Abstract
Objective: The objective of this study was to analyze the expression of TARBP1 and its clinical significance in hepatocellular carcinoma (HCC). Materials and Methods: 90 patients with primary hepatocellular carcinoma were included in this study. The tumor and paired adjacent non-tumor tissues were collected. TARBP1 expression was assessed by quantitative real-time polymerase chain reaction and immunohistochemistry. Associations of TARBP1 expression with the clinicopathological features were analyzed, and prognosis of HCC patients was evaluated. Results: The result show the expression of TARBP1 mRNA in liver cancer tissues were higher than in the adjacent normal liver tissues in 10 paired samples (P=0.0015). Compared with adjacent normal liver tissues, overexpression of TARBP1 was detected in 61.1% (55/90) HCC patients. TARBP1 expression was associated with the AJCC tumor stage (P=0.004) and clinical stage (P=0.005), and decreased overall survival (P=0.002). In multivariate analysis, TARBP1 expression was an independent prognostic factor for overall survival (Hazard ratio [HR]=2.773, 95% confidence interval [CI] 1.542-4.985; P=0.019). Conclusions: TARBP1 is up-regulated in HCC, and the expression of TARBP1 was associated with the pathological grading and clinical stage. TARBP1 maybe is an independent prognostic marker of HCC patients.
Keywords: Hepatocellular carcinoma-TARBP1- prognosis
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide, with the incidence on the rise [1]. Due to the high rate of recurrence and low detection rate at the curable stages, liver cancer has a poor prognosis. Statistics indicate that following hepatectomy, recurrence rates are high (approximately 50% at 3 years) [2], the survival rate of patients is 30-40% at five years [3]. In china, HCC is the second leading cause of cancer death, which accounts for about 50% of all HCC deaths worldwide [4-6]. In the United States, the incidence has approximately doubled in the past three decades [7]. The survival of HCC patients after resection remains poor, mainly attributing to frequent metastases and recurrence [8]. And one of the most important reason is lack of a universal HCC prognostic staging system [9]. Therefore, a comprehensive knowledge of the pathogenesis of liver cancer is urgently required and guide therapeutic options.
The TAR (transactivation response) RNA binding protein, TARBP1, is a cellular double-stranded RNA (dsRNA) binding protein that has been shown to promote the replication of human immunodeficiency virus types 1 and 2 (HIV-1 and -2) [10]. TARBP1 has more recently been found to be a constituent of the RNA-induced silencing complex (RISC) serving as a Dicer co-factor in the processing of the ~70 nucleotide pre-microRNAs (miRNAs) to 21-25 nucleotide mature miRNAs [11]. Recently, TARBP1 truncating mutations were found in human cancers with microsatellite instability [12]. Knockdown of TARBP1 can reduce the accumulation of hepatitis C virus RNA [13], and TARBP1 has been proposed as a target for antiviral therapies [10,14,15]. In this study, we sought to evaluate the expression of TARBP1 and its associated clinical significance in HCC.
Materials and methods
Patients and specimens
The ethics committee at the First Affiliated Hospital of Guangzhou Medical University approved this study, and all the patients gave written informed consent on the use of clinical specimens for medical research. 90 HCC patients undergoing curative resection at our institution between January 2007 and November 2009 were included in this study. The demographic features and clinicopathologic data were detailed in Table 1. The median age of patients was 49 years (range 27-80 years), and the median tumorsize was 5.4 cm (range 1.5-17.1 cm). All the patients were diagnosed with primary HCC, and 4 (4.4%) patients were associated with chronic viral hepatitis (HBV 3 patients and HCV 1 patients). None of patients received any type of neoadjuvant therapy, and all underwent curative surgery. Both tumor and adjacent non-tumor tissue (the adjacent non-tumor tissue was defined as at least 1-cm distance from the tumor edge) were processed immediately after operation. TARBP1 expression was evaluated by immunohistochemistry in all the 90 matched specimens. 10 paired specimens were evaluated by quantitative real-time polymerase chain reaction (RT-PCR) analysis.
