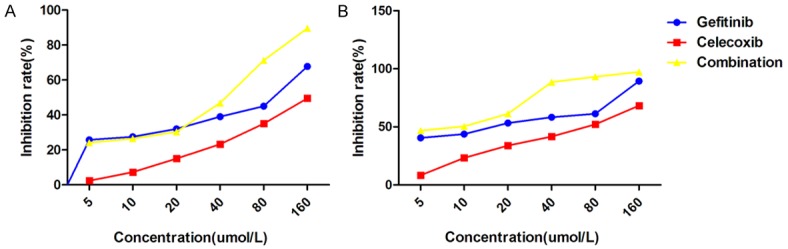
Effects of gefitinib and celecoxib on NSCLC cell growth. A549 (A) and HCC827 (B) cells were treated with serial dilutions of gefitinib (Ge, 0-160 μmol/L) and celecoxib (Ce, 0-160 μmol/L) individually and in combination (Ge+Ce). C, Control.
Table 3.
The q-value of growth inhibition of HCC827 and A549 cells treated with gefitinib and celecoxib
| C1+G1 | C2+G2 | C3+G3 | C4+G4 | C5+G5 | C6+G6 | |
|---|---|---|---|---|---|---|
| A549 | 0.95374233 | 1.015470455 | 1.0523125 | 1.344107872 | 1.777388889 | 1.482150552 |
| HCC827 | 1.20338845 | 1.282147263 | 1.306860592 | 1.690951786 | 1.721800021 | 1.160257822 |
The q-value was calculated using the formula by Jin (n = 3). C1-6: 5, 10, 20, 40, 80, 160 μmol/L celecoxib, respectively. G1-6: 5, 10, 20, 40, 80, 160 μmol/L gefitinib, respectively. Q-values > 1.15 are bolded.
Induction of apoptosis by gefitinib, celecoxib, and gefitinib plus celecoxib
Apoptosis assays were performed on the A549 and HCC827 cells to determine the mechanism of the observed growth inhibition. Administered individually, both gefitinib (40 µmol/L) and celecoxib (80 µmol/L) induced apoptosis in both cell lines (Figure 3). However, apoptosis was higher in HCC827 cells than in A549 cells following single-agent gefitinib and celecoxib treatment. Compared to their use as single agents, celecoxib and gefitinib combined significantly enhanced apoptosis in the HCC827 and A549 cells.
Figure 3.
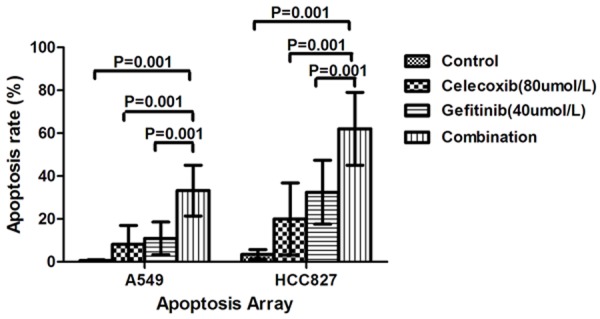
Induction of apoptosis by gefitinib and celecoxib in NSCLC cell lines. A549 and HCC827 cells were treated with gefitinib (40 μmol/L), celecoxib (80 μmol/L) individually and in combination. Flow cytometry revealed significant differences (P ≤ 0.05) when the combination treatment and each single treatment were compared with the control.
Modulation of COX-2 and p-EGFR
COX-2 and p-EGFR protein expression was determined in A549 and HCC827 cells treated with gefitinib (40 μmol/L), celecoxib (80 μmol/L), or gefitinib and celecoxib (Figure 4). No significant change in COX-2 expression was observed in either cell line following treatment with gefitinib alone, nor was there a significant change in p-EGFR expression in either cell line following treatment with celecoxib alone. COX-2 and p-EGFR protein levels were significantly decreased in both cell lines after the combined drug treatment.
Figure 4.

