Abstract
Background: The expression of microRNA-451 (miR-451) and its association with clinical pathological factors in GC remain still unclear. The purpose of this study was to investigate if miR-451 is a potential prognostic biomarker and tumor suppressor for gastric cancer. Methods: Real-time quantitative RT-PCR (qRT-PCR) was applied to detect miR-451 expression in GC cell lines and primary tumor and paired non-cancerous tissues. The association of miR-451 expression with clinicopathological factors and prognosis was statistically analyzed. Results: We found that miR-451 showed decreased expression in GC tissues and cell lines, and its down-regulation tended to be positively correlated with lymphatic metastasis, clinical staging and shorter overall survival of patients. In addition, forced expression of miR-451 in BGC-823 and MKN-45 cells did not impact on cell proliferation, but did reduce migration and invasion rates in BGC-823 cells. Conclusion: Our findings indicated that miR-451 may act as a novel prognostic marker and potential therapeutic target in human GC.
Keywords: MiR-451, gastric cancer, prognosis, metastasis
Introduction
Gastric cancer (GC) is now one of the most common type of malignancies and second leading cause of cancer-related deaths worldwide [1], with a particularly high incidence in eastern Asia [2]. According to the Chinese Cancer Registry Annual Report, GC is the third commonest malignancy and the second leading cause of cancer death in China [3]. Although various treatment improvements have been developed and the mortality rate of GC has gradually decreased over recent decades [4]. Unfortunately, the prognosis for advanced GC patients remains poor [5]. Hence, developing a novel therapeutic strategy is eagerly desired. Since GC is a polygenic disease arising as the result of multiple gene dysregulations, better understanding of GC biology and signaling pathways is imperative for improving GC therapy.
MicroRNAs (miRNAs) are a class of small non-coding RNAs, which is single-stranded, and has 19-25 nucleotides in length. They negatively regulate gene expression by base pairing to the 3’UTR of targeted messenger RNA (mRNA), in a post-transcriptional manner. MiRNAs are known as important factors in multiple biological processes, including development and differentiation. Functional studies in cancer cell lines or animal models of human cancers suggest that, miRNAs can function as tumor suppressors or oncogenes. They play critical roles in multiple biological processes such as cancer cell proliferation, metastasis, apoptosis, and angiogenesis [6]. Emerging evidence shows that specific miRNAs are aberrantly expressed in a variety of human cancers, such as GC, and specific signatures of deregulated miRNAs highlight the putative diagnostic and therapeutic potentials [7-9].
MicroRNA-451 (miR-451) is located on chromosome 17q11.2, which is close proximity to ERBB2 (17q12), a region had been reported to be amplified in some types of cancers [10,11]. Recently, miR-451 has attracted increasing attention due to its critical roles in the pathogenesis and development of several types of cancers, including glioblastoma, colorectal cancer and non-small cell lung cancer [12-14]. Two recent studies have highlighted the importance of miR-451 in GC, both of which concluded lower levels of miR-451 expression in GC cells than in neighboring non-cancerous cells [15,16]. One of the two reports was from Brenner and his colleagues’ findings. They found that miR-451 had the strongest prognostic impact on predicture of recurrence of GC [17]. However, to our knowledge, the clinical significance of miR-451 in GC tissues has not been clearly addressed yet. Besides, the role it plays in human GC cell lines remains unclear.
In the present study, we determined the expression of miR-451 in 107 human primary gastric tumors paired with their adjacent normal tissues, and four human GC cell lines compared with normal gastric cell line by using quantitative RT-PCR. Associations of miR-451 levels with clinicopathological factors and overall survival of the GC patients was also statistically analyzed. Furthermore, we investigated the effects of forced expression of exogenetic miR-451 on GC cell proliferation, migration and invasion abilities in vitro.
