Abstract
Lung cancer is the leading cause of cancer-related deaths worldwide and about 85% of these are non-small cell lung cancer (NSCLC). Several new chemotherapeutic agents have recently shown encouraging activity in NSCLC, especially docetaxel. MiRNAs (MicroRNAs) are closely related to cancer development. We studied miRNAs in NSCLC cell lines to identify those that can regulate and predict the effectiveness of docetaxel on NSCLC. CCK8, Annexin and V-FITC assays were carried out to evaluate the inhibitory effect of docetaxel on NSCLC cell linesA549 and H460, and qRT-PCR was used to detect and compare six miRNAs expression levels in the two cells with docetaxel or not. Knockdown of miR-7 by RNA interference and overexpression of miR-7 were taken to evaluatethe effect of miR-7 on docetaxel effectiveness. Western blotting was used to evaluate the effect of miR-7 on Bcl2 in A549 and H460 cells. Docetaxel induced non-small cell lung cancer cell apoptosis and suppressed cell proliferation in vitro. MiR-7 expression levels were increased by docetaxel in the two cell lines. MiR-7 overexpression improved anti-proliferative and pro-apoptotic effects of docetaxel on the NSCLC cells and that miR-7 down-regulation decreased those effects. Moreover, subsequent experiments showed that BCL-2 was downregulated by miR-7 at both transcriptional and translational levels. This study further extends the biological role of miR-7 in NSCLC A549 and H460 cells and identifies BCL-2 as a novel target possibly involved in miR-7-mediated growth suppression and apoptosis induction of NSCLC cells.
Keywords: miRNA, miR-7, non-small cell lung cancer, docetaxel
Introduction
Lung cancer is the leading cause of death worldwide [1], and non-small cell lung cancer (NSCLC) is the most commontype of this disease. At present, despite a number of randomized clinical trials and recent meta-analyses have demonstrated that survival in patients with advanced stage III or stage IV NSCLC can be prolonged with chemotherapy, more than one half of non-small-cell lung cancer (NSCLC) patients arenot diagnosed until the disease reaches an advanced stage. Thus, pathogenesis, diagnosis and medical treatments of NSCLC still are substantial clinical challenges.
Several new chemotherapeutic agents, such as docetaxel and pemetrexed, are useful as second- or third-line treatments for NSCLC. Docetaxel is a standard second-line chemotherapy regimen that is most widely used in Japan. A randomized phase III study comparing docetaxel and best supportive care demonstrated better overall survival (OS) for docetaxel patients (7.5 vs. 4.6 months, P = 0.047). Docetaxel is a cytotoxic anti-microtubule agent that binds to the β-tubulin subunit of microtubulin, resulting in stabilizing microtubules and preventing depolymerization, which leads to the inhibition of microtubule dynamics and cell cycle arrest and eventually apoptotic cell death [2-4]. More recent work suggests docetaxel has been widely used against different cancers such as ovarian cancer, lung cancer, breast cancer and is the first line treatment for castration-resistant prostate cancer [5-10]. However, the biological function and mechanisms of docetaxel in lung cancer, especially in NSCLC, remain to be further elucidated.
MicroRNAs (miRNAs) are a group of non-coding RNA (~22 nt) post-transcriptional regulators for gene expression [11]. MiRNAs are responsible for various biological and pathological processes, including cancer development and progression [12-15]. MiRNA is able to function as either a tumor suppressor or an oncogene [16,17]. Indeed, a number of differentially regulated miRNAs, such as miR-451 [18], let-7a [19], miR-21 [20], miR-205 [21,22], miR-126 [22] and miR-7 [23-27], have been identified to be functionally associated with cancer cell proliferation, invasion andmetastasis. Among them, miR-7 was first studied in Drosophila [28]. In 2008, it was identified as a tumor suppressor in glioblastomas [25], directly targeting EGFR as well as downregulating the AKT pathway to decrease viability and invasiveness of cancercells. This effect was confirmed in the A549 lung cancer cellsa year later [27]. Moreover, a recent study identified that miR-7 was reported to inhibit A549 cell growth by targeting BCL-2 [29]. Still, few studieshave assessed the relationship between miRNAs and the effect of Docetaxel on NSCLC.