COX-2 and p-EGFR expression in NSCLC cells treated with gefitinib, celecoxib, and gefitinib plus celecoxib. A549 and HCC827 cells were treated for 48 h with gefitinib (40 μmol/L), celecoxib (80 μmol/L), or gefitinib and celecoxib combined. There was significant COX-2 and p-EGFR downregulation both cell lines following treatment with gefitinib plus celecoxib compared to that following treatment with either drug alone.
Discussion
The EGFR signal transduction pathway has been a hotspot for research on cancer, lung cancer in particular. In the past few years, there has been rapid development of EGFR-TKIs for treating NSCLC, and reports on their efficacy [36-39]. However, EGFR-TKI efficacy is most common in patients with sensitive EGFR mutations [3-7]. EGFR mutations are associated with better prognosis in patients treated with EGFR-TKIs [40], and occur more frequently in patients with lung adenocarcinoma [41]. The samples used for detecting EGFR mutations are usually tumor tissue; however, insufficient tissue quantities limit detection for most cases of advanced NSCLC. A previous study showed that COX-2 expression was increased in human lung cancer, specifically in adenocarcinoma [42]. In the present study, we investigated the relationship between EGFR mutation status and serum COX-2 level, and found that serum COX-2 levels were independently associated with the presence of EGFR mutations and that the incidence rate of EGFR mutations significantly increased in tandem with serum COX-2 levels. We observed similar results in the NSCLC cell lines. Flow cytometry determined that expression of COX-2 and p-EGFR proteins in the HCC827 cells, which express mutant EGFR, was higher than that in the A549 cells expressing wild-type EGFR. High COX-2 levels appear to be related to EGFR mutation.
Elevated COX-2 levels have been implicated in apoptosis, tumor invasion, angiogenesis, and suppression of anti-tumor immunity [43-47]. COX-2 inhibitors play a role in the growth inhibition and apoptosis of many cancer cell lines, including NSCLC [48-53]. In our study, celecoxib inhibited proliferation of the NSCLC cell lines A549 and HCC827 in a dose-dependent manner. We also verified the pro-apoptotic effect of high-dose celecoxib single-agent treatment on both NSCLC cell lines.
Not all patients harboring sensitive EGFR mutations benefit from EGFR-TKI treatment [8-10]. Both EGFR and COX-2 are overexpressed in NSCLC and are associated with tumorigenesis, and the use of COX-2 inhibitors combined with EGFR-TKI treatment has generated much interest in recent years [54,55]. In colon, head and neck, and breast cancer, a preliminary synergistic growth inhibitory effect was observed when COX-2 inhibitors were combined with EGFR-TKIs [8-10]. However, in NSCLC, Gadgeel et al. showed that in unselected patients with platinum-refractory NSCLC, the response rate to combined celecoxib and gefitinib was similar to that following treatment with gefitinib alone. The authors reported that one female; non-smoker patient was free of tumor progression for > 3 years after study enrollment. The drug combination was well tolerated, and the most common adverse effects were skin rash and diarrhea [33-35]. These results suggest that combined gefitinib and celecoxib therapy may be more effective in patients with EGFR mutation. Another recent study reported that the efficacy of combining celecoxib with an EGFR-TKI was significantly greater in NSCLC cells with EGFR mutation than in NSCLC cells with wild-type EGFR; the authors attributed it to more complete inhibition of both pathways [56]. Chen et al. showed that when combined with ZD1839, celecoxib induced stronger inhibition of the related cell signal transduction pathways in NSCLC [57]. However, the authors did not determine EGFR mutation status. We performed this study to investigate the effects of gefitinib and celecoxib co-treatment on A549 and HCC827 cells. At high concentrations, celecoxib strengthened the cell proliferation inhibitory effects of gefitinib in both cell lines, and the q-value indicated that the effect of the two drugs in combination was greater than that for either drug as a single agent, indicating a synergistic growth inhibitory effect. At low drug concentrations, however, synergistic growth inhibitory effects were not observed in the A549 cell line. Subsequent flow cytometry analysis showed that high-dose gefitinib (40 µmol/L) and celecoxib (80 µmol/L) led to a synergistic apoptotic effect in both cell lines.