Materials and methods
Patients and tissue samples
A total of 107 patients with GC who underwent surgery without preoperative treatment at Shandong Provincial Hospital affiliated to Shandong University, from 2010 to 2012, were included in the present study. The receiving tumor tissues and paired adjacent normal tissues were promptly formalin-fixed and paraffin-embedded (FFPE). All surgical tissues were examined by a pathologist and final surgical pathology reports were obtained and recorded. The clinicopathological characters of the above patients referred to were summarized in Table 1. Overall survival (OS) was defined as the time from the initially surgery to death of the patient or, in cases of living patients, the date of the last follow-up. This study was approved by the medical ethics committee of Shandong Provincial Hospital affiliated to Shandong University. Written informed consent was received from all the patients.
Table 1.
Relationship between expression levels of miR-451 and clinicopathological factors of the 107 GC patients
| Clinical Characteristics | Increased miR-451 (n = 26) No (%) | Decreased miR-451 (n = 81) No (%) | Test of significance |
|---|---|---|---|
| Gender | |||
| Male | 21 (23.1%) | 70 (76.9%) | χ2 = 0.4941 |
| Female | 5 (31.2%) | 11 (68.8%) | P = 0.2274 |
| Age (years) | |||
| ≤ 60 | 14 (28.6%) | 35 (71.4%) | χ2 = 0.8970 |
| > 60 | 12 (20.7%) | 46 (79.3%) | P = 0.3436 |
| Diameter (cm) | |||
| ≤ 4 | 16 (34.8%) | 30 (65.2%) | χ2 = 4.8210 |
| > 4 | 10 (16.4%) | 51 (83.6%) | P = 0.0281 |
| Infiltrate depth | |||
| Mucous membrane | 4 (57.1%) | 3 (42.9%) | |
| Muscular layer | 5 (23.8%) | 16 (76.2%) | χ2 = 4.4397 |
| Fibrous membrane | 17 (21.5%) | 62 (78.5%) | P = 0.1086 |
| TNM stage | |||
| T stage | |||
| T1, T2 | 17 (43.6%) | 22 (56.4%) | χ2 = 12.4149 |
| T3, T4 | 9 (13.2%) | 59 (86.8%) | P = 0.0004 |
| Lymph node metastasis | |||
| No | 19 (32.8%) | 39 (67.2%) | χ2 = 4.9274 |
| Yes | 7 (14.3%) | 42 (85.7%) | P = 0.0264 |
| Clinical stage | |||
| I, II | 21 (31.3%) | 46 (68.7%) | χ2 = 4.8347 |
| III, IV | 5 (12.5%) | 35 (87.5%) | P = 0.0279 |
Using SAS V8 statistical software, a χ2 or Fisher’s exact test was performed to analyze the correlations of miR-451 expression levels with clinicopathological factors. P < 0.05 was considered statistically significant. TNM: tumor/node/metastasis.
Cell culture
Four established human GC cell lines (MKN-45, BGC-823, MKN-28, SGC-7901) and a non-malignant gastric cell line GES were ordered from the Cell Line Resource Center, Shanghai Institute of Biochemistry and Cell Biology, the Chinese Academy of Sciences (Shanghai, China). The cells were routinely cultured in RPMI-1640 media (Gibco BRL) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL), 100 U/ml penicillin and 0.1 mg/ml streptomycin at 37°C in an atmosphere consisting with 5% CO2 and 95% air in a humidified incubator. Cells were cultured in 10 cm culture dishes and allowed to grow approximately 70% confluence before experimentation.