In the present study, we observed that Docetaxel inhibited two NSCLC cell lines proliferation in vitro. MiR-7 expression levels were increased by docetaxel in the two cell lines. Regulation of miR-7 could affect the inhibition of proliferation and apoptosis of NSCLC cells induced by docetaxel. MiR-7 also increased Bcl2 protein expression in A549 and H460 cells. Collectively, our results suggest that miR-7 may be a target and an indicator of docetaxel’s effects on NSCLC.
Materials and methods
Cell lines and cell culture
A549, H460 and 293T cell lines were provided by Institute of Biochemistry and Cell Biology of Chinese Academy of Science (China) and originated from ATCC. All cells were grown in DMEM supplemented with 10% fetal bovine serum, 2 μM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin sulfates. Docetaxel (docetaxel) was purchased from Sigma (St. Louis, MO), dissolved in DMSO.
RNA extraction
Total RNA of cultured cells was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNAs were then stored at -80°C before RT-PCR analysis.
Quantitative RT-PCR (qRT-PCR) for miRNA
For mature miRNA expression analysis, approximately 10 ng of RNA was converted to cDNA using the ABI miRNA reverse transcription kit (Applied Biosystems, Foster City, CA) along with miR-7-specific primers (Applied Biosystems, Foster City, CA). After reverse transcription, quantitative polymerase chain reaction (PCR) was performed on the ABI 7500 thermocycler (Applied Biosystems, Foster City, CA) according to the manufacture’s protocol. U6 gene was used as a normalization control for all samples.
qRT-PCR for mRNA expression
Synthesis of cDNA was performed on 1 μg of total RNA per sample with the primerScript RT reagent Kit (TaKaRa, Dalian, China) according to the manufacturer’s manual. Quantitative reverse transcription-PCR was performed in triplicate for each sample by FastStart Universal SYBR Green Master kit (Roche, Switzerland) according to the manufacturer’s instructions. Oligonucleotides were designed by the PrimerExpress software. GAPDH was used as a housekeeping gene for normalization. The sequences of primers in this section are the followings: (1) BCL-2: 5’-atgtgtgtggagagcgtcaacc-3’ (forward) and 5’-tgagcagagtcttcagagacagcc-3’ (reverse); (2) GAPDH: 5’-tgcaccaccaactgcttagc-3’ (forward) and 5’-gcatggactgtggtcatgag-3’ (reverse).
MiRNAs mimic and transfection
The human miR-7 duplex mimic (miR-7) andnegative control oligonucleotide duplex mimic (miR-NC) were designed and provided by Ribo-bio (Guangzhou, Guangdong, China). 30-50% confluentcells were transfected with miRNAs by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to themanufacturer’s protocol. Total RNA was extracted 24 hours after transfection, and total cell protein wereextracted 48 or 72 hours after transfection.
Cell proliferation assay
Cell proliferation assay was determined by CellCounting Kit-8 assay (Dojindo, Japan), a redox assay similar to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) according to the manufacture’s protocol. Cell proliferation assay was carried out in hexakis.
Determination of apoptosis
The extent of apoptosis was determined by the flow cytometric measurement through AnnexinV-FITC apoptosis detection kit (Beyotime, China). Cells treated as above described. After 4 d, cells were harvested and washed twice with cold PBS. Then, cells were stained in 1 mL AnnexinV binding buffer with 10 μL of PI solution and 5 μL of AnnexinV-FITC for 10 min at RT and analyzed by flow cytometry.
Western blot analysis
Proteins extracted from cells were immunoblotted with different antibodies following a published protocol [30]. The primary antibodies used were BCL-2 (1:5000 dilutions) and GAPDH (1:5000 dilutions) (Cell Signaling, Danvers, MA, USA).
