Abstract
Hypoxia plays a critical role in the formation of diabetic retinopathy (DR). Hypoxia inducible factor-1α (HIF-1α) and survivin are upregulated following hypoxia, and contribute to the angiogenesis in cancer. The goal of our study was to investigate if the expression of HIF-1α and survivin is related to the retinal vascular change of rats with different stages of diabetes mellitus (DM). Wistar rats were randomly divided into DM for 1 month (DM1) group, DM3, DM6 group and normal control group. Streptozotocin (STZ) was used to induce diabetic rat models. Retinal vascular digest preparation was taken to observe the change of retinal microvessels. The expression of HIF-1α and survivin was determined by real-time PCR and Western blot. In DM3 group, capillaries became circuitous and irregular. Acellular capillary were detected in DM6 group. In the same time, the number of pericytes significantly decreased in DM3 and DM6 groups. The expressions of HIF-1α were more increased in the DM3 and DM6 groups. However, the expressions of survivin only were upregulated in DM6 group .These data suggest that HIF-1α induced the upregulation of survivin in the early stage of DR. Survivin contributed to the development and progression of DR through the HIF-1α pathway and become an important progression marker of DR.
Keywords: Diabetic retinopathy, hypoxia inducible factor-1α, survivin
Introduction
Diabetic retinopathy, the most common complication of diabetes mellitus, is the main cause of blindness in the working-age population worldwide [1]. There are variety of therapeutic strategies available for DR, One such strategy is laser photocoagulation, a conventional treatment that is effective in delaying the progression of angiogenesis in the short-term. However, this method is also destructive to the retinal photoreceptors, and does not reverse vision loss. Anti-vascular endothelial growth factor (VEGF), anti-inflammatory molecules, and protein kinas C inhibitors have shown undesirable side effects [2,3]. Therefore, searching more specific therapy targeting molecular mechanisms underlying retinal neovascularization may led to better treatment result and fewer severe side effects.
Although the mechanisms of diabetic retinopathy have not been completely indicated, hypoxia, resulting in worsening retinal ischemia, is often implicated as a key player. Hypoxia-mediated gene expression is regulated by HIF-1, which is generally regarded as the master regulators of the hypoxic response by regulating the transcription of a large number of target genes. HIF-1 is composed of HIF-1α and HIF-1β. HIF-1α is a key oxygen sensor and can activate transcription of many genes that are critical for cellular function under hypoxia conditions [4]. Evidences have indicated that HIF-1α overexpression is significantly associated with enhanced patient mortality in many different cancers and contribute to the chemoresistance of a wide range of solid tumors [5,6]. In addition, HIF-1α pathway is also activated in the retina in early diabetes [7]. Silencing HIF1α by RNA interference in hypoxia reduces VEGF expression and angiogenic factors, leading to inhibition of angiogenesis [8]. All these suggested that HIF-1α expression might serve as a regulator of tumor growth, anaerobic glycolysis, antiapoptosis and play an important role in regulating angiogenesis [9].
Survivin, an inhibitor of apoptosis protein family, has been reported to be correlated to hypoxia and unanimously viewed as one of the most prominent cancer genes. Surprisingly, survivin is barely expressed in normal tissues but is detected in many tumors [10]. In addition to its direct role in carcinogenesis, survivin may also play a critical role in tumor angiogenesis and inhibits apoptosis, increases proliferation, and elevates angiogenesis [11]. Furthermore, numerous studies have identified that overexpression of survivin is an indicator for poor prognosis and disease recurrence [12]. As far as we know, it has been shown that HIF-1α could co-operate with the regulation of survivin gene expression in multiple tumors, the down-regulation of HIF-1α decreases the levels of survivin expression in various types of cancer cells. Survivin expression is upregulated by the induction of HIF-1α resulting from tumor formation, possibly leading to tumor progression [13,14].
The relationship between HIF-1α and survivin expression in diabetic rat retinas has not been reported to our knowledge. In this study, we investigated the dynamic change of HIF-1α and survivin expression in the different stage of DM and the correlation of HIF-1α and survivin expression and retinal microvascular dysfunction.
Materials and methods
Mouse model of DR
Wistar rats (180-220 g) were purchased from the Laboratory Animal Center of China Medical University (Shenyang, China). All animal experiments were performed followed the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of Medical University Ophthalmic Center.
