Abstract
Objectives: To investigate the expression and correlation of matrix metalloproteinase (MMP)-7 and interleukin (IL)-15 in human osteoarthritis (OA). Methods: From October 2013 to December 2014, 30 patients with OA were enrolled. In addition, anther 30 patients with simple meniscus injury were collected as a control group. There were no significant differences in age and gender between the two groups. Articular cartilage tissue was obtained from both OA patients and control group patients. Protein, mRNA, and serum expression levels of MMP-7 and IL-15 in the both two groups were determined by immunohistochemical (IHC), in situ hybridization, and enzyme-linked immunosorbent assay (ELISA) assay, respectively. Additionally, correlation between MMP-7 and IL-15 expression level in cartilage tissue and serum was assessed using Pearson correlation analysis. Results: Protein, mRNA, and serum expression levels of MMP-7 and IL-15 in patients with OA were all significantly increased in OA patients compared with the control group. Besides, there were strong positive relationships between articular MMP-7 level and serum MMP-7 level (R2 = 0.573, P = 0.018), between articular IL-15 level and serum IL-15 level (R2 = 0.861, P = 0.023), and between serum IL-15 level and serum MMP-7 level (R2 = 0.602, P = 0.012). Conclusion: These results suggest that MMP-7 and IL-15 might play important roles in the pathogenesis of OA, and IL-15 and MMP-7 has positive correlation in OA.
Keywords: Matrix metalloproteinase-7, interleukin-15, osteoarthritis
Introduction
Osteoarthritis (OA) is the most common joint disorder, which particularly affects the weightbearing joints, predominantly in the knee [1]. It is characterized by progressive loss of cartilage matrix, subchondral bone remolding, and osteophyte formation, etc. [2,3]. It has been reported that approximately 10% people over age 60 years in the world suffer from OA [2], and the prevalence is estimated to continue to grow [4]. Besides, OA not only impacts on an individual’s health, but also carries out a significantly financial and societal burden for families and the society [3,5]. However, effective management of OA has not been acquired due to the unclear pathophysiology mechanism.
Recently, roles of matrix metalloproteinase (MMPs) and cytokines in OA have been increasingly paid attention. MMPs and cytokines are considered as potential candidates for biochemical mediators [6]. The mediator either acted individually, or with another mediator acted in networks could greatly regulate cellular responses (both catabolic and anabolic activities) in joint tissues [7]. MMPs are a family of Zn2+-dependent endopeptidases that participate in the degradation of extracellular matrix (ECM). Previous studies have indicated that MMP-1, -2, -3, -8, -9, and -13 are involved in OA [8-11], and MMP-13 has been regarded as a crucial target gene for the progression of OA [12]. Besides, a study conducted by Ohta suggested that MMP-7 is overproduced in human OA cartilage and MMP-7 may play a significant role in the ECM degradation in OA [13]. Cytokines, such as interleukin (IL)-1, IL-6, IL-15 and tumor necrosis factor (TNF)-α, have been involved in the pathogenic mechanism of OA [14-17]. Among the cytokines, IL-15 has been regarded as a candidate therapeutic target for OA [17]. However, little information is available regarding the associations of MMP-7 expression and IL-15 expression in articular and serum of OA patients.
Therefore, we aimed to investigate the MMP-7 expression and IL-15 expression in both articular and serum of OA patients, as well as the associations between articular and serum of MMP-7, the associations between articular and serum of IL-15 level, and the associations between serum of MMP-7 expression and IL-15 expression. Our study might contribute to explore the pathogenesis mechanism of OA.
Materials and methods
Participants and study design
This study was approved by the hospital medical ethics committees, and informed consent was obtained from all participants. Between October 2013 and December 2014, thirty patients with knee OA (13 males and 17 females; mean age 65.16 ± 17.66, range 35-76) attending at our hospital were enrolled. Besides, thirty patients with simple meniscus injury (16 males and 14 females; mean age 63.60 ± 15.23, range 30-79) were collected as a control group. Diagnosis of OA was based on clinical and radiological evaluations revealed by the 1986 American College of Rheumatology (ACR) criteria [18]. Patients with obvious joint injury or with inflammatory arthritis, previous knee surgery were excluded from the study. Also, patients who received intra-articular corticosteroid or hyaluronan injections within 3 months of surgery were excluded.