Cell signal transduction systems involved in tumorigenesis are complex and are targeted by many new therapeutic drugs, including EGFR-TKI and COX-2 inhibitors. In a recent review, Chen and colleagues suggested that EGFR and COX-2 potentially interact in cell signal transduction [57]. We used western blotting to detect changes in p-EGFR and COX-2 expression in A549 and HCC827 cells following treatment with gefitinib or celecoxib as single agents, or in combination. There have been many studies on the biology of EGFR signal transduction in the past 40 years [58,59]. We found that using gefitinib to inactivate EGFR in the A549 and HCC827 cells downregulated p-EGFR expression. COX-2 is induced by many stimuli, including oncogenes, tumor promoters, and growth factors [60]. Recent studies have indicated that EGFR activation might lead to COX-2 expression [61-63]. In our study, gefitinib decreased COX-2 expression in the A549 and HCC827 cells, indicating that the EGFR signal transduction pathway may participate in COX-2 stimulation. Conversely, other studies have shown that the PGE2-activated signal transduction pathways are implicated in carcinogenesis [64-68]. In our study, p-EGFR expression was downregulated when A549 and HCC827 cells were treated with high concentrations of celecoxib, indicating that COX-2 inhibitors can inhibit EGFR activation. When the A549 and HCC827 cells were treated with the combined celecoxib and gefitinib, we detected enhanced inhibition of p-EGFR and COX-2 expression, indicating the synergistic effect of the drug combination in NSCLC cells.
In conclusion, our results demonstrate that high serum COX-2 levels may predict EGFR mutations and that combining celecoxib with gefitinib enhances growth inhibition and apoptosis in NSCLC cell lines with mutated EGFR; at high drug concentrations, inhibition and apoptosis in NSCLC cell lines with wild-type EGFR are enhanced. We also show that in NSCLC cell lines, combining gefitinib and celecoxib results in more effective inhibition of both the EGFR and COX-2 pathways compared to the effect of either agent alone. Our results suggest that this combination will be beneficial for treating NSCLC with mutated EGFR; in NSCLC with wild-type EGFR, the benefit is likely to be limited to the use of higher drug concentrations. We plan to conduct animal studies to develop our in vitro findings and to conduct further in vitro studies to better define the mechanisms underlying our observations.
Acknowledgements
We are grateful to the native English speaking scientists at ELIXIGEN (Huntington Beach, CA) for proofreading the manuscript. This study was supported by the Graduate Student Innovation Project of Xinjiang Uighur Autonomous Region (XJGRI2012067) and the Department of Cell Research of Shihezi University (YL2012-S021).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N, Duffield EL, Rukazenkov Y, Speake G, Jiang H, Armour AA, To KF, Yang JC, Mok TS. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J. Clin. Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24:54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 10.Mok T, JJ Yang, KC Lam. Treating patients with EGFR-sensitizing mutations: first line or second line--is there a difference? J. Clin. Oncol. 2013;31:1081–1088. doi: 10.1200/JCO.2012.43.0652. [DOI] [PubMed] [Google Scholar]
- 11.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 12.Uefuji K, Ichikura T, Mochizuki H. Increased expression of interleukin-1alpha and cyclooxygenase-2 in human gastric cancer: a possible role in tumor progression. Anticancer Res. 2005;25:3225–3230. [PubMed] [Google Scholar]
