Abstract
Oral squamous cell carcinoma (OSCC) is one of the most lethal malignant tumors. The cadherin/catenin cell-cell adhesion complex plays a major role in cancer development and progression. p120-catenin (p120) is a cytoplasmic molecule closely associated with E-cadherin which activates phospholipase C-γ1 (PLC-γ1). Our previous studies indicate that activation of PLC-γ1 plays a critical role in epidermal growth factor (EGF)-induced migration and proliferation of squamous cell carcinoma (SCC) cells and phosphatidylinositol 3-kinase enhancer (PIKE) is highly expressed in SCC cells and mediates EGFR-dependent SCC cell proliferation. Our current study was to determine whether the expression of E-cadherin, p120, PLC-γ1, and PIKE, is associated with OSCC. To address this issue, we assessed levels and localization of E-cadherin, p120, PLC-γ1, and PIKE in specimen of 92 patients with OSCC by immunohistochemistry. The results showed that the expression of E-cadherin, and p120 negatively correlated with the tumor differentiation and the expression of PLC-γ1 and PIKE positively correlated with the tumor differentiation. The expression of PLC-γ1 and PIKE in OSCC stage T3 + T4 or in OSCC with lymph node metastasis was significantly higher than that in OSCC stage T1 + T2 or in OSCC without lymph node metastasis. The expression of p120 positively correlated with levels of E-cadherin but negatively correlated with levels of PLC-γ1 and PIKE in OSCC. These data indicate that increased expression of PLC-γ1 and PIKE and decreased expression of E-cadherin and p120 are associated with the aggressiveness of OSCC.
Keywords: E-cadherin, p120, PLC-γ1, PIKE, OSCC
Introduction
Oral squamous cell carcinoma (OSCC) arises from malignant transformation of keratinocytes in the oral epithelium and represents 90% of oral cancer. It is the sixth most common cancer worldwide. An estimated 263,900 new cases and 128,000 deaths from oral cavity cancer occurred in 2008 worldwide [1]. The 5-year survival rate for patients with advanced OSCC has not changed significantly over the last 30 years, and still remains about 50%. Identifying markers for tumor aggressiveness is needed for improving risk assessment to customize therapeutic approaches.
E-cadherin is a calcium-dependent transmembrane glycoprotein which promotes cell-cell adherence and is involved in various biological processes, including embryo development, morphogenesis and neoplasm metastasis [2,3]. E-cadherin regulates the adherence reaction among cells, maintains cell polarity, participates in differentiation modulation, and maintains the shape and integrity of tissue configuration [4]. p120 coexists in E-cadherin complexes with either β-or γ-catenin and has been shown to play an important role in stabilizing the E-cadherin-catenin complex [5,6]. Alteration of cadherin or catenin in the complex reduces adhesion and cell polarity, and makes cancer cells easy to invade and metastasize. E-cadherin and p120 expression have been extensively studied in many human cancers, including breast [7], bladder [8,9], renal [10], colorectal [11], colorectal polyps [12], lung [13], prostate [14], gastric [15], pancreas [16], and OSCC [17-29].
Phospholipase C-γ1 (PLC-γ1) is the most abundant member of the phospholipase C family in keratinocytes. Previous studies have demonstrated that PLC-γ1 mediates calcium-induced keratinocyte differentiation [40]. In the presence of high extracellular calcium, the E-cadherin-β-catenin-p120 catenin complex recruits phosphatidylinositol-4-phosphate 5-kinase1α (PIP5K1α) and phosphatidylinositol-3-kinase (PI3K) to the plasma membrane. These kinases promote production of phosphtidylinositol (4,5)-bisphosphate (PIP2) and phosphatidylinositol (3,4,5)-triphosphate (PIP3) and subsequently the recruitment of PLC-γ1 to the plasma membrane. PLC-γ1 in turns hydrolyzes PIP2 to produce diacylglycerol and inositol trisphosphate (IP3) which increases intracellular calcium concentration to ultimately promote differentiation of keratinocytes. On the other hand, EGF induces tyrosine phosphorylation of PLC-γ1. Activation of the SH3 domain of PLC-γ1 promotes proliferation of keratinocytes and activation of the catalytic domain of PLC-γ1 promotes migration of keratinocytes [30,31]. Overexpression of PLC-γ1 in OSCC has been observed in our previous studies [32]. However, it is unknown whether overexpression of PLC-γ1 is associated with the aggressiveness of OSCC.
Phosphatidylinositol 3-kinase enhancer (PIKE) is a recently identified brain specific nuclear GTPase, which binds PI 3-kinase and stimulates its lipid kinase activity. PIKE GTPase is activated by PLC-γ1 in the nucleus, the SH3 domain of PLC-γ1 acts as a guanine nucleotide exchange factor for PIKE, the short form of which is a nuclear GTPase and enhances the activity of nuclear class Ia PI3K required for proliferation [31,33]. Our previous studies have shown that PLC-γ1 is required for epidermal growth factor (EGF)-induced squamous cell carcinoma (SCC) mitogenesis [32]. PIKE has been identified as a downstream target of PLC-γ1. We have also shown that PIKE plays a critical role in EGF-induced proliferation of OSCC cells and is overexpressed in OSCC [34]. However, it is unclear whether overexpression of PIKE expression is associated with the aggressiveness of OSCC.