Table 1.
Patient characteristics and clinicopathologic correlation of TARBP1 expression levels
| Characteristics | Total | TARBP1 | P value | |
|---|---|---|---|---|
|
|
||||
| Positive | Negative | |||
| Gender | 0.731 | |||
| Male | 81 | 50 | 31 | |
| Female | 9 | 5 | 4 | |
| Age (years) | 0.189 | |||
| ≥60 | 22 | 11 | 11 | |
| <60 | 67 | 44 | 23 | |
| Hepatitis | 0.641 | |||
| HBV/HCV | 4 | 2 | 2 | |
| Negative | 86 | 53 | 33 | |
| Live cirrhosis | 0.708 | |||
| Presence | 33 | 21 | 12 | |
| Absence | 57 | 34 | 23 | |
| Tumor size (cm) | 0.318 | |||
| ≥5 | 53 | 35 | 18 | |
| <5 | 36 | 20 | 16 | |
| AJCC tumor stage | 0.004 | |||
| I | 9 | 3 | 6 | |
| II | 48 | 25 | 23 | |
| III | 33 | 27 | 6 | |
| Clinical stage | 0.005 | |||
| 1 | 11 | 3 | 8 | |
| 2 | 29 | 15 | 14 | |
| 3 | 42 | 32 | 10 | |
HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer.
RT-PCR analysis
Total RNA from human liver tissues was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized from 1 ug of total RNA using the First-Strand Synthesis System (Fermentas). Real-time PCR procedure was carried out using a CFX96 Real-Time System (BIO-RAD). 2X SYBR green master mixture (BIO-RAD) was used in a total volume of 10 ul. The primer sequences were as follows: TARBP1 sense 5’-TGCAACATTTCACCCACTCAA-3’, antisense 5’-CCCGCAGCTAAAGGAACATC-3’. GAPDH sense 5’-TGTTGCCATCAATGACCCC-3’, antisense 5’-CTCCACGACGTACTCAGC-3’) was used as an internal control. All reactions were run in triplicate in three independent experiments.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed according to the manufacturer’s protocol (Zymed®, Life Technologies, Carlsbad, CA, USA). All sections were baked 1 h at 60°C and deparaffined with xylenes and rehydrated through graded ethanol series to distilled water. Then, the sections were submerged in sodium citrate buffer and heated for antigenic retrieval. The sections were blocked by the endogenous peroxidase with 0.3% H2O2 for 15 min at room temperature, and then incubated with normal goat serum for 30 min at room temperature to reduce the nonspecific binding. Next, the sections were incubated with rabbit polyclonal anti-TARBP1 antibody (1:100 LSBIO) overnight at 4°C. After 3 washings in sterile phosphate-buffered saline, the sections were incubated with a biotinylated anti-rabbit secondary antibody (Zymed) followed by further incubation with streptavidin-horseradish peroxidase (Zymed) at 37°C for 30 min. Diaminobenzidine (DAB) was used for color reaction, and the antibody was replaced by normal goat serum for negative controls. Tissue section immunoreactivities were viewed and scored separately by two independent pathologists, who were blind to the histopathological features and patient information of the samples. Scores given by the two pathologists were averaged for further comparative evaluation of TARBP1 expression. Immunoreactivities were scored by the intensity of staining (0, no staining; 1, weak = light yellow; 2, moderate = yellow brown; 3, strong = brown) and the percentage of stained cells (0, no staining; 1, 1-10%; 2, 11-35%; 3, 36-70%; 4, >70%). By multiplication of both values, a final score ranging between 0 and 12 was obtained. TARBP1 overexpression was defined as final score more than zero.