- 13.Barisik NO, Keser SH, Gul AE, Sensu S, Kandemir NO, Kucuk HF, Gumus M, Karadayı N. The value of COX-2 expression in the prognostic parameters of invasive ductal carcinoma of the breast. Med Oncol. 2011;28:703–708. doi: 10.1007/s12032-010-9503-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Guo XZ, Li HY, Zhao JJ, Shao XD, Wu CY. Prognostic significance of cyclooxygenase-2 protein in pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35:10301–10307. doi: 10.1007/s13277-014-2260-y. [DOI] [PubMed] [Google Scholar]
- 15.He S, Xiao Z, Zeng G, Chen L. Letter regarding the article of Jiao G. J. et al. entitled “Prognostic significance of cyclooxygenase-2 in osteosarcoma: a meta-analysis”. Tumour Biol. 2014;35:6187–6189. doi: 10.1007/s13277-014-2041-7. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Park JH, Suh JH, Nam KH, Choe JY, Jung HY, Chae JY, Moon KC. Cyclooxygenase-2 expression and its prognostic significance in clear cell renal cell carcinoma. Korean J Pathol. 2012;46:237–245. doi: 10.4132/KoreanJPathol.2012.46.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee TS, Jeon YT, Kim JW, Won JK, Park NH, Park IA, Juhnn YS, Kang SB, Lee HP, Song YS. Increased cyclooxygenase-2 expression associated with inflammatory cellular infiltration in elderly patients with vulvar cancer. Ann N Y Acad Sci. 2007;1095:143–153. doi: 10.1196/annals.1397.018. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Chen Q, Xiao J, Liu C, Zhao X. Prognostic significance of cyclooxygenase-2 in cervical cancer: a meta-analysis. Int J Cancer. 2013;132:363–373. doi: 10.1002/ijc.27686. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 20.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 21.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 22.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 23.Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006–2011. [PubMed] [Google Scholar]
- 24.Sawaoka H, Kawano S, Tsuji S, Tsujii M, Murata H, Hori M. Effects of NSAIDs on proliferation of gastric cancer cells in vitro: possible implication of cyclooxygenase-2 in cancer development. J Clin Gastroenterol. 1998;27:S47–S52. doi: 10.1097/00004836-199800001-00009. [DOI] [PubMed] [Google Scholar]
- 25.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–2103. [PubMed] [Google Scholar]
- 26.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 27.Tomozawa S, Nagawa H, Tsuno N, Hatano K, Osada T, Kitayama J, Sunami E, Nita ME, Ishihara S, Yano H, Tsuruo T, Shibata Y, Muto T. Inhibition of haematogenous metastasis of colon cancer in mice by a selective COX-2 inhibitor, JTE-522. Br J Cancer. 1999;81:1274–1279. doi: 10.1038/sj.bjc.6694262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 29.Tortora G, Caputo R, Damiano V, Melisi D, Bianco R, Fontanini G, Veneziani BM, De Placido S, Bianco AR, Ciardiello F. Combination of a selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 and protein kinase A antisense causes cooperative antitumor and antiangiogenic effect. Clin Cancer Res. 2003;9:1566–1572. [PubMed] [Google Scholar]
- 30.Chen Z, Zhang X, Li M, Wang Z, Wieand HS, Grandis JR, Shin DM. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase-2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:5930–5939. doi: 10.1158/1078-0432.CCR-03-0677. [DOI] [PubMed] [Google Scholar]
- 31.Torrance CJ, Jackson PE, Montgomery E, Kinzler KW, Vogelstein B, Wissner A, Nunes M, Frost P, Discafani CM. Combinatorial chemoprevention of intestinal neoplasia. Nat Med. 2000;6:1024–1028. doi: 10.1038/79534. [DOI] [PubMed] [Google Scholar]