In the present study, we examined expression levels of E-cadherin, p120, PLC-γ1 and PIKE in the specimen of OSCC from 92 patients to evaluate whether the expression levels of these proteins are associated with the clinicopathologic features.
Materials and methods
Specimens and clinicopathologic materials
The study comprised of 92 OSCC subjects, aged 52.6 ± 11.2 years, with pathological stages I-IV, hospitalized in the Second Xiangya Hospital of Central South University in China from June 2009 to June 2011. Seventy eight cases (84.8%) were male and 14 (15.2%) were female. Specimens were classified in accordance with the guidelines set by the World Health Organization for the histological classification of OSCC. Thirty-two cases were well differentiated, 45 cases were moderately differentiated, and 15 cases were poorly differentiated. Fifty three cases were at stage I-II and 39 cases were at stage III-IV. Thirty six patients showed cervical lymph node metastasis and none of the patients had received radiotherapy or chemotherapy before biopsy. All specimens taken from malignant oral primary tumors were fixed by 4% formaldehyde, followed by conventional paraffin-embedded sectioning. The written consents were signed by the patients for their specimen and the study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University in China.
Immunohistochemical staining
Paraffin-embedded 4-micrometer-thick specimens were dewaxed in turpentine and rehydrated through decreased concentrations of ethanol. Endogenous peroxidase activity was blocked by using 3% H2O2 in methanol for 15 min. The sections were incubated with trisodium citrate dihydrate liquid (0.125%, pH 6.0) for 15 min, and then soaked with phosphate buffered saline (PBS) liquid (pH 7.2-7.4) three times for 5 min. The sections were then pre-incubated with sheep serum for 10 min to block non-specific antigen. The pretreated slides were incubated overnight at 4°C in a humidified chamber with rabbit polyclonal primary antibodies against mouse E-cadherin, p120, PLC-γ1, or PIKE. Antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA) include rabbit polyclonal antibodies against PLC-γ1 (Cat# sc-81, dilution 1:100), E-cadherin (Cat# sc-7870, dilution 1:100), or p120 (Cat# sc-13957, dilution 1:100). Antibodies purchased from upstate biotechnology (Lakeplacid, NY) include rabbit polyclonal the antibody against PIKE (Cat# 52-5817, dilution 1:100). After incubation with these antibodies, the slides were rinsed with PBS three times and the slides were incubated with appropriate biotinylated secondary antibodies for 20 min followed by avidin (Maixin Biological Technology Development Company) and diaminobenzidine (Maixin Biological Technology Development Company). Hematoxylin was used as counter-staining. In the negative controls, PBS (pH 7.4) was used instead of the primary antibody.
Statistical analysis
The expression of E-cadherin or p120 were assessed by counting the number of cells in which E-cadherin or p120 were positively stained in the plasma membrane and the total number of cells at 400 × magnification in five representative regions of the tumor. The expression of PLC-γ1 and PIKE were assessed by counting the number of cells in which PLC-γ1 and PIKE were positively stained in the nucleus or cytoplasm and total number of cancer cells at 400 × magnification in five representative regions of the tumor. Results are expressed as the proportion of positively stained cells over the total number of cells [35]. For evaluation of the nucleus staining of p120, PLC-γ1, and PIKE, each section was assessed by counting the number of cells in which p120, PLC-γ1, and PIKE were positively stained in the nucleus and the total number of cells at 400 × magnification in five representative regions of the tumor. Results are expressed as the proportion of positively stained cells over the total number of cells. For routine histological analysis, the results were examined under a light microscope by two Board-certified pathologists at the Second Xiangya Hospital. Data are presented as mean ± standard deviation. Analysis was assessed using the Analysis of Variance (ANOVA) and chi-square test. Significance was defined as P < 0.05.