About 80 healthy Wistar rats were randomly divided into DM for 1 month (DM1) group, DM for 3 months (DM3) group, DM for 6 months (DM6) group and normal control group (n=20, each group). Wistar rats received a single 60 mg/kg intraperitoneal injection of streptozotocin (STZ; Sigma, St. Louis, MO) in 10 mmol/l sodium citrate buffer (pH 4.5). Control animals were fasted and received the buffer alone. After receiving STZ for 7 days, animals with plasma glucose values of 16.7 mmol/L (approximately 300 mg/dL) were considered to be diabetic. Blood glucose levels were checked weekly. Diabetic and non-diabetic controls were sacrificed after 1, 3 and 6 months of diabetes. The eyes were used for sub cellular fractionation and western blot and for immunohistology.
Retinal histopathology
Fours rats in each group were killed by a lethal dose of sodium pentobarbital through intraperitoneal injection. As described previously [15], briefly both eyes were removed and the retina was incubated for up to 4 hr in phosphate-buffered saline, and then all but the vascular elements were digested using 3.0% trypsin buffer (pH=7.8) at 37°C, the retinal vessels were stained by the periodic acid-Schiff (PAS) reaction and hematoxylin and eosin (H&E). Retinal vascular structures were observed, and the pericytes were counted under masked conditions.
RNA extraction, cDNA synthesis, and real-time quantitative PCR
The expression levels of HIF-1α mRNA and Survivin mRNA were detected using Real-time quantitative PCR. Eight rats from each group were sacrificed, the eye balls were enucleated immediately, and the retinas excised in iced plate and frozen in liquid nitrogen. Total RNA was extracted from each retina sample by 0.5 mL of Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Concentration of total RNA was determined by ultraviolet absorbance spectroscopy. 2.5 μg of total RNA was reverse transcribed using a Prime Script RT reagent Kit (TaKaRa, Dalian, China). β-actin was used as the internal control. Real- time PCR was performed using the SYBR Green Kit (Toyobo, Shang Hai China) in a thermal cycler (Applied Biosystems Step One™, America). The following primers were used: Survivin (forward: 5’-AGGACCACCGGATCTACACCTTCA-3’, reverse: 5’-CTCGGTAGGGCAGTGGATGAAGC-3’); HIF-1α (forward: 5’-ACCGCAACTGCCACCACTGA-3’, reverse: 5’-TGGTGAGGCTGTCCGACTGTGA-3’); β-actin (forward: 5’-CCTCTATGCCAACACAGTGC-3’, reverse: 5’-CATCGTACTCCTGCTTGCTG-3’).
The PCR reactions were presented as the following: 1 min at 95°C, 35 cycles at 95°C for 10 seconds, 60°C for 30 seconds, and 72°C for 20 seconds.
Protein isolation and western blot analysis
Retinas extracted from each group (n=8) were lysed in 200 μL of lysis buffer with protease inhibitors (Sigma Chemical Co., St. Louis, MO).Protein concentration was assessed by the BCA protein assay kit (Pierce Biotechnology, Rockford. IL). After that, total protein extract from each sample were loaded onto SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane and then were blocked in 5% nonfat milk for 1 hour, and then the membrane was incubated with primary antibodies of rabbit polyclonal anti-HIF-1α antibody (1:500, Santa Cruz, CA) or polyclonal anti-survivin antibody (1:400, Santa Cruz, CA) and β-actin monoclonal antibody (1:400 Santa Cruz, CA) overnight at 4°C. Horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit antibody, 1:1200, Santa Cruz, CA) was used to incubate at room temperature. The proteins were detected with enhanced chemiluminescence detection (Santa Cruz, CA). β-actin served as the internal standard. Experiments were repeated three times.
Statistical analysis
The results were expressed on the basis of mean ± standard deviation. Comparisons between groups were performed using Student’s t-tests. Statistical analyses were performed using SPSS 10.0 software and P values <0.05 were considered to be statistically significant. All the experiments were carried out three times.
Results
Difference of retinal histopathology
DM1 group: There was no distinct difference in retinal vascular digest preparation compare with normal control group. The whole vascular network could be detectable and the retinal capillary diameter is uniform (Figure 1A, 1B). There are two kinds of cells in the capillary. The nucleuses of the pericytes were small and round, being triangle, located at one side of the capillary. The nucleuses of the endothelial cells were big, oval or irregular which paralleled with the vasculature in long axis (Figure 2). The number of the pericytes was 8.56±0.34 and 8.60±0.15 in the control and DM1 groups, respectively (P>0.05).