Statistical analysis
Data are reported as mean ± standard deviation (SD). Statistical significance was determined using double-sided Student’s t test. Multiple groups were analyzed using ANOVA. A P value of less than 0.05 was considered to be significant.
Results
Effects of docetaxel on proliferation and apoptosis of NSCLC cells in vitro
A549 cell was treated with increasing doses of docetaxel, and cell numbers were counted after 48 h treatment. As shown in Figure 1A, docetaxel showed a dose-dependent inhibitory effect on the NSCLC cell growth. One of the mechanisms of docetaxel-mediated cytotoxicity is inducing apoptotic cell death. We therefore measured apoptosis induced by docetaxel in these cells. As shown in Figure 1B, docetaxel treatment induces considerably apoptosis in A549 cells in a dose dependent manner. Collectively, these results demonstrate that docetaxel could inhibit in vitro proliferation of NSCLC cells.
Figure 1.

Docetaxel suppressed cell proliferation and induced apoptosis of A549 cells and H460 cells. Cells were treated with different doses of docetaxel (0, 0.025, 0.05, 0.1, 0.2, 0.4 and 1.0 μM) for 48 h. A. The cell viability was determined using a Cell Counting kit-8 assay. B. Apoptosis of A549 and H460 cells were detected by Annexin V/PI assay following 72-h exposure to docetaxel. Results are shown as mean ± SEM. Data is representative of three independent experiments.
We also tested whether docetaxel could induce the proliferative and apoptosis capacity of another NSCLC cell line, H460. H460 showed a similar proliferation rate and apoptotic rate even after 72 hours in culture (Figure 1A, 1B). These findings indicate that docetaxel may play an important role in the proliferation and apoptosis of NSCLC cells.
MiR-7 expression levels were upregulated by docetaxel in NSCLC cells
Some studies have reported that miRNAs can regulate the efficacy of chemotherapy drugs and molecule-targeted drugs [31]. They may become a novel type of biomarkerto predict and regulate chemotherapy. Thus, we took qRT-PCR to evaluate the changes of six miRNAs expression relative with NSCLC induced by docetaxel treatment. As shown in Figure 2, qRT-PCR assays demonstrated that miR-7 expression levels were increased after treatment with docetaxel A549 and H460 cells, and all other 5 miRNAs have no changes in both A549 and H460 cell lines.
Figure 2.
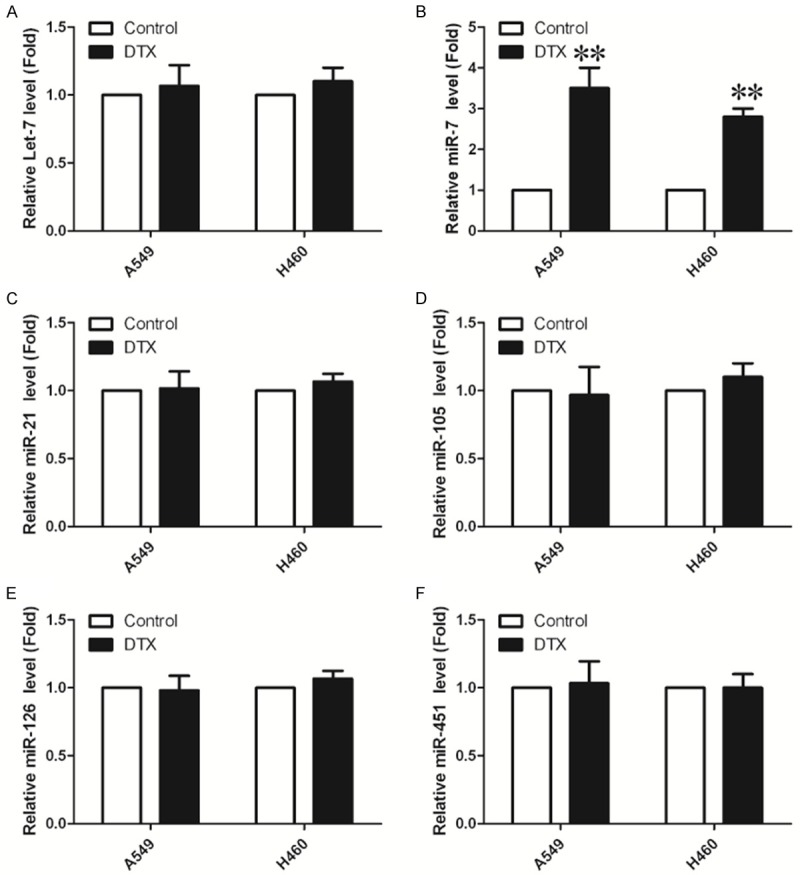
Expression of miR-7 is upregulated by docetaxel in NSCLC cell lines. Six miRNAs expression in NSCLC cell lines were measured by quantitative polymerase chain reaction (*P<0.05, **P<0.01).