Results
Association of the levels of E-cadherin, p120, PLC-γ1, and PIKE with OSCC clinicopathologic features
To determine whether levels of E-cadherin, p120, PLC-γ1, and PIKE are associated with OSCC clinicopathologic features, we examined the levels of these proteins in OSCC and adjacent non-cancerous tissues. Figure 1A shows the representative staining image of p120, E-cadherin, PIKE, and PLC-γ1 in OSCC with different levels of differentiation and adjacent non-cancerous tissue. The poorly differentiated OSCC had the lowest levels of E-cadherin and p120 in the plasma membrane and highest levels of PLC-γ1 and PIKE in the cytoplasm and nucleus (P < 0.05). In contrast, the well differentiated group had the highest levels of E-cadherin and p120 in the plasma membrane and the lowest levels of PLC-γ1 and PIKE in the cytoplasm and nucleus. p120 was mainly localized in the plasma membrane in the non-cancerous tissue. However, the staining of p120 in the plasma membrane was reduced and became evident in the nucleus of OSCC cells, especially in poorly differentiated OSCC cells. E-cadherin was mainly localized in the plasma membrane in the non-cancerous tissue and reduced in OSCC. Unlike p120, no nuclear staining of E-cadherin was seen in OSCC, even in poorly differentiated OSCC cells. Figure 1B shows the quantitation of these proteins in OSCC and adjacent non-cancerous tissue. The positivities of p120 in poorly differentiated, moderately differentiated, well differentiated, and non-cancerous tissue were 10 ± 5%, 25 ± 8%, 38 ± 9%, and 68 ± 7% respectively. The positivities of E-cadherin in poorly differentiated, moderately differentiated, well differentiated, and non-cancerous tissue were 11 ± 6 %, 27 ± 8%, 41 ± 10%, and 72 ± 10% respectively. The positivities of PIKE in poorly differentiated, moderately differentiated, well differentiated, and non-cancerous tissue were 72 ± 8%, 55 ± 7%, 42 ± 7%, and 24 ± 4% respectively. The positivities of PLC-γ1 in poorly differentiated, moderately differentiated, well differentiated, and non-cancerous tissue were 73 ± 8%, 56 ± 6%, 45 ± 7%, and 20 ± 5% respectively.
Figure 1.
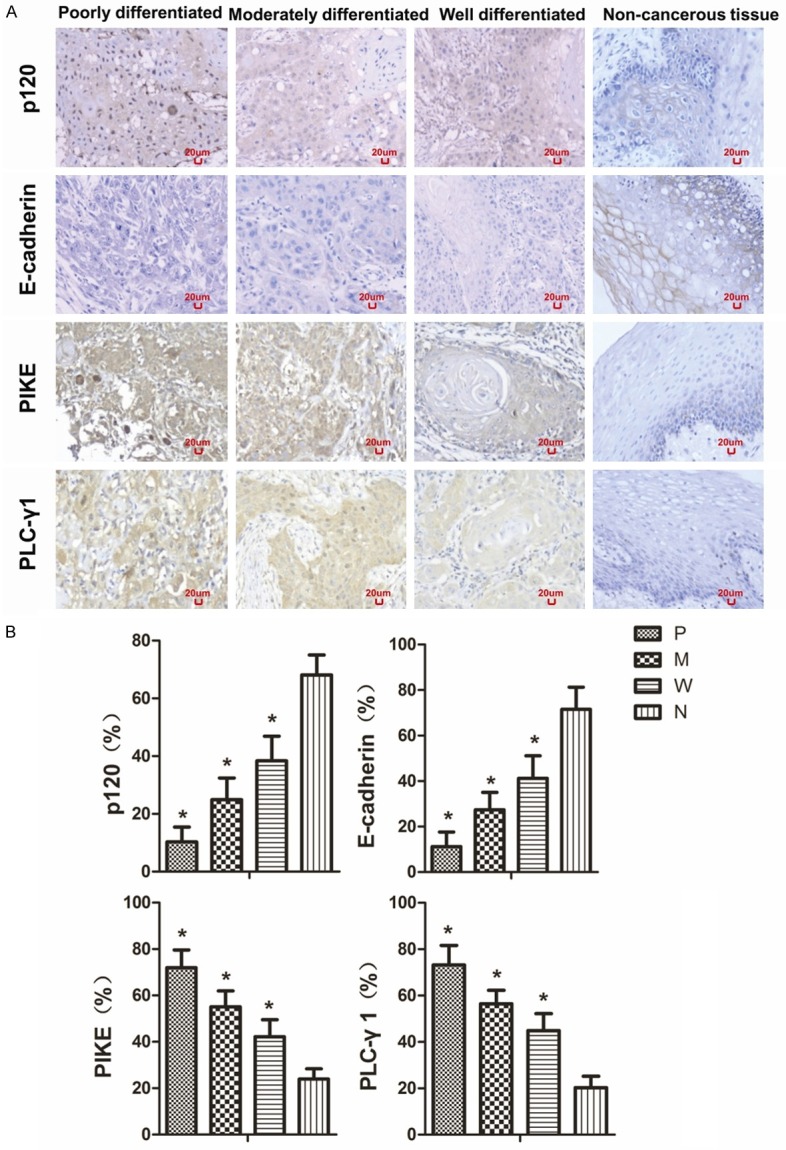
The expression levels of p120, E-cadherin, PIKE, and PLC-γ1 protein in OSCC with different levels of differentiation. Positive expression is shown in brown and the counterstaining is shown in blue. The representative section shows the average levels of p120, E-cadherin, PIKE, and PLC-γ1 in poorly differentiated, moderately differentiated, well differentiated OSCC, and adjacent non-cancerous tissue (A). The expression of p120, E-cadherin, PIKE, and PLC-γ1 levels in poorly differentiated, moderately differentiated, well differentiated OSCC, and non-cancerous tissue is shown as bar graphs (B). The quantitation of positive expression in each section was obtained by counting the number of positive cells and total number of cells in the corresponding region in five representative regions in each section. The proportion of positively staining cells over the total number of cells was calculated as describe in Materials and Methods. The data are expressed as mean ± SD, *P < 0.05 (compared with the normal epithelium). P, poorly differentiated; M, moderately differentiated; W, well differentiated; N, non-cancerous tissue.