Figure 1.
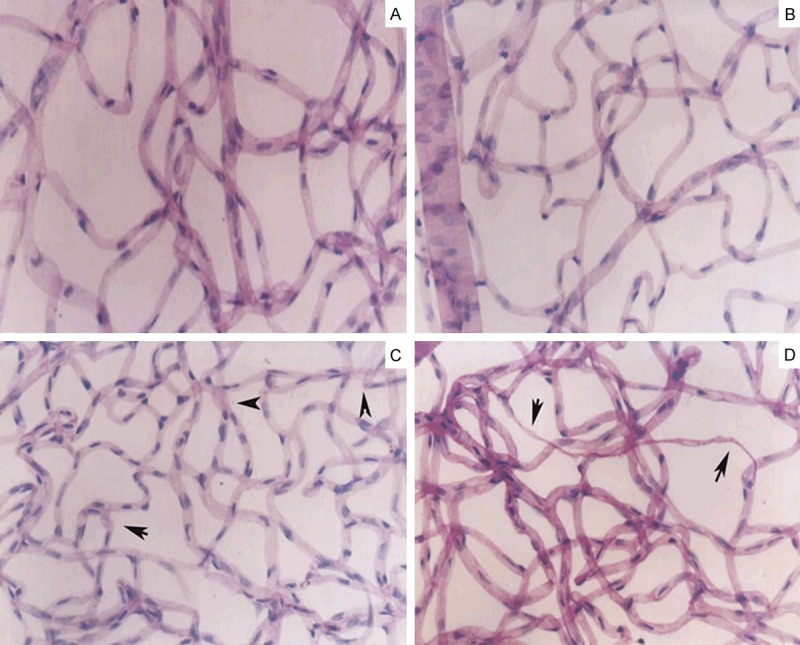
Retinal histopathology: There are intact vascular network and the uniform diameter of retinal capillary in the normal control group and DM1 group (A, B). In the DM3 group, the vascular net was in uniform distribution and the irregular with tortuous and dilatations of vascular cavity (C, arrow denoted). Acellular capillaries were mainly present in the DM6 group (D, arrow denoted) Magnification × 20.
Figure 2.
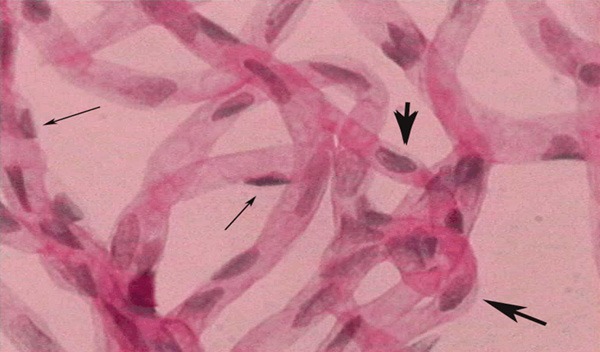
Two kinds of cells were seen in the capillary. Pericyte nuclei were small and round, being triangle, located at one side of the capillary (narrow arrow). Endothelial cell nuclei were large, oval or irregular which paralleled with the vasculature in long axis (wide arrow) Magnification × 400.
DM3 group: The vascular net was showed in uniform distribution and the vascular cavity was disorder with tortuous and dilatations, but no angioma and intracellular thrombi were found (Figure 1C). The number of the pericytes was reduced compared with normal control group (6.13±0.24, P<0.05).