Overexpression of miR-7 suppressed the expression of BCL-2 in vitro
A recent study identified that miR-7 was reported to inhibit A549 cell growth by targeting BCL-2. To directly test whether miR-7 can repress BCL-2, we transiently transfected A549 and H460 cells with miR-7 mimics. As a control, cancer cells were transfected with negative control mimic (miR-NC) without specifically targeting any human gene products. As shown in Figure 3A, after transfection with miR-7 mimic in A549, the expression of miR-7 was up-regulated by about 5.5-fold compared with control group and miR-7 showed similar up-regulated expression level in H460 cells.
Figure 3.
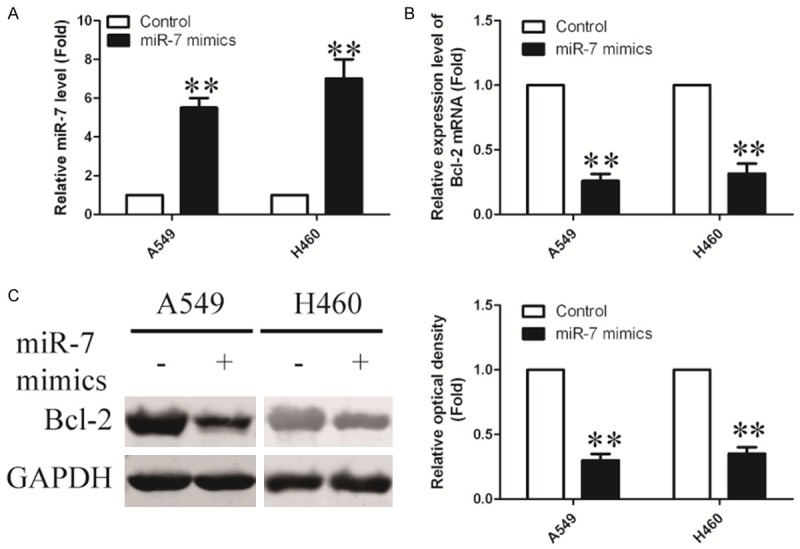
Overexpression of miR-7 in NSCLC cell lines. A. MiR-7 expression in A549 and H460 cells, following transfection with miR-7 or miR-NC, was determined by quantitative polymerase chain reaction. B. A549 and H460 cells were transfected either with miR-7 or miR-NC for 24 hours. BCL-2 mRNA levels were measured by real time RT-PCR, normalized with endogenous GAPDH and expressed as fold change compared with control. C. BCL-2 protein levels were examined by Western blot using GAPDH as a loading control. Results are shown as mean ± SEM. Data are representative of three independent experiments (*P<0.05, ***P<0.01).