RNA extraction and quantitive real-time PCR
For cultured cell lines, total RNA was isolated by using Trizol (Invitrogen), according to the manufacturer’s instructions. For the above referred to 107 pairs of FFPE samples, total RNA was purified by using the miRNeasy FFPE kit (Qiagen) according to the manufacturer’s protocols. cDNA was synthesized by the M-MLV reverse transcriptase (Promega), using the indicated primers. The reactions were incubated in a PTC-200 thermal cycler (Bio-Rad) in a 96-well plate for 5 min at 70°C, followed by 3 min at 4°C, 60 min at 42°C, and then held at 4°C. The expression levels of miR-451 were quantified by qRT-PCR with the SYBR Premix Ex Taq Kit (Takara). qRT-PCR was performed in a DNA Engine Opticon2 system (Bio-Rad). The following PCR protocol was used: denaturation at 95°C for 3 min, amplification for 40 cycles of 95°C for 12 s and 62°C for 40 s. The melting curve was plotted from 62°C to 95°C, read every 0.2°C with a 2 s hold). U6 small nuclear RNA was used as an internal control. The results were represented as fold changes, which were calculated by the 2-ΔCT method.
Construction of miR-451 over-expressed stable cell lines
High titer of LV-NC and LV-miR-451 lentiviruses were purchased from Gene Pharma (Shanghai, China). Concentrated lentiviral solutions of LV-NC and LV-miR-451 were added into two wells of cultured BGC-823 or MKN-45 cells, respectively, once the cells reached a 30~40% confluence. Enhanced infection solution was then added to reach a total incubation volume of 2 ml. After 12 h of incubation, the cell culture medium was replaced with fresh medium. 72 h later, the infected cells were selected by 2 g/ml puromycin resistance for 72-96 h.
MTT assay
MTT assay was carried out to determine cell proliferation rate in this study. Briefly, the NC or miR-451 overexpressed stable BGC-823 or MKN-45 cell lines were trypsinzed and seeded into 96-well plates at a density of 2,500 cells in 200 µL of medium per well and then incubated at 37°C. Following incubation overnight, the medium was changed with fresh cell culture medium containing 0.5 mg/ml MTT. The cells were then incubated for another 4 hours. Finally the medium was carefully aspirated and 150 µL of DMSO was added into each well to dissolve the crystals. Absorbance was detected at 490 nm using a microplate reader. Experiments were carried out in triplicates and repeated at least three times independently.
Transwell migration and invasion assays
The migration and invasion assays were performed to assess the transition of cells into the outside surface of the upper chamber, using 24-well transwell chambers. For the migration assay, cells were resuspended in serum-free medium, and 2 × 105 cells were seeded onto the upper chambers of a transwell (filter with 8 m pores; Corning). 0.5 mL cell culture medium 10% FBS was added into the bottom chambers. After 24 hours of incubation, cells on the upper surface of the membrane were scrubbed off. Cells that had migrated through the membrane were fixed, stained with 0.1% crystal violet, and photographed under a light microscope. The invasion assay protocol was similar to that of the migration assay except that the upper chambers were first covered with 1 mg/mL matrigel.
Statistical analysis
Results were analyzed with SPSS 16.0 (version 16.0, SPSS Inc, IL, USA). Data were expressed as mean ± standard deviation (SD). The paired-samples t-test was used in the analysis of differential miR-451 expression between tumor and normal tissues. Survival curves were estimated by the Kaplan-Meier method. For assessing the significance of difference between the treatment and control groups in vitro experiments, independent-samples t-test was used. P value < 0.05 was considered as statistically significant.
Results
Decreased expression of miR-451 in GC tumor tissues and cell lines
To explore if miR-451 might have a role in tumorigensis of human GC, qRT-PCR was first applied to determine its expression level in tumor tissues and adjacent non-cancerous tissues, and normalized to U6 small RNA expression. The results showed that, the expression levels of miR-451 in human GC tissues were significantly decreased than in the paired normal tissues (P < 0.001). Pair-wise comparison indicated that nearly 30% of tumors showed greater than 2-fold reduction of miR-451 expression compared to their matching controls, with only two pairs showing increase (Figure 1A). Expression of miR-451 was also decreased in a number of human gastric cancer cell lines, including MKN-45, BGC-823, MKN-28, and SGC-7901 cells, when compared to that in the normal gastric GES cells (Figure 1B).