Cartilage tissue samples
Articular cartilage was acquired from both OA patients and meniscus injury patients who were undergoing arthroscopic examination or arthroplastic surgery. The tissue sample was fixed in 4% paraformaldehyde immediately for 24 h after biopsy. Then the tissue was embedded in paraffin, sectioned, and dewaxed according to the standard methods [13]. Consecutive 4-μm- thickness sections were prepared.
Immunohistochemical (IHC) staining
IHC staining was performed using monoclonal anti-MMP-7 antibody (diluted 1:50; Chemicon, Temecula, CA) and anti-IL-15 antibody (diluted 1:200; Santa Cruz Biotechnologies). The slides were pretreated by microwave heating before the incubation. After incubation with primary antibody, biotinylated secondary antibody was performed. Negative controls procedures were also performed (the primary antibody was replaced by phosphate-buffered saline (PBS) solution). Finally, chromogen 3,3-diaminobenzidine-hydrogen peroxide (DAB, Sigma- Alderich, S.R.L) was performed for visualized reaction.
In situ hybridization
In situ hybridization was performed on 4-μm-thickness sections according to a standard method [19] with a MMP-7 kit (HPV-0610, Fuzhou Maixin Biotech. Co. Ltd) and a IL-15 kit (MK1165, Boster, Wuhan, China). Briefly, the sections were deparaffinized, treated with 0.01% Triton X-100 for 2 min, deproteinated with proteinase K (0.25 g/L) for 5 min and washed in 2 × saline sodium citrate (SSC) at 42°C . Then the slides were prehybridized at 42°C for 3-4 h in prehybridization solution. The probe of MMP-7 and IL-15, and 20 pg tRNA were mixed with the prehybridization solution and incubated at 42°C overnight, and washed with 2 × SSC. Thereafter, 0.1% Triton X-100 was performed for 2 min and washed with buffer solution 1 (1 mol/L NACL, 0.1 mol/L Tris, PH7.5). The slides were then incubated with buffer solution 2 (buffer solution 1 and 3 g bovine serum albumin (BSA)) for 1 h. After the buffer solution 2 was discarded, avidin and alkaline phosphatase was added to the mixture for 20 min at 42°C. Finally, autoradiography was carried out by nitroblue tetrazolium (NBT).
Computer-assisted image analysis
Five representative areas of each sample (assessed by both IHC and in situ hybridization) were randomly chosen under a light microscope (Olympus, Tokyo, Japan). Quantitative analysis was performed with Image-Pro Plus 6.0 (Media Cybernetics, GE, USA). The positive areas were regarded as specific brown yellow color (for IHC) or blueviolet (for in situ hybridization). The integrated optical density (IOD; pixel area × the optical density) was measured after standard optical density (OD) calibration.
Enzyme-linked immunosorbent assay (ELISA) assay
Fasting blood samples (5 mL) were obtained from an antecubital vein. After clotting, the blood samples were separated by centrifugation at 1,000 g for 15 min at 4°C. Serum samples were subsequently stored at -80°C until use. The serum levels of MMP-7 and IL-15 were determined by an ELISA kit (Calbiochem, Cambridge, MA, USA) according to the manufacturer’s protocol. Briefly, 96-well plates were coated with either anti-MMP-7 or anti-IL-15 antibody, and the appropriate diluent serum samples were added in duplicate, followed with incubation for 2 h at room temperature. After washing with wash buffer, the corresponding secondary antibody was applied. After incubation for another 2 h, the plates were washed again. Spectrophotometric microplate reader was employed to determine the optical density at 490 nm. The concentration of MMP-7 and IL-15 was calculated from a standard curve.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD). Statistical analysis was performed with statistical package for the social sciences (SPSS) software (version 17.0; SPSS Inc., Chicago, IL). The 2-sample Student’s t test was used to compare continuous variables. Correlation analysis was performed using Pearson test. A statistical significance was defined when P < 0.05.
Results
MMP-7and IL-15 protein and mRNA expression
IHC and in situ hybridization were performed to examine the protein and mRNA expression levels of MMP-7 and IL-15 in human cartilage tissue, respectively. Here we set the baseline of relative IOD in control group to be 1. The results showed that the expression protein levels of MMP-7 and IL-15 were both significantly increased in OA patients compared with control group (P < 0.05) (Figure 1A and 1B). Similarly, the results of in situ hybridization demonstrated that the expression mRNA levels of MMP-7 and IL-15 were also both statistically increased in patients with OA compared with the control group (P < 0.05) (Figure 2A and 2B).
Figure 1.