To assess whether miR-7 had a functional role in downregulation of endogenous BCL-2 expression, A549 and H460 cells were transfected with miR-7 mimic for 24 h and then analyzed the BCL-2 expression by real-time PCR. As a result, overexpression of miR-7 significantly reduced the expression of BCL-2 at mRNA level (Figure 3B). Protein level of BCL-2 expression was further analyzed by Western blot. Compared with control groups, BCL-2 expression was remarkably reduced in response to miR-7 transfection for 48 h (Figure 3C). Taken together, these results suggested that miR-7 could decrease expression of BCL-2.
Knockdown of miR-7 induces apoptosis by upregulation of Bcl-2 in NSCLC cells
Since miR-7 could decrease expression of BCL-2, we would like to further investigate the influence of such kind of alteration on A549 and H460 cells. MiR-7 inhibitor was used to knock down endogenous miR-7 (Figure 4A). Following transfection with the miR-7 or miR-7 inhibitor, A549 and H460 cells were treated with DTX at various concentrations. As expected, the combined treatment of A549 and H460 cells with a miR-7 inhibitor and DOCETAXEL partly restored the DOCETAXEL-mediated suppression of cell viability (Figure 4B). The inhibition of miR-7 was associated with an increased mRNA and protein expression of Bcl2 in A549 and H460 cells treated with docetaxel (Figure 4C). Meanwhile, the percentage of cells with apoptotic nuclei was also increased by Annexin-V FITC/PI double staining assay (Figure 4D). These data indicate that Bcl2 may mediate the molecular mechanism of NSCLC sensitization to docetaxel by miR-7.
Figure 4.
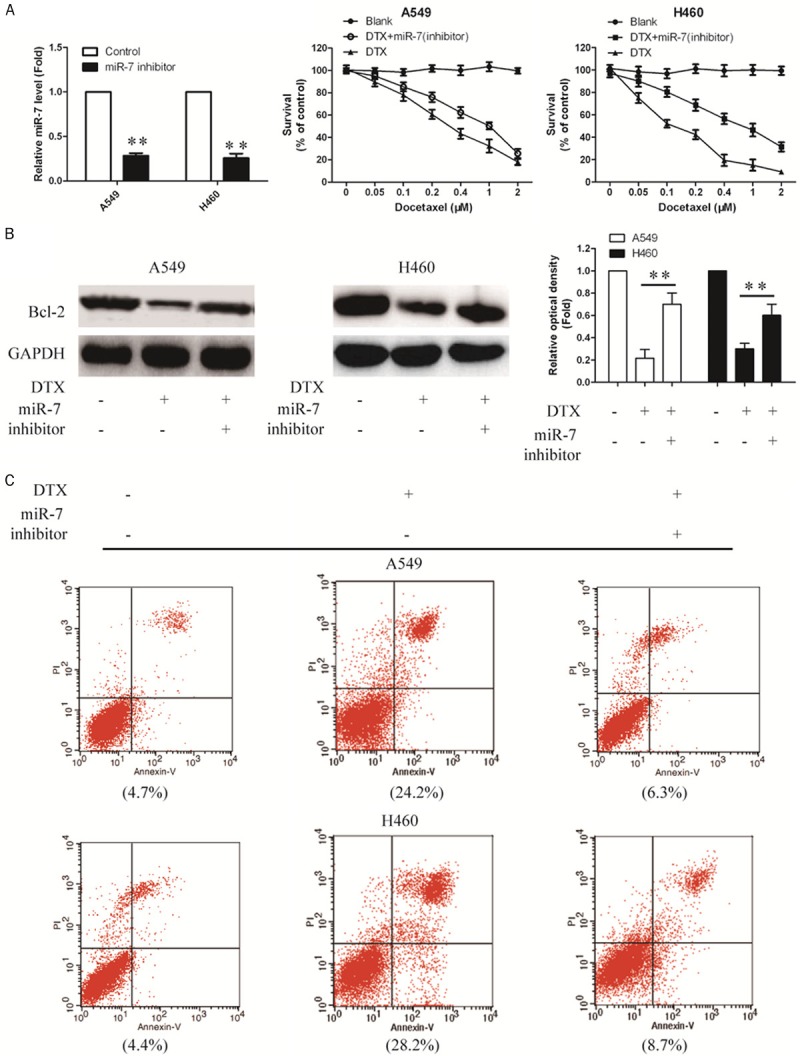
Knockdown of endogenous miR-7 expression enhances the protein expression of Bcl-2 in A549 and H460 cells treated with docetaxel. A. MiR-7 expression in A549 and H460 cells following transfection with the miR-7 or miR-7 inhibitor was detected by quantitative polymerase chain reaction. B. Cell viability of A549 and H460 cells was evaluated by Cell Counting kit-8 (CCK-8) assay following transfection with the miR-7 inhibitor and docetaxel combined treatment. C. Bcl-2 protein level was examined by Western blotting using GAPDH as a loading control. D. Apoptosis of A549 and H460 cells were determined by Annexin V/PI assays 48 h after transfection with the miR-7 inhibitor and docetaxel combined treatment (*P<0.05, **P<0.01).