A significantly higher expression of PLC-γ1 (64 ± 9% positivity) and PIKE (62 ± 10% positivity) proteins and a lower expression of p120 (18 ± 8% positivity) and E-cadherin (17 ± 8% positivity) protein were found in patients with locally advanced OSCC (stage T3 + T4) compared with PLC-γ1 (48 ± 7% positivity), PIKE (46 ± 8% positivity), p120 (35 ± 8 % positivity), and E-cadherin (38 ± 9% positivity) of early stage OSCC (stage T1 + T2) (P < 0.05) (Figure 2). Univariate analysis revealed that the OSCC with lymph node metastasis had higher expression of PLC-γ1 (63 ± 10% positivity) and PIKE (62 ± 10% positivity) but lower expression of E-cadherin (20 ± 9% positivity) and p120 (18 ± 9% positivity) compared to PLC-γ1 (49 ± 9% positivity), PIKE (47 ± 9% positivity), p120 (34 ± 10% positivity) and E-cadherin (37 ± 10% positivity) of the OSCC without lymph node metastasis (P < 0.05) (Figure 3).
Figure 2.
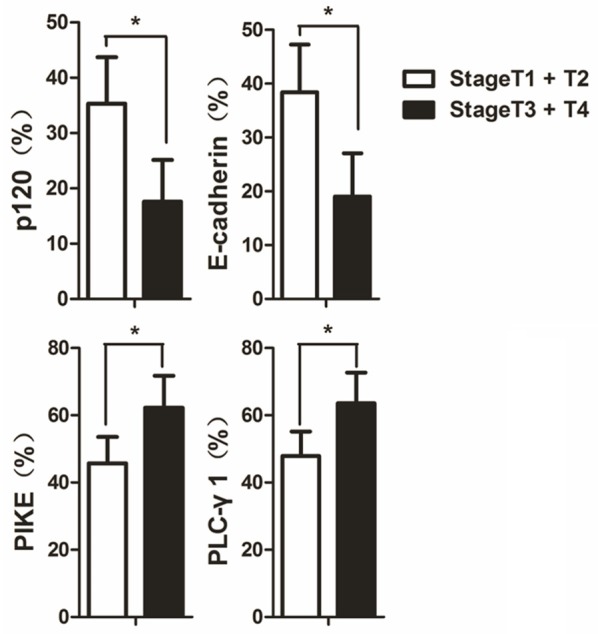
The expression levels of p120, E-cadherin, PIKE, and PLC-γ1 in OSCC with different clinical stages. The expression levels of p120, E-cadherin, PIKE, and PLC-γ1 in early stage OSCC (stage T1 + T2) and advanced OSCC (stage T3 + T4) are shown as bar graphs. The quantitation was obtained as described in Figure 1. The data are expressed as mean ± SD, *P < 0.05.
Figure 3.
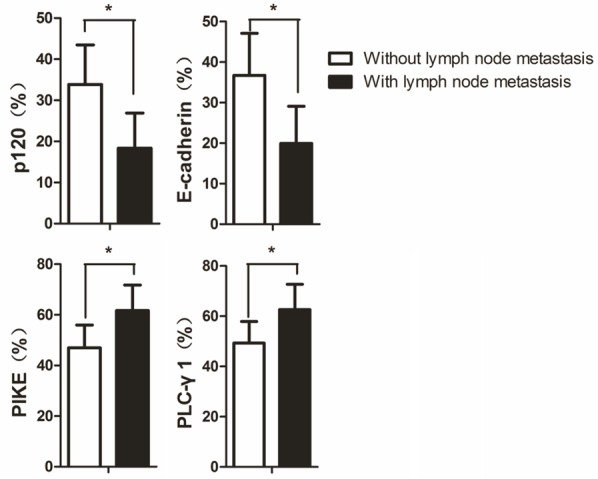
The expression levels of p120, E-cadherin, PIKE, and PLC-γ1 in OSCC with or without lymph node metastases. The expression levels of p120, E-cadherin, PIKE, and PLC-γ1 in OSCC with lymph node metastasis and OSCC without lymph node metastasis are shown as bar graphs. The quantitation was obtained as described in Figure 1. The data are expressed as mean ± SD, *P < 0.05.