Statistical analysis
TARBP1 mRNA transcriptional levels in the tumor and matched non-tumor tissue were compared by paired sample t test. TARBP1-positive patients and TARBP1-negative patients were compared using the chi-square tests for categorical variables, and the Student t test for continuous data. Overall survival (OS) was defined from the date of operation to the date of death. Kaplan-Meier method was used to analyze overall survival (OS) and comparisons were analyzed by log-rank test. Cox’s proportional hazards model was used for multivariate analysis, adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated. All the statistical analyses were evaluated using the Statistical Software Package for the Social Sciences (SPSS Inc. Chicago, IL, USA). All statistical tests were two-sided and a P value of less than 0.05 was considered statistically significant.
Results
TARBP1 is up-regulated in HCC patients
To determine whether the TARBP1 expression levels were differentially between liver carcinoma and normal liver samples, we first queried the Oncomine database, in the meta-analysis, TARBP1 expression significantly higher than the corresponding normal tissues in liver carcinoma with a median rank of 98.0 and a P-value of 6.55E-19 (Figure 1). To confirm this result, we obtained 10 paired liver carcinoma samples for real-time RT-PCR analysis. The result as shown in Figure 2, the expression level of TARBP1 mRNA is significantly higher in tumor tissues. Furthermore, these results were confirmed by immunohistochemistry. TARBP1 positive rate was 61.1% (55/90) in tumor tissue. In non-cancerous adjacent tissues and normal tissues, TARBP1 protein was not staining. TARBP1 was predominantly present in the cytoplasm of tumor cells (Figure 3). These data showed that TARBP1 was significantly elevated in HCC tumor tissue.
Figure 1.
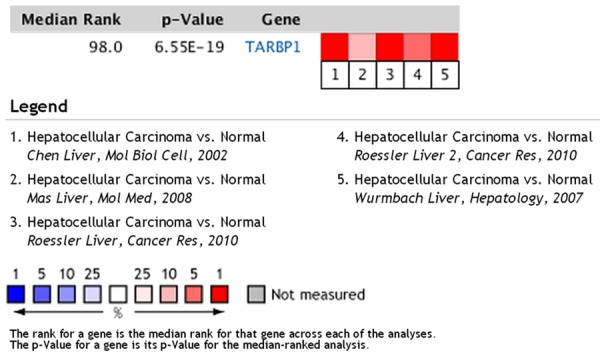
In Oncomine database, TARBP1 expression is up-regulated in human liver carcinomas. Oncomine heat map of TARBP1 gene expression in clinical liver carcinoma samples compared with the normal liver tissues.
Figure 2.
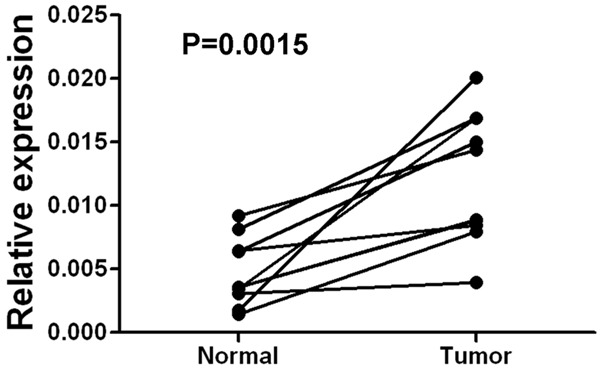
Expression levels of TARBP1 mRNA in liver cancer tissues. Expression levels of TARBP1 mRNA in ten paired liver cancer tissues by real-time PCR. Normal, para carcinoma (normal) liver tissues. Tumor, liver carcinoma tissues.
Figure 3.