Discussion
In the present study, we found that docetaxel induced non-small cell lung cancer cell apoptosis and suppressed cell proliferation. We also found that miRNAs expression levels were different between A549 and H460 cells, among them miR-7 expression levels were increased by docetaxel in the two cell lines. Furthermore, we demonstrated that combined treatment with docetaxel and miR-7 mimics reduced Bcl2 expression at the mRNA and protein levels. By contrast, the miR-7 inhibitor increased Bcl2 expression in docetaxel-treated cells. These results indicated that the downregulation of BCL-2 by miR-7 may be involved in the pro-apoptotic functionof miR-7.
Docetaxel, a semisynthetic analog of paclitaxel, is one of the first-line chemotherapy regimens for advanced NSCLC, with genotoxic effects caused by microtubule stabilizing, apoptotic induction throughmicrotubule bundling, and Bcl-2 blocking [32]. In this study, we investigated the potential mechanisms of docetaxel that occurred after docetaxel treatment in NSCLC cells. Our results demonstrate that docetaxel showed a dose-dependent inhibitory effect on the NSCLC cell growth and induces apoptotic cell death. These results suggest that docetaxel induced cell death is partly dueto induction of apoptosis in NSCLC cells.
Indeed, recent studies have demonstrated miRNAs as potential agents involved in thesensitivity of lung cancer cells to cytotoxic therapy. Several groups reported that the correlation of high miR-21 level, intumors or sera, with poor survival of lung cancer patients [33]. Wang et al. found that ectopic expression of miR-451 was able to induce an increase in apoptosis in a caspase-3 dependent manner [18]. MiR-7 is a putative tumor suppressor in a large range of solid tumors, and is often downregulated in NSCLC [34]. Ronghua et al. found the role of miR-7 in promoting chemosensitivity of cancer cells to PTX [35]. These findings imply that miRNAs are involved in NSCLC development and progression and could serve as potential diagnostic markers and therapeutic targets. In the present study, we found that the level of miR-7 expression was significantly increased in A549 and H460 with docetaxel.
Members of the evolutionarily conserved BCL-2 family are thought to be the central regulators of apoptosis. The expression level of BCL-2 differs for different cell types, however, high levels and aberrant patterns of BCL-2 expression were reported in a wide variety of human cancers, including lung cancer [29]. Elevation of Bcl-2 protein expression contributes not only to the development of cancer but also to resistanceagainst a wide variety of anticancer agents [36,37]. In our study, combined treatment with docetaxel and miR-7 mimics led to a significant reduction in endogenous BCL-2 mRNA and protein levels. By contrast, the miR-7 inhibitor increased Bcl2 expression in docetaxel-treated cells. This result is consistent with the previously report [29]. These results indicated that the downregulation of BCL-2 by miR-7 may be involved in the pro-apoptotic function of miR-7.