- 32.Shin DM, Zhang H, Saba NF, Chen AY, Nannapaneni S, Amin AR, Müller S, Lewis M, Sica G, Kono S, Brandes JC, Grist WJ, Moreno-Williams R, Beitler JJ, Thomas SM, Chen Z, Shin HJ, Grandis JR, Khuri FR, Chen ZG. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: preclinical and clinical studies. Clin Cancer Res. 2013;19:1244–1256. doi: 10.1158/1078-0432.CCR-12-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reckamp KL, Krysan K, Morrow JD, Milne GL, Newman RA, Tucker C, Elashoff RM, Dubinett SM, Figlin RA. A phase I trial to determine the optimal biological dose of celecoxib when combined with erlotinib in advanced non-small cell lung cancer. Clin Cancer Res. 2006;12:3381–3388. doi: 10.1158/1078-0432.CCR-06-0112. [DOI] [PubMed] [Google Scholar]
- 34.Gadgeel SM, Ruckdeschel JC, Heath EI, Heilbrun LK, Venkatramanamoorthy R, Wozniak A. Phase II study of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), and celecoxib, a cyclooxygenase-2 (COX-2) inhibitor, in patients with platinum refractory non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2:299–305. doi: 10.1097/01.JTO.0000263712.61697.69. [DOI] [PubMed] [Google Scholar]
- 35.Reckamp KL, Krysan K, Morrow JD, Milne GL, Newman RA, Tucker C, Elashoff RM, Dubinett SM, Figlin RA. A phase I trial to determine the optimal biological dose of celecoxib when combined with erlotinib in advanced non-small cell lung cancer. Clin Cancer Res. 2006;12:3381–3388. doi: 10.1158/1078-0432.CCR-06-0112. [DOI] [PubMed] [Google Scholar]
- 36.Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82:241–250. doi: 10.1016/s0163-7258(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 37.Valentini V, De Paoli A, Gambacorta MA, Mantini G, Ratto C, Vecchio FM, Barbaro B, Innocente R, Rossi C, Boz G, Barba MC, Frattegiani A, Lupattelli M, Doglietto GB. Infusional 5-fluorouracil and ZD1839 (Gefitinib-Iressa) in combination with preoperative radiotherapy in patients with locally advanced rectal cancer: a phase I and II Trial (1839IL/0092) Int J Radiat Oncol Biol Phys. 2008;72:644–649. doi: 10.1016/j.ijrobp.2008.01.046. [DOI] [PubMed] [Google Scholar]
- 38.Mackenzie MJ, Hirte HW, Glenwood G, Jean M, Goel R, Major PP, Miller WH Jr, Panasci L, Lorimer IA, Batist G, Matthews S, Douglas L, Seymour L. A phase II trial of ZD1839 (Iressa) 750 mg per day, an oral epidermal growth factor receptor-tyrosine kinase inhibitor, in patients with metastatic colorectal cancer. Invest New Drugs. 2005;23:165–170. doi: 10.1007/s10637-005-5862-9. [DOI] [PubMed] [Google Scholar]
- 39.Dawson NA, Guo C, Zak R, Dorsey B, Smoot J, Wong J, Hussain A. A phase II trial of gefitinib (Iressa, ZD1839) in stage IV and recurrent renal cell carcinoma. Clin Cancer Res. 2004;10:7812–7819. doi: 10.1158/1078-0432.CCR-04-0310. [DOI] [PubMed] [Google Scholar]
- 40.Toyooka S, Takano T, Kosaka T, Hotta K, Matsuo K, Ichihara S, Fujiwara Y, Soh J, Otani H, Kiura K, Aoe K, Yatabe Y, Ohe Y, Mitsudomi T, Date H. Epidermal growth factor receptor mutation, but not sex and smoking, is independently associated with favorable prognosis of gefitinib-treated patients with lung adenocarcinoma. Cancer Sci. 2008;99:303–308. doi: 10.1111/j.1349-7006.2007.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 42.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 43.Koki AT, Masferrer JL. Celecoxib: a specific COX-2 inhibitor with anticancer properties. Cancer Control. 2002;9:28–35. doi: 10.1177/107327480200902S04. [DOI] [PubMed] [Google Scholar]
- 44.Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci U S A. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclooxygenase-2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 46.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 48.Koki AT, Khan NK, Woerner BM, Seibert K, Harmon JL, Dannenberg AJ, Soslow RA, Masferrer JL. Characterization of cyclooxygenase-2 (COX-2) during tumorigenesis in human epithelial cancers: evidence for potential clinical utility of COX-2 inhibitors in epithelial cancers. Prostaglandins Leukot Essent Fatty Acids. 2002;66:13–18. doi: 10.1054/plef.2001.0335. [DOI] [PubMed] [Google Scholar]