Pairwise association of E-cadherin, p120, PLC-γ1, and PIKE in OSCC
The pairwise association between aberrant expressions of E-cadherin, p120, PLC-γ1, and PIKE in OSCC is shown in Table 1. A positive association between p120 and E-cadherin levels (r = 0.407, P < 0.01) and also between PLC-γ1 and PIKE levels (r = 0.572, P < 0.01) was observed. However, negative correlations between levels of p120 and levels of PLC-γ1 (r = -0.651, P < 0.01) and PIKE (r = -0.733, P < 0.01) in OSCC and between levels of E-cadherin and levels of PLC-γ1 (r = -0.702, P < 0.01) and PIKE (r = -0.740, P < 0.01) in OSCC were seen.
Table 1.
Pairwise association of aberrant expressions of E-cadherin, p120, PLC-γ1, and PIKE in OSCC
| p120 | PLC-γ1 | PIKE | E-cadherin | |
|---|---|---|---|---|
| p120 | 1 | -0.651 | -0.733 | 0.407 |
| PLC-γ1 | - | 1 | 0.572 | -0,702 |
| PIKE | - | - | 1 | -0.740 |
| E-cadherin | - | - | - | 1 |
Association of p120, PLC-γ1, and PIKE staining in the nucleus with OSCC clinicopathologic features
The percentages of positive cells for nuclear staining of p120 (27%), PLC-γ1 (60%), and PIKE (27%) in the poorly differentiated OSCC group was obviously stronger than that (13% for p120, 31% for PLC-γ1, and 16% for PIKE) of the moderately differentiated group (P < 0.05) (Figure 4). However, the percentages of positive cells for nuclear staining of p120 (3%), PLC-γ1 (12%), and PIKE (9%) in the well differentiated OSCC group were obviously weaker than that (13% for p120, 31% for PLC-γ1, and 16% for PIKE) of the moderately differentiated group (P < 0.05) (Figure 4). A significantly higher level of nuclear staining of p120 (23%), PLC-γ1 (41%), and PIKE (23%) was found in patients with locally advanced stage of OSCC (stage T3 + T4) compared with that (4% for p120, 21% for PLC-γ1, and 9% for PIKE) of early stage OSCC (stage T1 + T2) (P < 0.05) (Figure 4). The OSCC with lymph node metastasis displayed a higher level of nuclear staining of p120 (19%), PLC-γ1 (33%), and PIKE (19%) than that (7% for p120, 27% for PLC-γ1, and 13% for PIKE) of OSCC without lymph node metastasis (P < 0.05) (Figure 4).
Figure 4.
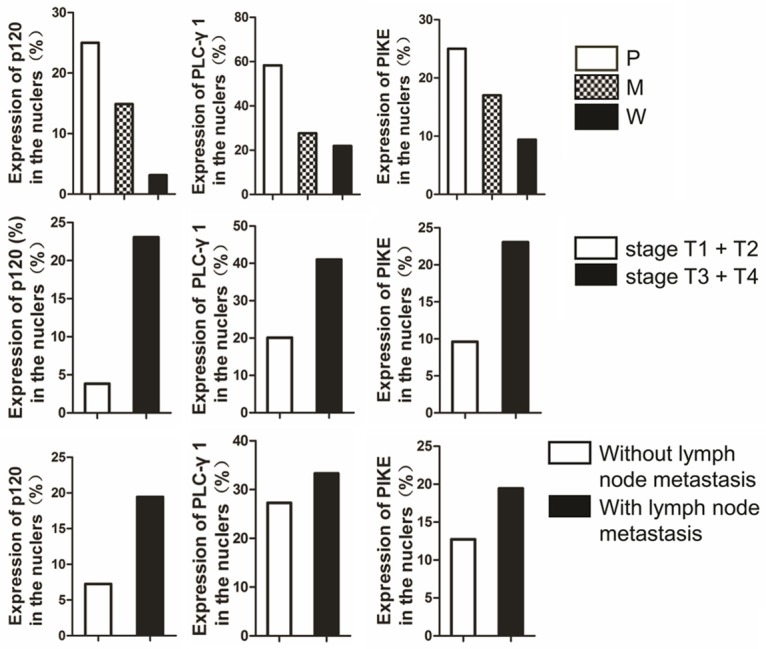
Nuclear staining of p120, PLC-γ1, and PIKE in OSCC. The percentages of positive cells for nuclear staining of p120, PLC-γ1, and PIKE in OSCC with different levels of differentiation, clinical stages and with or without lymph node metastasis are shown as bar graphs. P, poorly differentiated; M, moderately differentiated; W, well differentiated; N, non-cancerous tissue.
Discussion
Previous studies have demonstrated an association of the reduction of E-cadherin and p120 expression in several advanced malignancies [7-12]. Data from the present study showed that the expression of p120 and E-cadherin positively correlated with the level of OSCC differentiation. The data also showed a lower expression level of E-cadherin and p120 in OSCC stage T3 + T4 compared to that in OSCC stage T1 + 2 and a lower expression level of E-cadherin and p120 in OSCC without lymph node metastasis. These data suggest that increased levels of E-cadherin and p120 are associated with increased differentiation and decreased progression and metastasis in OSCC.