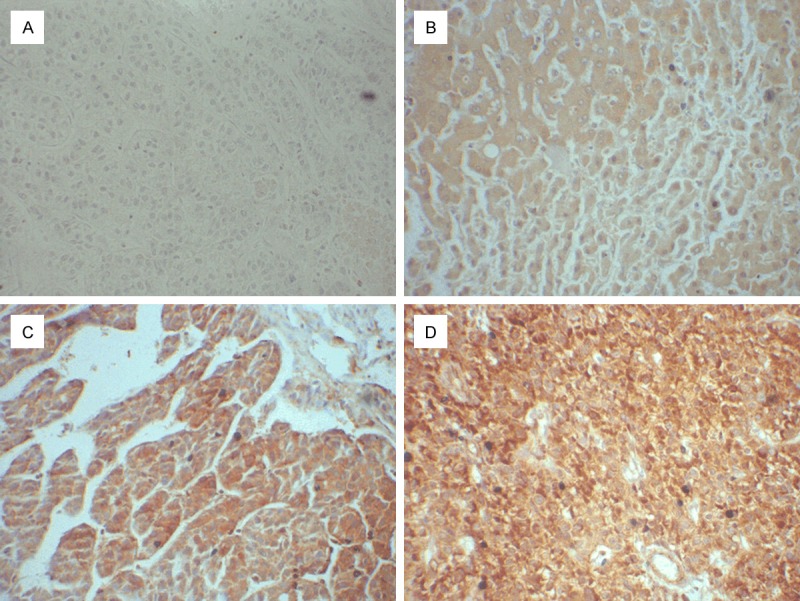
The expression of TARBP1 protein by immunohistochemistry. TARBP1 expression was mainly localized in the cytoplasm of tumor cells. In normal epithelial cells, TARBP1 is not expressed, in HCC cells, TARBP1 was over-expression. A. Staining of TARBP1 in normal liver tissues; B-D. Low and high expression of TARBP1 in HCC tissues.
TARBP1 correlates with clinicopathological features of HCC
For better understanding of the potential roles of TARBP1 in HCC development and progression, patients were grouped according to TARBP1 expression. TARBP1 expression was significantly associated with AJCC tumor stage (P=0.004) and clinical stage (P=0.005). However, there was no correlation of TARBP1 expression with other clinical features, such as age, gender, and tumor size (Table 1).
Expression of TARBP1 in HCC patients correlates with worse overall survival
After a median follow-up of 29 months (range 1-80 months), 52 (57.8%) patients died. The estimated 3-year overall survival (OS) was 42.10% (95% CI 35.22-48.98). In univariate analysis, TARBP1 expression was significantly associated with worse OS, patients with negative TARBP1 expression had a 3-year OS rate of 55.90% (95% CI 45.85-65.93), whereas those with positive TARBP1 expression had a 3-year OS rate of 33.02% (95% CI 24.65-41.38; P=0.002; Figure 4). In multivariate analysis (Table 2), factors associated with worse OS were stage III tumor (HR=3.812; 95% CI (1.579-9.423); P=0.004), and TARBP1 expression (HR=2.773, 95% CI 1.542-4.985; P=0.019). These results indicated that TARBP1 expression was an independent prognostic marker for OS of HCC patients.
Figure 4.
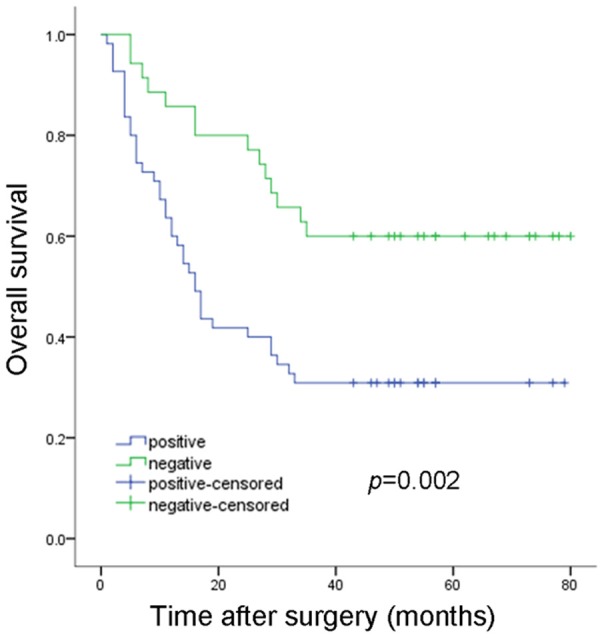
3-year overall survival according to TARBP1 expression.