In summary, this study further extends the biological role of docetaxel in NSCLC A549 cells and H460 and identified BCL-2 as a novel target possibly involved in miR-7-mediated growth suppression and apoptosis induction of A549 cells. The identification of miR-7 as a potential sensitizer in docetaxel therapy provides a fundamental basis for new approaches in the development of novel and docetaxel therapeutic strategies.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Caraglia M, Giuberti G, Marra M, Di Gennaro E, Facchini G, Caponigro F, Iaffaioli R, Budillon A, Abbruzzese A. Docetaxel induces p53-dependent apoptosis and synergizes with farnesyl transferase inhibitor r115777 in human epithelial cancer cells. Front Biosci. 2005;10:2566–2575. doi: 10.2741/1720. [DOI] [PubMed] [Google Scholar]
- 3.Katsumata N. Docetaxel: an alternative taxane in ovarian cancer. Br J Cancer. 2003;89(Suppl 3):S9–S15. doi: 10.1038/sj.bjc.6601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaswamy B, Puhalla S. Docetaxel: a tubulin-stabilizing agent approved for the management of several solid tumors. Drugs Today (Barc) 2006;42:265–279. doi: 10.1358/dot.2006.42.4.968648. [DOI] [PubMed] [Google Scholar]
- 5.Escobar PF, Rose PG. Docetaxel in ovarian cancer. Expert Opin Pharmacother. 2005;6:2719–2726. doi: 10.1517/14656566.6.15.2719. [DOI] [PubMed] [Google Scholar]
- 6.Galsky MD, Vogelzang NJ. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann Oncol. 2010;21:2135–2144. doi: 10.1093/annonc/mdq050. [DOI] [PubMed] [Google Scholar]
- 7.Markman M. Pharmaceutical management of ovarian cancer: current status. Drugs. 2008;68:771–789. doi: 10.2165/00003495-200868060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Ramalingam S. First-line chemotherapy for advanced-stage non-small-cell lung cancer: focus on docetaxel. Clin Lung Cancer. 2005;7(Suppl 3):S77–82. doi: 10.3816/clc.2005.s.014. [DOI] [PubMed] [Google Scholar]
- 9.Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–241. doi: 10.1513/pats.200809-110LC. [DOI] [PubMed] [Google Scholar]
- 10.Vici P, Viola G, Botti C, Rossi S, Vitucci C, Corsetti S, Di Lauro L, Sergi D, Foggi P, Perri P, Tirelli C, Mottolese M, Fattoruso SI, Lopez M. Docetaxel in the adjuvant therapy of HER-2 positive breast cancer patients. Clin Ter. 2008;159:449–452. [PubMed] [Google Scholar]
- 11.Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell. 2007;28:328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang P, Liu R, Zheng Y, Liu X, Chang L, Xiong S, Chu Y. MiR-34a inhibits lipopolysaccharide-induced inflammatory response through targeting Notch1 in murine macrophages. Exp Cell Res. 2012;318:1175–1184. doi: 10.1016/j.yexcr.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Xiong S, Jiang P, Liu R, Liu X, Qian J, Zheng X, Chu Y. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: a novel anti-inflammation mechanism. Free Radic Biol Med. 2012;52:1307–1317. doi: 10.1016/j.freeradbiomed.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 16.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin PY, Yu SL, Yang PC. MicroRNA in lung cancer. Br J Cancer. 2010;103:1144–1148. doi: 10.1038/sj.bjc.6605901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian HB, Pan X, Yang JS, Wang ZX, De W. Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549) J Exp Clin Cancer Res. 2011;30:20. doi: 10.1186/1756-9966-30-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu CC, Chen YW, Chiou GY, Tsai LL, Huang PI, Chang CY, Tseng LM, Chiou SH, Yen SH, Chou MY, Chu PY, Lo WL. MicroRNA let-7a represses chemoresistance and tumourigenicity in head and neck cancer via stem-like properties ablation. Oral Oncol. 2011;47:202–210. doi: 10.1016/j.oraloncology.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya H, Iinuma H, Shimada R, Horiuchi A, Watanabe T. Clinicopathological and prognostic value of microRNA-21 and microRNA-155 in colorectal cancer. Oncology. 2010;79:313–320. doi: 10.1159/000323283. [DOI] [PubMed] [Google Scholar]