- 49.Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 50.Chang HC, Weng CF. Cyclooxygenase-2 level and culture conditions influence NS398-induced apoptosis and caspase activation in lung cancer cells. Oncol Rep. 2001;8:1321–1325. doi: 10.3892/or.8.6.1321. [DOI] [PubMed] [Google Scholar]
- 51.Sun SY, Schroeder CP, Yue P, Lotan D, Hong WK, Lotan R. Enhanced growth inhibition and apoptosis induction in NSCLC cell lines by combination of celecoxib and 4HPR at clinically relevant concentrations. Cancer Biol Ther. 2005;4:407–413. doi: 10.4161/cbt.4.4.1618. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Alcazar JA, Bradbury DA, Pang L, Knox AJ. Cyclooxygenase (COX) inhibitors induce apoptosis in non-small cell lung cancer through cyclooxygenase independent pathways. Lung Cancer. 2003;40:33–44. doi: 10.1016/s0169-5002(02)00530-5. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–1780. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 54.Richardson CM, Sharma RA, Cox G, O’Byrne KJ. Epidermal growth factor receptors and cyclooxygenase-2 in the pathogenesis of non-small cell lung cancer: potential targets for chemoprevention and systemic therapy. Lung Cancer. 2003;39:1–13. doi: 10.1016/s0169-5002(02)00382-3. [DOI] [PubMed] [Google Scholar]
- 55.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J. Clin. Oncol. 2005;23:254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 56.Gadgeel SM, Ali S, Philip PA, Ahmed F, Wozniak A, Sarkar FH. Response to dual blockade of epidermal growth factor receptor (EGFR) and cycloxygenase-2 in nonsmall cell lung cancer may be dependent on the EGFR mutational status of the tumor. Cancer. 2007;110:2775–2784. doi: 10.1002/cncr.23100. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, He Y, Huang H, Liao H, Wei W. Selective COX-2 inhibitor celecoxib combined with EGFR-TKI ZD1839 on non-small cell lung cancer cell lines: in vitro toxicity and mechanism study. Med Oncol. 2008;25:161–171. doi: 10.1007/s12032-007-9015-1. [DOI] [PubMed] [Google Scholar]
- 58.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 59.Sako Y, Minoghchi S, Yanagida T. Single-molecule imaging of EGFR signalling on the surface of living cells. Nat Cell Biol. 2000;2:168–172. doi: 10.1038/35004044. [DOI] [PubMed] [Google Scholar]
- 60.Xie W, Herschman HR. Vsrc induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase and the c-Jun transcription factor. J Biol Chem. 1995;270:27622–27628. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]
- 61.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, Chinery R, Kirkland SC, DuBois RN, Jetton TL, Morrow JD. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yucel-Lindberg T, Ahola H, Carlstedt-Duke J, Modéer T. Involvement of tyrosine kinases on cyclooxygenase expression and prostaglandin E2 production in human gingival fibroblasts stimulated with interleukin-1beta and epidermal growth factor. Biochem Biophys Res Commun. 1999;257:528–532. doi: 10.1006/bbrc.1999.0523. [DOI] [PubMed] [Google Scholar]
- 63.Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, Dannenberg AJ. Retinoids suppress epidermal growth factor-induced transcription of cyclooxygenase-2 in human oral squamous carcinoma cells. Cancer Res. 1997;57:2890–2895. [PubMed] [Google Scholar]
- 64.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 65.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 66.Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218–5223. [PubMed] [Google Scholar]
- 67.Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohadwala M, Dubinett SM. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–6281. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 68.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]