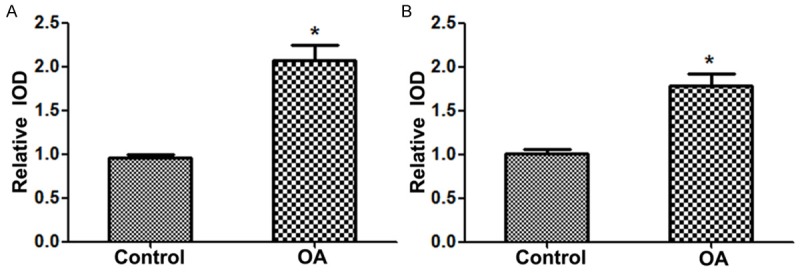
MMP-7 and IL-15 protein expression level in the two groups. A. MMP-7 protein expression level in the two groups; B. IL-15 protein expression level in the two groups. MMP, matrix metalloproteinase; interleukin, IL; OA, osteoarthritis. *P < 0.05 vs. control group.
Figure 2.
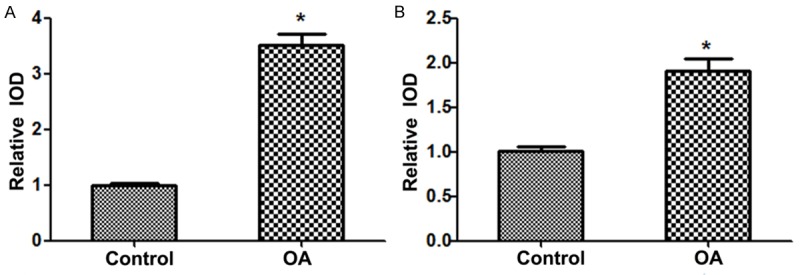
MMP-7 and IL-15 mRNA expression level in the two groups. A. MMP-7 mRNA expression level in the two groups; B. IL-15 mRNA expression level in the two groups. MMP, matrix metalloproteinase; interleukin, IL; OA, osteoarthritis. *P < 0.05 vs. control group.
MMP-7and IL-15 serum expression
The serum expression levels of MMP-7 and IL-15 were showed in Figure 3A and 3B, respectively. As shown in Figure 3A, the average serum MMP-7 level was 5.617 ± 2.300 ng/ml (range 2.2-8.8 ng/ml) in the control group, whereas the average serum MMP-7 level was 20.117 ± 7.083 ng/ml (range 11.0-29.4 ng/ml). There were significant differences in the serum MMP-7 level between the two groups. Also, the serum IL-15 level (average 129.6 ± 39.922 pg/ml; range 85.0-176.0 pg/ml) in OA patients was significantly higher than that in the control group (average 576.8 ± 194.574 pg/ml; range 369.0-875.0 pg/ml) (Figure 3B).
Figure 3.
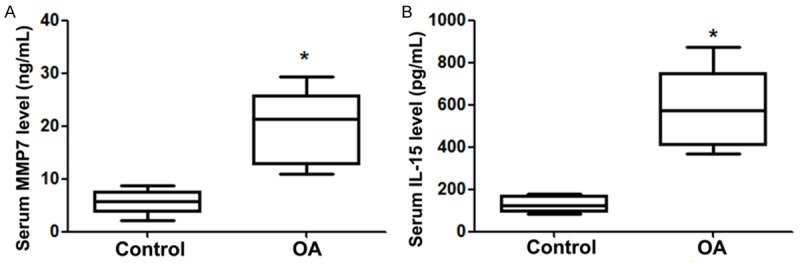
MMP-7 and IL-15 serum expression level in the two groups. A. MMP-7 serum expression level in the two groups; B. IL-15 serum expression level in the two groups. MMP, matrix metalloproteinase; interleukin, IL; OA, osteoarthritis. *P < 0.05 vs. control group.
Correlation analyses between MMP-7and IL-15
In order to confirm the clinical diagnostic value of MMP-7 and IL-15 in OA, we further performed the correlation analysis between the expression level of serum and articular cartilage specimens in OA patients. The results showed that there were strong relationships between articular MMP-7 level and serum MMP-7 level (R2 = 0.573, P = 0.018), between articular IL-15 level and serum IL-15 level (R2 = 0.861, P = 0.023), and between serum IL-15 level and serum MMP-7 level (R2 = 0.602, P = 0.012) (Figure 4A-C).
Figure 4.