We have previously shown that PLC-γ1 and PIKE mediate EGF-induced OSCC proliferation and the expression levels of PLC-γ1 and PIKE are elevated in OSCC [32,34]. Our present results showed that PLC-γ1 and PIKE negatively correlated with the OSCC differentiation and the expression of PLC-γ1 and PIKE in OSCC stage T3 + T4 was higher than that in OSCC stage T1 + T2. A higher expression of PLC-γ1 and PIKE in OSCC with lymph node metastasis compared to that in OSCC without lymph node metastasis was also noted. These data suggest that increased expression of PLC-γ1 and PIKE is associated with OSCC progression and metastasis.
The correlation analysis showed that p120 expression positively correlated with E-cadherin but negatively correlated with PLC-γ1 and PIKE expression. There was also a positive correlation between PLC-γ1 and PIKE. These results suggest that decreased expression of PLC-γ1 and PIKE is associated with increased expression of E-cadherin and p120. Our previous studies have shown that activation of PLC-γ1 is a major downstream component in the pathway of calcium-induced keratinocyte differentiation mediated by E-cadherin-p120 [36]. One could speculate that PLC-γ1 expression might be upregulated in the well-differentiated OSCC. However, our present results showed that PLC-γ1 was downregulated in the well differentiated OSCC and upregulated in the poorly differentiated OSCC. This seems to be discrepant from what we have found in our previous studies. The possible explanation for this discrepancy is that keratinocyte differentiation is mediated by the activation of PLC-γ1 in the plasma membrane and proliferation of keratinocytes may require elevated PLC-γ1 in the different cellular pool. Elevated expression of PLC-γ1 in the cytoplasm of OSCC cells compared to the adjacent non-cancerous tissue as shown in our previous studies support this explanation.
Our results showed that the levels of the nuclear staining of p120, PLC-γ1 and PIKE in moderately differentiated OSCC group were higher than that in well differentiated group and lower than that in poorly differentiated group. In addition, higher levels of nuclear staining of p120, PLC-γ1, and PIKE were detected in patients with locally advanced stage of OSCC (stage T3 + T4) compared to that with early stage of OSCC (stage T1 + T2). The OSCC with lymph node metastasis displayed higher nuclear staining of PLC-γ1 and PIKE than OSCC without lymph node metastasis. Our data suggest that the nuclear staining of p120, PLC-γ1 and PIKE is associated with tumor progression.
E-cadherin and p120 were normally localized in the plasma membrane and PLC-γ1 in the cytoplasm. PIKE was normally localized in both the nucleus and cytoplasm. In the present study, p120 was seen in the nucleus and cytoplasm and PLC-γ1 was observed in the nucleus. It is known that p120 functions as a transcription factor, but the role of which in the nucleus is not quite clear. It is likely that p120 behaves like β-catenin, plays different roles in different cellular compartments [37,38].
PIKE identified originally in neuronal cells has three alternatively spliced forms: the short form exclusively in the nucleus and the long form and PIKE-activating Akt (PIKE-A) in both nuclear and cytosolic compartments. The short form of PIKE is localized in the nucleus where it binds to PLC-γ1 in response to EGF and required for neuronal cell proliferation [39]. We have previously demonstrated that PIKE is highly expressed in OSCC cells and mediates EGFR-dependent SCC cell proliferation [34]. However, the expression of PIKE is not regulated by EGFR activation. PIKE sits in the nucleus to bind to PLC-γ1 which is translocated from the plasma membrane in the presence of EGFR activation [34]. Based on the present and past observations [34,36,40-43], we hypothesize that p120 suppresses OSCC cell proliferation via sequestering PLC-γ1 away from the nucleus, thereby losing its ability to activate PIKE in the nucleus. Further studies are required to test this hypothesis.
In conclusion, increased expression of PLC-γ1 and PIKE and decreased expression of E-cadherin and p120 are associated with tumor progression and metastasis. Our data support the emerging hypothesis that the signaling pathway of E-cadherin-β-catenin-p120 complex-dependent activation of PLC-γ1 and PIKE could play an important role in the progression and metastasis of OSCC. A thorough understanding of the mechanism might open new avenues for oral cancer research.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 81072219, 81272973 and 81471055).
Disclosure of conflict of interest
None.