Figure 1.
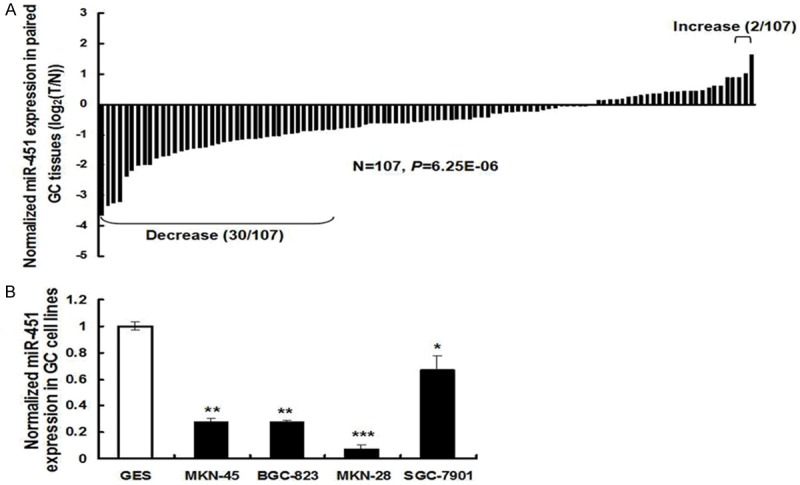
Expression of miR-451 in gastric cancer (GC) tissues and cell lines. The expression levels of miR-451 were determined by qRT-PCR. A. Pairwise comparison of miR-451 expression between GC and matching non-cancerous tissues showing miR-451 expression was reduced in 28% (30/107) of the sample pairs. The expression level of miR-451 was calculated by the 2-ΔCt method, after normalized to U6 RNA. B. MiR-451 expression was reduced in four human GC cell lines: MKN-45, BGC-823, MKN-28, and SGC-7901 cells, when compared to that in normal gastric GES cells. Data were represented as mean ± SD from three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001).
Clinicopathological significance of miR-451 expression in GC
Using the equal expression of miR-451 in tumor verses normal tissues in all 107 patients as a cutoff, the patients were divided into high miR-451 expression group (in which the expression ratios of tumor/normal ≥ 1, n = 26), and low miR-451 expression group (in which the expression ratios of tumor/normal < 1, n = 81). To access the clinical value of miR-451 expression in GC tissues, the association of its expression and clinicopathological characters of 107 patients was statistically analyzed by 2 tests or Fisher’s exact test (Table 1). The results showed that, decreased expression of miR-451 positively correlated with tumor size, lymphatic metastasis, TNM malignancy staging and clinical stage, but not with age, gender or infiltrate depth (Table 1). These data suggested that decreased expression of miR-451 was associated with significantly aggressive pathologic features, indicating that miR-451 might inhibit GC progression.
Down-regulation of miR-451 confers poor prognosis in GC patients
We next performed survival analysis to evaluate whether miR-451 expression had prognostic potential for overall survival (OS) of GC patients with clinical fellow-up information. Survival curves were plotted using the Kaplan-Meier method. The results showed that patients with lower miR-451 expression had significantly worse OS rates than those who had cancers with high expression of this miRNA (log-rank test P = 0.042, shown in Figure 2).
Figure 2.
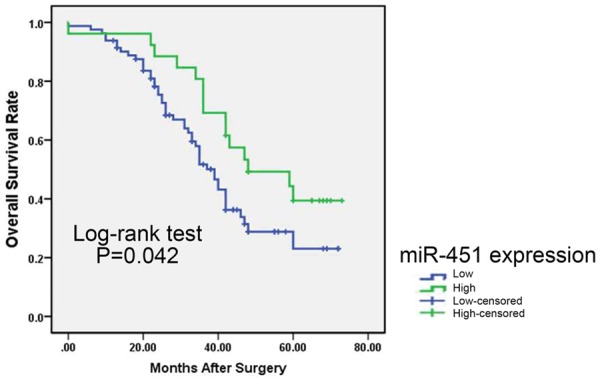
Overall survival curves for two groups defined as low and high expression of miR-451 in GC patients. Lower miR-451 expression was significantly associated with poorer outcome of patients (P < 0.05, log-rank test).