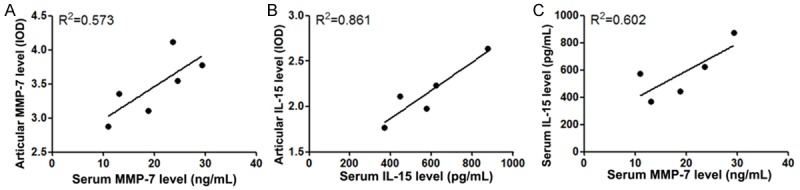
Correlation analyses between MMP-7and IL-15. A. The associations between articular MMP-7 level and serum MMP-7 level; B. The associations between articular IL-15 level and serum IL-15 level; C. The associations between articular MMP-7 level and serum MMP-7 level. MMP, matrix metalloproteinase; interleukin, IL; OA, osteoarthritis.
Discussion
In the present study, the expression and correlation of MMP-7 and IL-15 in human OA was investigated. The results showed that the protein, mRNA, and serum expression levels of MMP-7 and IL-15 in OA patients were all significantly higher than those in the control group. Besides, there were strong positive relationships between articular MMP-7 level and serum MMP-7 level, between articular IL-15 level and serum IL-15 level, and between serum IL-15 level and serum MMP-7 level. Our results indicated that MMP-7 and IL-15 might play important roles in the pathogenesis of OA, and IL-15 and MMP-7 has positive correlation in OA.
MMPs are a group of proteolytic enzymes responsible for the degradation of ECM, which is involved in multiple physiological and pathological progressions of different tissues [20-22]. The degradation of ECM plays essential roles in the tissue resorption and remodeling [23]. It has been reported that approximately 95% of the dry weight of articular cartilage is ECM [24]. The imbalance of the cartilage ECM results in OA, therefore, a delicate balance between the breakdown and synthesis of ECM is of importance [25]. MMP-7, also known as matrilysin, is a unique member of the MMP family. Unlike other members, MMP-7 is the smallest member that only consists of the common catalytic domain and zinc-binding region [26]. It has a specific ability to degrade various ECM components such as cartilage proteoglycan [27]. Since Ohta has firstly reported that MMP-7 is overexpressed in human OA cartilage [13], the functions and roles of MMP-7 in OA have been paid attention [28,29]. In consistent with previous studies, our results also suggested that the expression levels of MMP-7 were significantly higher than those in the control group, either in articular cartilage or in serum. Additionally, we found that there was a strong positive relationship between articular MMP-7 level and serum MMP-7 level.
The expression of MMPs is regulated by cytokines [30]. Inflammatory reaction has been well demonstrated in the development and progression of OA, even in the early stage of the disease [31]. IL-15, a proinflammatory cytokine, has been considered as a contributor to inflammation in OA [6]. Previous study has indicated that IL-15 has both potential diagnostic and prognostic biochemical marker for OA [28]. The expression level of IL-15 was found significantly increased in OA patient’s serum samples; besides, IL-15 level was positively correlated with a Western Ontario McMaster University Osteoarthritis Index (WOMAC) pain scores [32]. Moreover, IL-15 has been reported to induce MMP production, especially MMP-1 and MMP-9 [33]. Additionally, previous studies suggested that there were associations between IL-15 level with MMP-1 and MMP-3 [17]. Also, our study confirmed the higher expression of IL-15 in OA patients than that in control patients, which was in line with previous studies. In addition to the results, we found that serum IL-15 level was positively related with articular IL-15 level and serum MMP-7 level. One possible reason for that is MMP-7 is may be induced by IL-15, and both of them participate in the pathogenesis of OA.
In conclusion, our results suggest that both MMP-7 and IL-15 play important roles in the pathogenesis of OA, and IL-15 and MMP-7 has positive correlation in OA.
Disclosure of conflict of interest
None.