Abbreviations
- OSCC
oral squamous cell carcinoma
- E-cad
E-cadherin
- p120
p120-catenin
- PIP5K1α
phosphatidylinositol-4-phosphate 5-kinase1α
- PLC-γ1
phospholipase C-γ1
- PIKE
phosphatidylinositol 3-kinase enhancer
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm’s length. Am J Physiol Cell Physiol. 2004;286:C987–97. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- 3.Xiong H, Hong J, Du W, Lin YW, Ren LL, Wang YC, Su WY, Wang JL, Cui Y, Wang ZH, Fang JY. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem. 2012;287:5819–5832. doi: 10.1074/jbc.M111.295964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillon DA, D’Aquila T, Reynolds AB, Fearon ER, Rimm DL. The expression of p120ctn protein in breast cancer is independent of alpha- and beta-catenin and E-cadherin. Am J Pathol. 1998;152:75–82. [PMC free article] [PubMed] [Google Scholar]
- 8.Shimazui T, Schalken JA, Giroldi LA, Jansen CF, Akaza H, Koiso K, Debruyne FM, Bringuier PP. Prognostic value of cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120cas) in bladder tumors. Cancer Res. 1996;56:4154–4158. [PubMed] [Google Scholar]
- 9.Syrigos KN, Karayiannakis A, Syrigou EI, Harrington K, Pignatelli M. Abnormal expression of p120 correlates with poor survival in patients with bladder cancer. Eur J Cancer. 1998;34:2037–2040. doi: 10.1016/s0959-8049(98)00279-2. [DOI] [PubMed] [Google Scholar]
- 10.Shimazui T, Bringuier PP, van Berkel H, Ruijter E, Akaza H, Debruyne FM, Oosterwijk E, Schalken JA. Decreased expression of alpha-catenin is associated with poor prognosis of patients with localized renal cell carcinoma. Int J Cancer. 1997;74:523–528. doi: 10.1002/(sici)1097-0215(19971021)74:5<523::aid-ijc8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Skoudy A, Gomez S, Fabre M, Garcia de Herreros A. p120-catenin expression in human colorectal cancer. Int J Cancer. 1996;68:14–20. doi: 10.1002/(SICI)1097-0215(19960927)68:1<14::AID-IJC3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Valizadeh A, Karayiannakis AJ, el-Hariry I, Kmiot W, Pignatelli M. Expression of E-cadherin-associated molecules (alpha-, beta-,and gamma- catenins and p120) in colorectal polyps. Am J Pathol. 1997;150:1977–1984. [PMC free article] [PubMed] [Google Scholar]
- 13.Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115–124. doi: 10.1016/s0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 14.Kallakury BV, Sheehan CE, Ross JS. Co-downregulation of cell adhesion proteins alpha- and beta-catenins, p120CTN, E-cadherin, and CD44 in prostatic adenocarcinomas. Hum Pathol. 2001;32:849–855. doi: 10.1053/hupa.2001.26463. [DOI] [PubMed] [Google Scholar]
- 15.Karayiannakis AJ, Syrigos KN, Alexiou D, Kalahanis N, Rosenberg T, Bastounis E, Pignatelli M. Expression patterns of the novel catenin p120cas in gastrointestinal cancers. Anticancer Res. 1999;19:4401–4405. [PubMed] [Google Scholar]
- 16.Chetty R, Jain D, Serra S. p120 catenin reduction and cytoplasmic relocalization leads to dysregulation of E-cadherin in solid pseudopapillary tumors of the pancreas. Am J Clin Pathol. 2008;130:71–76. doi: 10.1309/FEYD99TXC4LMYVA5. [DOI] [PubMed] [Google Scholar]
- 17.Soares MQ, Mendonça JA, Morais MO, Leles CR, Batista AC, Mendonça EF. E-cadherin, β-catenin, and α2β1 and α3β1 integrin expression in primary oral squamous cell carcinoma and its regional metastasis. Histol Histopathol. 2015;30:1213–22. doi: 10.14670/HH-11-616. [DOI] [PubMed] [Google Scholar]
- 18.Afrem MC, Mărgăritescu C, Crăiţoiu MM, Ciucă M, Şarlă CG, Cotoi OS. The immunohistochemical investigations of cadherin “switch” during epithelial-mesenchymal transition of tongue squamous cell carcinoma. Rom J Morphol Embryol. 2014;55:1049–1056. [PubMed] [Google Scholar]
- 19.Silva SD, Morand GB, Alobaid FA, Hier MP, Mlynarek AM, Alaoui-Jamali MA, Kowalski LP. Epithelial-mesenchymal transition (EMT) markers have prognostic impact in multiple primary oral squamous cell carcinoma. Clin Exp Metastasis. 2015;32:55–63. doi: 10.1007/s10585-014-9690-1. [DOI] [PubMed] [Google Scholar]
- 20.Zaid KW. Immunohistochemical assessment of E-cadherin and β-catenin in the histological differentiations of oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2014;15:8847–8853. doi: 10.7314/apjcp.2014.15.20.8847. [DOI] [PubMed] [Google Scholar]