Table 2.
Univariate and Multivariate analysis of the effect of covariates on overall survival for all patients
| Factor | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | ||||
| Male | Reference | |||
| Female | 0.382 (0.229-1.761) | 0.634 | ― | ― |
| Age | ||||
| ≥60 | Reference | |||
| <60 | 1.658 (0.831-3.309) | 0.151 | ― | ― |
| Tumor size (cm) | ||||
| <5 | Reference | |||
| ≥5 | 0.914 (0.523-1.596) | 0.751 | ― | ― |
| Hepatitis | ||||
| Negative | Reference | |||
| HBV/HCV | 2.053 (1.090-8.553) | 0.054 | ― | ― |
| Live cirrhosis | ||||
| Absence | Reference | |||
| Presence | 0.777 (0.431-1.402) | 0.403 | ― | ― |
| TARBP1 expression | ||||
| Negative | Reference | Reference | ||
| Positive | 0.402 (0.217-0.744) | 0.004 | 2.773 (1.542-4.985) | 0.0019 |
| AJCC tumor stage | ||||
| I | Reference | Reference | ||
| II | 0.150 (0.036-0.631) | 0.010 | ― | |
| III | 0.393 (0.201-0.768) | 0.006 | 3.812 (1.579-9.423) | 0.004 |
| Clinical stage | ||||
| 1 | Reference | |||
| 2 | 0.301 (0.071-1.282) | 0.104 | ||
| 3 | 0.787 (0.450-1.376) | 0.401 | ― | ― |
HBV, hepatitis B virus; HCV, hepatitis C virus; AJCC, American Joint Committee on Cancer.
Discussion
Hepatocellular carcinoma (HCC) is a disease with a high incidence and mortality rate worldwide. HCC is the most common primary liver malignancy in adults [16], in China, 360,000 incident cases and 350,000 deaths secondary to HCC occur annually [17]. HCC is a late complication of chronic liver disease, the main risk factors for the development of HCC include infection with hepatitis B virus (HBV) or hepatitis C virus (HCV). Hepatitis infection is believed to be the main etiologic factor in >80% of cases [18]. An overall 10 years study in Singapore show, the overall incidence of HCC in cirrhotics was 29.7% (3.0%/year) [19], Other risk factors though the management of HCC has significantly changed due to improved diagnostic technologies, development of evidence-based staging systems, and the availability of effective treatment during the past few decades, the outcome after curative resection for HCC is still poor, more than 50% of patients develop disease recurrence within 3 years after curative surgery [20]. Now there are many new strategies and target therapies in HCC, Saber Ismail show NF-ĸB and PRDX3 may serve as early and sensitive biomarkers for early detection of HCC and nuclear factor kappa B (NF-ĸB) as a target for treatment of liver fibrosis and HCC [21]. However, the mechanisms underlying its pathogenesis are still elusive, and there is still lack of marker for predicting the recurrence of HCC after resection. It is necessary to find useful markers of recurrence and survival.
Human transactivation responses (TAR) RNA binding protein was initially identified as protein that bind the HIV type 1 (HIV-1) TAR RNA and activate long terminal repeat (LTR) expression in the absence and in the presence of the viral transactivator Tat [22]. It contains two double-stranded RNA binding domains (RBD). TARBP1 is a multifaceted protein with two well-characterized functions [23]. Firstly, it is an integral component of Dicer-containing complexes, and secondary, it assists Dicer in generating small RNA-induced silencing complex (RISC) [24,25]. Consistent with the notion of miRNA-mediated restriction of viral infection in mammalian cells, loss of TARBP1 activity through its sequestration by TAR RNA resulted in the enhanced replication of HIV-1 in human cells [26]. TARBP1 has a physiological role in spermatogenesis and growth control during development. They also bind the interferon-induced dsRNA-activated protein kinase PKR, with the PKR activator PACT, and with the tumor suppressor [14].