References
- 1.Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-Art management of knee osteoarthritis. World J Clin Cases. 2015;3:89–101. doi: 10.12998/wjcc.v3.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19:1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blalock D, Miller A, Tilley M, Wang J. Joint instability and osteoarthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:15–23. doi: 10.4137/CMAMD.S22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004:S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 6.Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6:95–105. doi: 10.5312/wjo.v6.i1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 8.Bluteau G, Conrozier T, Mathieu P, Vignon E, Herbage D, Mallein-Gerin F. Matrix metalloproteinase-1, -3, -13 and aggrecanase-1 and -2 are differentially expressed in experimental osteoarthritis. Biochim Biophys Acta. 2001;1526:147–158. doi: 10.1016/s0304-4165(01)00122-2. [DOI] [PubMed] [Google Scholar]
- 9.Galasso O, Familiari F, De Gori M, Gasparini G. Recent findings on the role of gelatinases (matrix metalloproteinase-2 and -9) in osteoarthritis. Adv Orthop. 2012;2012:834208. doi: 10.1155/2012/834208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fosang AJ, Last K, Knauper V, Murphy G, Neame PJ. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 11.Fosang AJ, Last K, Knauper V, Neame PJ, Murphy G, Hardingham TE, Tschesche H, Hamilton JA. Fibroblast and neutrophil collagenases cleave at two sites in the cartilage aggrecan interglobular domain. Biochem J. 1993;295:273–6. doi: 10.1042/bj2950273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, Chen D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta S, Imai K, Yamashita K, Matsumoto T, Azumano I, Okada Y. Expression of matrix metalloproteinase 7 (matrilysin) in human osteoarthritic cartilage. Lab Invest. 1998;78:79–87. [PubMed] [Google Scholar]
- 14.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youssef PP, Triantafillou S, Parker A, Coleman M, Roberts-Thomson PJ, Ahern MJ, Smith MD. Variability in cytokine and cell adhesion molecule staining in arthroscopic synovial biopsies: quantification using color video image analysis. J Rheumatol. 1997;24:2291–2298. [PubMed] [Google Scholar]
- 16.Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, van Wijnen AJ, Im HJ. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanzello CR, Umoh E, Pessler F, Diaz-Torne C, Miles T, Dicarlo E, Potter HG, Mandl L, Marx R, Rodeo S, Goldring SR, Crow MK. Local cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage. 2009;17:1040–1048. doi: 10.1016/j.joca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 19.Prosser IW, Stenmark KR, Suthar M, Crouch EC, Mecham RP, Parks WC. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989;135:1073–1088. [PMC free article] [PubMed] [Google Scholar]
- 20.Peng WJ, Yan JW, Wan YN, Wang BX, Tao JH, Yang GJ, Pan HF, Wang J. Matrix metalloproteinases: a review of their structure and role in systemic sclerosis. J Clin Immunol. 2012;32:1409–14. doi: 10.1007/s10875-012-9735-7. [DOI] [PubMed] [Google Scholar]
- 21.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases A review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 22.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 23.Gronski TJ Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997;272:12189–94. doi: 10.1074/jbc.272.18.12189. [DOI] [PubMed] [Google Scholar]
- 24.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 25.Grimaud E, Heymann D, Redini F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002;13:241–257. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama Y, Grunebach F, Schmidt SM, Heine A, Hantschel M, Stevanovic S, Rammensee HG, Brossart P. Matrilysin (MMP-7) is a novel broadly expressed tumor antigen recognized by antigen-specific T cells. Clin Cancer Res. 2008;14:5503–5511. doi: 10.1158/1078-0432.CCR-07-4041. [DOI] [PubMed] [Google Scholar]
- 27.Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–136. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 28.Ling SM, Patel DD, Garnero P, Zhan M, Vaduganathan M, Muller D, Taub D, Bathon JM, Hochberg M, Abernethy DR, Metter EJ, Ferrucci L. Serum protein signatures detect early radiographic osteoarthritis. Osteoarthritis Cartilage. 2009;17:43–48. doi: 10.1016/j.joca.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flannelly J, Chambers MG, Dudhia J, Hembry RM, Murphy G, Mason RM, Bayliss MT. Metalloproteinase and tissue inhibitor of metalloproteinase expression in the murine STR/ort model of osteoarthritis. Osteoarthritis Cartilage. 2002;10:722–733. doi: 10.1053/joca.2002.0818. [DOI] [PubMed] [Google Scholar]
- 30.Matrisian LM, Hogan BL. Growth factor-regulated proteases and extracellular matrix remodeling during mammalian development. Curr Top Dev Biol. 1990;24:219–259. doi: 10.1016/s0070-2153(08)60089-7. [DOI] [PubMed] [Google Scholar]
- 31.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 32.Sun JM, Sun LZ, Liu J, Su BH, Shi L. Serum interleukin-15 levels are associated with severity of pain in patients with knee osteoarthritis. Dis Markers. 2013;35:203–206. doi: 10.1155/2013/176278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constantinescu CS, Grygar C, Kappos L, Leppert D. Interleukin 15 stimulates production of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by human peripheral blood mononuclear cells. Cytokine. 2001;13:244–247. doi: 10.1006/cyto.2000.0818. [DOI] [PubMed] [Google Scholar]