- 21.Lebanony D, Benjamin H, Gilad S, Ezagouri M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H, Nonaka D, Li J, Spector Y, Rosenfeld N, Chajut A, Cohen D, Aharonov R, Mansukhani M. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J. Clin. Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Peng XC, Zheng XL, Wang J, Qin YW. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Shalom-Feuerstein R, Riley J, Zhang SD, Tucci P, Agostini M, Aberdam D, Knight RA, Genchi G, Nicotera P, Melino G, Vasa-Nicotera M. miR-7 and miR-214 are specifically expressed during neuroblastoma differentiation, cortical development and embryonic stem cells differentiation, and control neurite outgrowth in vitro. Biochem Biophys Res Commun. 2010;394:921–927. doi: 10.1016/j.bbrc.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Liu X, Chen Z, Jin Y, Heidbreder CE, Kolokythas A, Wang A, Dai Y, Zhou X. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem J. 2010;432:199–205. doi: 10.1042/BJ20100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S, Purow B. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 26.Saydam O, Senol O, Wurdinger T, Mizrak A, Ozdener GB, Stemmer-Rachamimov AO, Yi M, Stephens RM, Krichevsky AM, Saydam N, Brenner GJ, Breakefield XO. miRNA-7 attenuation in Schwannoma tumors stimulates growth by upregulating three oncogenic signaling pathways. Cancer Res. 2011;71:852–861. doi: 10.1158/0008-5472.CAN-10-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284:5731–5741. doi: 10.1074/jbc.M804280200. [DOI] [PubMed] [Google Scholar]
- 28.Stark A, Brennecke J, Russell RB, Cohen SM. Identification of Drosophila MicroRNA targets. PLoS Biol. 2003;1:E60. doi: 10.1371/journal.pbio.0000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong S, Zheng Y, Jiang P, Liu R, Liu X, Chu Y. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int J Biol Sci. 2011;7:805–814. doi: 10.7150/ijbs.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong SD, Yu K, Liu XH, Yin LH, Kirschenbaum A, Yao S, Narla G, DiFeo A, Wu JB, Yuan Y, Ho SM, Lam YW, Levine AC. Ribosome-inactivating proteins isolated from dietary bitter melon induce apoptosis and inhibit histone deacetylase-1 selectively in premalignant and malignant prostate cancer cells. Int J Cancer. 2009;125:774–782. doi: 10.1002/ijc.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhuri K, Chatterjee R. MicroRNA detection and target prediction: integration of computational and experimental approaches. DNA Cell Biol. 2007;26:321–337. doi: 10.1089/dna.2006.0549. [DOI] [PubMed] [Google Scholar]
- 32.Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao W, Yu Y, Cao H, Shen H, Li X, Pan S, Shu Y. Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed Pharmacother. 2010;64:399–408. doi: 10.1016/j.biopha.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Duncavage E, Goodgame B, Sezhiyan A, Govindan R, Pfeifer J. Use of microRNA expression levels to predict outcomes in resected stage I non-small cell lung cancer. J Thorac Oncol. 2010;5:1755–1763. doi: 10.1097/JTO.0b013e3181f3909d. [DOI] [PubMed] [Google Scholar]
- 35.Liu R, Liu X, Zheng Y, Gu J, Xiong S, Jiang P, Jiang X, Huang E, Yang Y, Ge D, Chu Y. MicroRNA-7 sensitizes non-small cell lung cancer cells to paclitaxel. Oncol Lett. 2014;8:2193–2200. doi: 10.3892/ol.2014.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher TC, Milner AE, Gregory CD, Jackman AL, Aherne GW, Hartley JA, Dive C, Hickman JA. bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is independent of classical resistance pathways. Cancer Res. 1993;53:3321–3326. [PubMed] [Google Scholar]
- 37.Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]