Based on previous studies, we investigated the expression of TARBP1 in HCC and its prognostic values in predicting tumor recurrence following curative resection in the present study. Our results clearly showed that TARBP1 is up-regulated at mRNA level in ten HCC tissues compared with paired normal tissues. In addition, IHC analysis showed that TARBP1 was overexpressed in 55 of 90 (61.1%) HCC specimens. Similar observations were also made in other diseases, such as HIV [14] and epithelial skin cancer [27]. The difference in the expression of TARBP1 between tumor and non-tumor tissues indicates that TARBP1 may be a key regulator in tumorigenesis.
In this study, TARBP1 was up regulated in HCC, and it was associated with AJCC tumor stage (P=0.004) and clinical stage (P=0.005). The advance tumor stage considered as a sign of tumor progression and increased malignant potential. Our study clearly indicated that TARBP1 expression in HCC tumors was significantly associated with advanced tumor stage. In this study, we found that TARBP1 over-expression was associated with worse outcome, and it was an independent prognosis factor for HCC patients after curative resection (HR=2.773, 95% CI 1.542-4.985; P=0.019).These results suggest TARBP1 could be a new biomarker for HCC and a potential therapeutic target. However, the molecular mechanism connecting worse outcome and TARBP1 over-expression is unclear and remains to be elucidated.
In conclusion, HCC is the most common primary malignancy of the liver and the major cause of mortality. TARBP1, over-expression in HCC, was correlated with AJCC tumor stage and clinical stage. TARBP1 is associated with worse prognosis in HCC and could be a potential prognostic biomarker for HCC. Further studies are warranted to reveal the molecular mechanism of TARBP1 in the development and progression of HCC. To our knowledge, this is the first report to identify TARBP1 as an independent prognostic factor for HCC patients.
Acknowledgements
Our work is supported by the National Natural Science Foundation of China (No. 81201695) and Key Clinical Disciplines of Guangdong Province (20111219).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Mulcahy MF. Management of hepatocellular cancer. Curr Treat Options Oncol. 2005;6:423–435. doi: 10.1007/s11864-005-0045-7. [DOI] [PubMed] [Google Scholar]
- 3.Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11:7391–7400. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72–78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Chen WQ, Zheng RS, Zhang SW. Liver cancer incidence and mortality in China, 2009. Chin J Cancer. 2013;32:162–169. doi: 10.5732/cjc.013.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 8.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 9.Huitzil-Melendez FD, Capanu M, O’Reilly EM, Duffy A, Gansukh B, Saltz LL, Abou-Alfa GK. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J. Clin. Oncol. 2010;28:2889–2895. doi: 10.1200/JCO.2009.25.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen HS, Daher A, Soye KJ, Frankel LB, Alexander MR, Laine S, Bannwarth S, Ong CL, Chung SW, Campbell SM, Purcell DF, Gatignol A. Small interfering RNAs against the TAR RNA binding protein, TRBP, a Dicer cofactor, inhibit human immunodeficiency virus type 1 long terminal repeat expression and viral production. J Virol. 2007;81:5121–5131. doi: 10.1128/JVI.01511-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi YH, Semmes OJ, Jeang KT. A proteomic study of TAR-RNA binding protein (TRBP)-associated factors. Cell Biosci. 2011;1:9. doi: 10.1186/2045-3701-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melo SA, Ropero S, Moutinho C, Aaltonen LA, Yamamoto H, Calin GA, Rossi S, Fernandez AF, Carneiro F, Oliveira C, Ferreira B, Liu CG, Villanueva A, Capella G, Schwartz S Jr, Shiekhattar R, Esteller M. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nat Genet. 2009;41:365–370. doi: 10.1038/ng.317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Zhang C, Huys A, Thibault PA, Wilson JA. Requirements for human Dicer and TRBP in microRNA-122 regulation of HCV translation and RNA abundance. Virology. 2012;433:479–488. doi: 10.1016/j.virol.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Sanghvi VR, Steel LF. The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR. J Virol. 2011;85:12614–12621. doi: 10.1128/JVI.05240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eekels JJ, Geerts D, Jeeninga RE, Berkhout B. Long-term inhibition of HIV-1 replication with RNA interference against cellular co-factors. Antiviral Res. 2010;89:43–53. doi: 10.1016/j.antiviral.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Pons-Renedo F, Llovet JM. Hepatocellular carcinoma: a clinical update. MedGenMed. 2003;5:11. [PubMed] [Google Scholar]
- 17.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, Khin MW, Koo WH Asian Oncology Summit. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 18.Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11:383–393. doi: 10.1111/j.1365-2893.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 19.Poh Z, Goh BB, Chang PE, Tan CK. Rates of cirrhosis and hepatocellular carcinoma in chronic hepatitis B and the role of surveillance: a 10-year follow-up of 673 patients. Eur J Gastroenterol Hepatol. 2015;27:638–643. doi: 10.1097/MEG.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Ismail S, Mayah W, Battia HE, Gaballah H, Jiman-Fatani A, Hamouda H, Afifi MA, Elmashad N, Saadany SE. Plasma nuclear factor kappa B and serum peroxiredoxin 3 in early diagnosis of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2015;16:1657–1663. doi: 10.7314/apjcp.2015.16.4.1657. [DOI] [PubMed] [Google Scholar]
- 22.Kozak CA, Gatignol A, Graham K, Jeang KT, McBride OW. Genetic mapping in human and mouse of the locus encoding TRBP, a protein that binds the TAR region of the human immunodeficiency virus (HIV-1) Genomics. 1995;25:66–72. doi: 10.1016/0888-7543(95)80110-8. [DOI] [PubMed] [Google Scholar]
- 23.Daniels SM, Gatignol A. The multiple functions of TRBP, at the hub of cell responses to viruses, stress, and cancer. Microbiol Mol Biol Rev. 2012;76:652–666. doi: 10.1128/MMBR.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redfern AD, Colley SM, Beveridge DJ, Ikeda N, Epis MR, Li X, Foulds CE, Stuart LM, Barker A, Russell VJ, Ramsay K, Kobelke SJ, Hatchell EC, Payne C, Giles KM, Messineo A, Gatignol A, Lanz RB, O’Malley BW, Leedman PJ. RNA-induced silencing complex (RISC) Proteins PACT, TRBP, and Dicer are SRA binding nuclear receptor coregulators. Proc Natl Acad Sci U S A. 2013;110:6536–6541. doi: 10.1073/pnas.1301620110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Zenno S, Ishibashi O, Takizawa T, Saigo K, Ui-Tei K. Interactions between the non-seed region of siRNA and RNA-binding RLC/RISC proteins, Ago and TRBP, in mammalian cells. Nucleic Acids Res. 2014;42:5256–5269. doi: 10.1093/nar/gku153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennasser Y, Yeung ML, Jeang KT. HIV-1 TAR RNA subverts RNA interference in transfected cells through sequestration of TAR RNA-binding protein, TRBP. J Biol Chem. 2006;281:27674–27678. doi: 10.1074/jbc.C600072200. [DOI] [PubMed] [Google Scholar]
- 27.Sand M, Skrygan M, Georgas D, Arenz C, Gambichler T, Sand D, Altmeyer P, Bechara FG. Expression levels of the microRNA maturing microprocessor complex component DGCR8 and the RNA-induced silencing complex (RISC) components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in epithelial skin cancer. Mol Carcinog. 2011;51:916–922. doi: 10.1002/mc.20861. [DOI] [PubMed] [Google Scholar]