MiR-451 does not inhibit gastric cancer cell proliferation
To determine if miR-451 had the propensity to suppress gastric cancer tumorigenesis, we introduced pre-miR-451 or negative control (NC) oligonucleotides respectively into BGC-823 and MKN-45 cell lines by lentivirus and constructed miR-451 or NC overexpression stable cell lines. Stem-loop qRT-PCR confirmed the elevated level of miR-451 in the transfected BGC-823 and MKN-45 cells (Figure 3A). We then monitored the rate of cell growth using the tetrazolium dye, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method. Results from the MTT assay indicated that the growth rate of these cells demonstrated no significant change between each other in the grouped cell lines, over a 5-day period (Figure 3B). These findings indicated that miR-451 might negatively impact on GC tumorigensis, at least not through the proliferation pathway.
Figure 3.
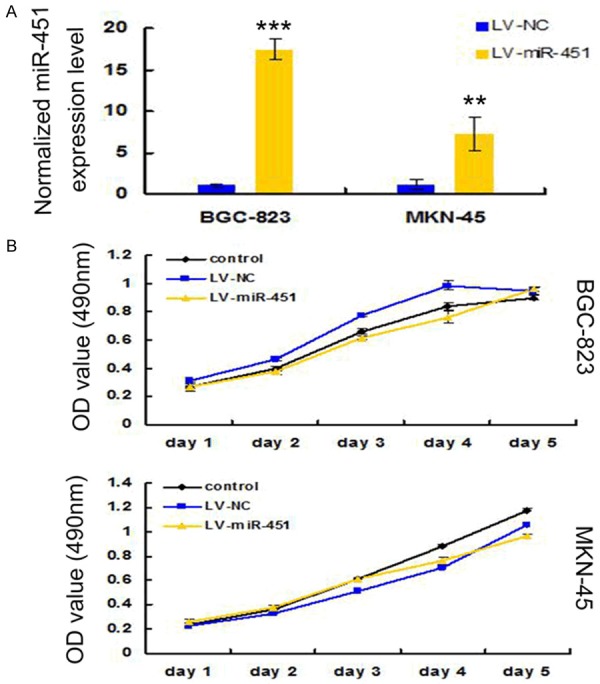
Effects of miR-451 over-expression on GC cell proliferation. A. The expression level of miR-451 in miR-451 containing lentivirus infected cells was significantly higher compared with negative control (NC) transfected cells. qRT-PCR was done to detect the expression of miR-451 in three independent experiments. U6 small nuclear RNA was used as the internal control. ***P < 0.001, **P < 0.01. B. Cell proliferation was measured by MTT assays in BGC-823 and MKN-45 cells infected with miR-451 or NC containing lentivirus. The plates were read every one day for 5 consecutive days. Data were represented as mean ± SD from the experiments performed in triplicate.
MiR-451 inhibits gastric cancer cell migration and invasion
Since decreased expression of miR-451 was associated with metastasis characters of 107 GC patients (Table 1), suggesting its vital role in migratory or invasion processes. We then carried out transwell migration and invasion assays using the miR-451-expressing stable BGC-823 cells. The results showed that enforced overexpression of miR-451 caused a suppression of cell migration ability in BGC-823 cells. Ectopic overexpression of miR-451 further inhibited BGC-823 cell invasion as demonstrated by the matrigel invasion assay (Figure 4). Hence, miR-451 displayed a suppressive property in cancer cell migration and invasion assays in vitro.
Figure 4.