- 21.Balasundaram P, Singh MK, Dinda AK, Thakar A, Yadav R. Study of β-catenin, E-cadherin and vimentin in oral squamous cell carcinoma with and without lymph node metastases. Diagn Pathol. 2014;9:145. doi: 10.1186/1746-1596-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SW, Kallarakkal TG, Abraham MT. Changed expression of E-cadherin and galectin-9 in oral squamous cell carcinomas but lack of potential as prognostic markers. Asian Pac J Cancer Prev. 2014;15:2145–2152. doi: 10.7314/apjcp.2014.15.5.2145. [DOI] [PubMed] [Google Scholar]
- 23.Mohtasham N, Anvari K, Memar B, Saghravanian N, Ghazi N, Bagherpour A, Ramtin M. Expression of E-cadherin and matrix metalloproteinase-9 in oral squamous cell carcinoma and histologically negative surgical margins and association with clinicopathological parameters. Rom J Morphol Embryol. 2014;55:117–121. [PubMed] [Google Scholar]
- 24.Luo SL, Xie YG, Li Z, Ma JH, Xu X. E-cadherin expression and prognosis of oral cancer: a meta-analysis. Tumour Biol. 2014;35:5533–5537. doi: 10.1007/s13277-014-1728-0. [DOI] [PubMed] [Google Scholar]
- 25.Pannone G, Santoro A, Feola A, Bufo P, Papagerakis P, Lo Muzio L, Staibano S, Ionna F, Longo F, Franco R, Aquino G, Contaldo M, De Maria S, Serpico R, De Rosa A, Rubini C, Papagerakis S, Giovane A, Tombolini V, Giordano A, Caraglia M, Di Domenico M. The role of E-cadherin down-regulation in oral cancer: CDH1 gene expression and epigenetic blockage. Curr Cancer Drug Targets. 2014;14:115–127. doi: 10.2174/1568009613666131126115012. [DOI] [PubMed] [Google Scholar]
- 26.Fan CC, Wang TY, Cheng YA, Jiang SS, Cheng CW, Lee AY, Kao TY. Expression of E-cadherin, Twist, and p53 and their prognostic value in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2013;139:1735–1744. doi: 10.1007/s00432-013-1499-9. [DOI] [PubMed] [Google Scholar]
- 27.de Freitas Silva BS, Yamamoto-Silva FP, Pontes HA, Pinto Júnior Ddos S. E-cadherin downregulation and Twist overexpression since early stages of oral carcinogenesis. J Oral Pathol Med. 2014;43:125–131. doi: 10.1111/jop.12096. [DOI] [PubMed] [Google Scholar]
- 28.Ma LW, Zhou ZT, He QB, Jiang WW. Phosphorylated p120-catenin expression has predictive value for oral cancer progression. J Clin Pathol. 2012;65:315–319. doi: 10.1136/jclinpath-2011-200516. [DOI] [PubMed] [Google Scholar]
- 29.Mahomed F, Altini M, Meer S. Altered E-cadherin/beta-catenin expression in oral squamous carcinoma with and without nodal metastasis. Oral Dis. 2007;13:386–392. doi: 10.1111/j.1601-0825.2006.01295.x. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z, Peng J, Pennypacke SD, Chen Y. Critical role for the catalytic activity of phospholipase C-gamma1 in epidermal growth factor-induced cell migration. Biochem Biophys Res Commun. 2010;399:425–428. doi: 10.1016/j.bbrc.2010.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Z, Chen Y, Pennypacker SD, Zhou Z, Peng D. The SH3 domain, but not the catalytic domain, is required for phospholipase C-gamma1 to mediate epidermal growth factor-induced mitogenesis. Biochem Biophys Res Commun. 2010;398:719–722. doi: 10.1016/j.bbrc.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Z, Chen Y, Liao EY, Jiang Y, Liu FY, Pennypacker SD. Phospholipase C-gamma1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem Biophys Res Commun. 2010;397:296–300. doi: 10.1016/j.bbrc.2010.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH. Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature. 2002;415:541–544. doi: 10.1038/415541a. [DOI] [PubMed] [Google Scholar]
- 34.Xie Z, Y Jiang, Liao EY, Chen Y, Pennypacker SD, Peng J, Chang SM. PIKE mediates EGFR proliferative signaling in squamous cell carcinoma cells. Oncogene. 2012;31:5090–5098. doi: 10.1038/onc.2012.10. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Cai JH, Zhang J, Tang YX, Wan L. Effects of cyclooxygenase inhibitors in combination with taxol on expression of cyclin d1 and ki-67 in a xenograft model of ovarian carcinoma. Int J Mol Sci. 2012;13:9741–9753. doi: 10.3390/ijms13089741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 37.Peifer M, Yap AS. Traffic control: p120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells. J Cell Biol. 2003;163:437–40. doi: 10.1083/jcb.200310090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH. Phospholipase C-c1 is aphysiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature. 2002;415:541–544. doi: 10.1038/415541a. [DOI] [PubMed] [Google Scholar]
- 40.Xie Z, Bikle DD. Phospholipase C-gamma1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- 41.Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Mol Biol Cell. 2009;20:1695–1704. doi: 10.1091/mbc.E08-07-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]