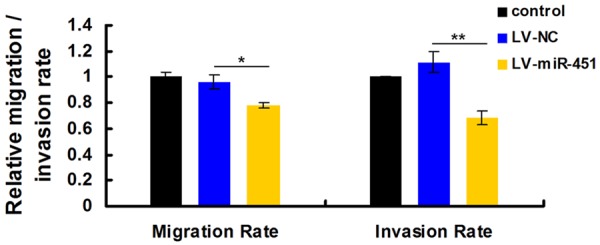
MiR-451 inhibits GC cell migration and invasion in vitro. Over-expression of miR-451 significantly inhibited abilities of cell migration and invasion in miR-451-expressing stable BGC-823 cells compared with NC group. Data were expressed as the mean ± SD of at least three independent experiments (*P < 0.05). The migration and matrigel invasion assays showed that the number of migrated or invaded cells was significantly lower in the miR-451-overexpressed group than in the NC group (**P < 0.01).
Discussion
Despite having improved treatment modalities over the last decade, only modest improvements have been seen in overall survival for most GC patients. It is of great importance to investigate the cellular and molecular mechanisms of GC progression, and to identify novel genetic or protein biomarkers for accurate diagnosis and prediction of prognosis.
This study found a significant low expression of miR-451 in GC tissues as compared to corresponding adjacent normal tissues, which has also been reported before. What’s new in our findings is that low expression level of miR-451 tends to be associated with lymphatic metastasis, TNM malignancy staging and clinical stage. We further found GC patients with low levels of miR-451 expression have a shorter survival (P = 0.042). As a result, miR-451 may be a potential biomarker for diagnoses and prognoses of GC. Finally, in vitro functional assays demonstrated that forced expression of miR-451 expression in BGC-823 cells was able to reduce cell migration and invasion. We didn’t show the migration or invasion results of MKN-45 cells because of its bad metastasis nature. To the authors’ knowledge, this is the first report regarding the clinical significance and functional attributes of miR-451 in GC.
Evidence of miR-451 as a tumor suppressor has been reported in several cancers: Bandres et al initially found that miR-451 was decreased in gastric and colorectal cancer versus non-cancerous tissues, and its down-regulation was associated with worse prognosis, which was also in line with our results. Specifically, they demonstrated that forced expression of miR-451 in gastric and colorectal cancer cells could inhibit cell proliferation and increase sensitivity to radiotherapy. Furthermore, they identified the novel oncogene macrophage migration inhibitory factor (MIF) as a potential target of miR-451 [15]. While we proved here that miR-451 seems not to influence cell proliferation. So the growth-inhibitory function of miR-451 might be a context-dependent effect. Interestingly, Godlewski et al found reduced expression of miR-451 in migrating glioma cells. And its over-expression was proved to inhibit migration but promote cell proliferation. In glioblastoma patients, they found that increased expression of miR-451 was associated with shorter survival [18]. Nan et al also pointed out that miR-451 was a tumor suppressor in human glioma cells. But they found that increased expression of miR-451 inhibited cell growth, induced G0/G1 phase arrest and increased cell apoptosis [12]. Wang et al found that miR-451 functioned as a tumor suppressor in human non-small cell lung cancer (NSLC) by targeting ras-related protein 14 (RAB14) [13,19]. Li et al reported that miR-451 could inhibit cell proliferation in human hepatocellular carcinoma or colorectal carcinoma cells through direct suppression of IKK-β, or downregulation of PI3k/Akt pathway, respectively [14,20]. All these findings indicated that miR-451 may function as a tumor suppressor in various cancers.
In this study, we showed decreased expression of miR-451 in GC may be associated with lymph node metastasis and clinical stage. In vitro experiments showed that miR-451 did not modulate GC cell proliferation, but did inhibit GC cell migration and invasion. While in this study, we had not identified the underlying molecular mechanisms of the indicated phenotype. In future studies, more attention should be paid on its known targets in various other types of cancers, as well as the signaling pathways it had been reported to influence on. In conclusion, we demonstrated that, miR-451 may be a meaningful prognostic biomarker and potential therapeutic target in GC treatment. Our findings may have a therapeutic potential to suppress GC cell metastasis.
Acknowledgements
This study is supported by Department of science and technology of Shandong Province (No. 2013GSF11862).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Moore MA, Eser S, Igisinov N, Igisinov S, Mohagheghi MA, Mousavi-Jarrahi A, Ozentürk G, Soipova M, Tuncer M, Sobue T. Cancer epidemiology and control in North-Western and Central Asia-past, present and future. Asian Pac J Cancer Prev. 2010;11(Suppl 2):17–32. [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 5.Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12:3237–3242. doi: 10.3748/wjg.v12.i20.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Ren C, Han C, Wang D, Chen Y, Fu D. Expression and Prognostic Value of miR-486-5p in Patients with Gastric Adenocarcinoma. PLoS One. 2015;10:e0119384. doi: 10.1371/journal.pone.0119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Sun J, Bai Z, Li H, He S, Chen R, Che X. MicroRNA-153 acts as a prognostic marker in gastric cancer and its role in cell migration and invasion. Onco Targets Ther. 2015;8:357–364. doi: 10.2147/OTT.S78236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Tang Y, Wu JH, Liu FH. Role of microRNAs in diagnosis and treatment of the pathogenesis of gastric cancer. Int J Clin Exp Med. 2014;7:5947–5957. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Mahlamäki EH, Bärlund M, Tanner M, Gorunova L, Höglund M, Karhu R, Kallioniemi A. Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosomes Cancer. 2002;35:353–358. doi: 10.1002/gcc.10122. [DOI] [PubMed] [Google Scholar]
- 11.Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, Frierson H Jr, Powell SM, Knuutila S, Kallioniemi A, El-Rifai W. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625–2629. [PubMed] [Google Scholar]
- 12.Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang Y, Yue X, Pu P, Zhong Y, Kang C. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 13.Wang XC, Tian LL, Jiang XY, Wang YY, Li DG, She Y, Chang JH, Meng AM. The expression and function of miRNA-451 in non-small cell lung cancer. Cancer Lett. 2011;311:203–209. doi: 10.1016/j.canlet.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Li HY, Zhang Y, Cai JH, Bian HL. MicroRNA-451 inhibits growth of human colorectal carcinoma cells via downregulation of Pi3k/Akt pathway. Asian Pac J Cancer Prev. 2013;14:3631–3634. doi: 10.7314/apjcp.2013.14.6.3631. [DOI] [PubMed] [Google Scholar]
- 15.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, Jimenez P, Rodriguez J, Garcia-Foncillas J. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 16.Takagi T, Iio A, Nakagawa Y, Naoe T, Tanigawa N, Akao Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology. 2009;77:12–21. doi: 10.1159/000218166. [DOI] [PubMed] [Google Scholar]
- 17.Brenner B, Hoshen MB, Purim O, David MB, Ashkenazi K, Marshak G, Kundel Y, Brenner R, Morgenstern S, Halpern M, Rosenfeld N, Chajut A, Niv Y, Kushnir M. MicroRNAs as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2011;17:3976–3985. doi: 10.3748/wjg.v17.i35.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37:620–632. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang R, Wang ZX, Yang JS, Pan X, De W, Chen LB. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14) Oncogene. 2011;30:2644–2658. doi: 10.1038/onc.2010.642. [DOI] [PubMed] [Google Scholar]
- 20.Li HP, Zeng XC, Zhang B, Long JT, Zhou B, Tan GS, Zeng WX, Chen W, Yang JY. miR-451 inhibits cell proliferation in human hepatocellular carcinoma through direct suppression of IKK-β. Carcinogenesis. 2013;34:2443–2451. doi: 10.1093/carcin/bgt206. [DOI] [PubMed] [Google Scholar]