DM6 group: The morphology of retinal vasculature is damaged seriously. The vascular cavity was more irregular than that of DM3 group; the acellular capillaries could be seen (Figure 1D). The number of pericytes were significantly decreased compared with that of normal control group (3.17±0.42, P<0.001).
mRNA and protein levels of HIF-1a and survivin in rat retinas
The results of differential expression of HIF-1α and survivin mRNA are shown in Figure 3, There were less mRNA levels of HIF-1α in control group compared to the DM1 group (P>0.05). HIF-1α mRNA elevated gradually with the progress of diabetes. Significant rise of HIF-1α expression was detected in the DM3 and DM6 groups compared to normal group (P<0.05, P<0.01, respectively). Meanwhile the mRNA of survivin was significantly increased in the DM6 group compared with the normal control group (P<0.01). There were no or very little expression of survivin in DM1 and DM3 groups, comparing to the control group (>0.05). We performed western blot assay to confirm the expression of HIF-1α and survivin at protein level. As shown in Figure 4, the protein expression was consistent with mRNA expression. In the normal group, protein expression of survivin was almost impossible to detect and HIF-1α expression was only detectable. However, the expression of HIF-1α and survivin was relatively increased with the progression of DM. The increased expression of survivin was mainly in DM6 group compare to the normal groups (P<0.01).
Figure 3.
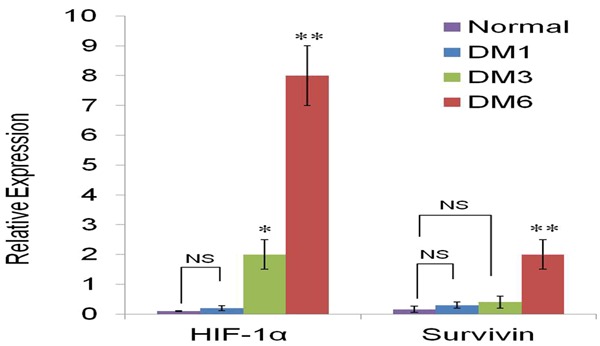
Real-time PCR was performed to detect the mRNA level of HIF-1α and survivin in the all experiment groups. Expression of HIF-1α was increased in the DM3 and DM6 groups compared to that in normal group, and survivin expression was increased significantly in the DM6 groups. β-actin served as internal control. *P<0.05, **P<0.01 visa to normal group.
Figure 4.
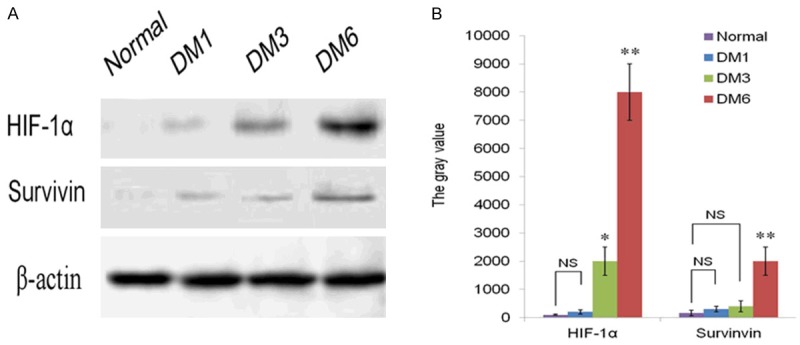
HIF-1α and Survivin protein levels were detected in the DM1, DM3 and DM6 groups compared to that in the control group. (A) Western Blot shows that HIF-1α and Survivin was barely detected in the control group and DM1, but the expression of HIF-1α was elevated in the DM3 and DM6 groups, the level of Survivin was increased markedly in DM6 group. (B) Quantification of the data in (A) shows the expression intensity of HIF-1α and Survivin. β-actin served as internal control. *P<0.05, **P<0.01 vies to normal group.
Discussion
Pathological retinal neovascularisation is the classic hallmark of diabetic retinopathy. Although the definite pathogenesis of DR remains unclear, selective disappearances of the pericytes, ischemia and hypoxia in the early stage of DR are accepted generally [16]. In the present study, we used the retinal vascular digest preparation to detect the change of the pericytes and obliterated retinal capillaries, which is direct and obvious.
Tissue hypoxia plays a critical role in the formation of retinal NV [17,18]. Hypoxia stimulates a lot of angiogenic growth factors and cytokines, which involve in the pathogenesis of NV. HIF-1α is the oxygen-regulated a-subunit of HIF-1. HIF-1 triggers the activation of many gene-encoding proteins, such as VEGF, erythropoietin (Epo) and angiopoietins (Angs) that regulate angiogenesis [19]. Previous studies have suggested that overexpression of HIF-1α in tumor tissues is associated with tumor angiogenesis, aggressive tumor growth and poor patient prognosis [20]. HIF1α and VEGF levels are induced in the vitreous fluid of patients with proliferative diabetic retinopathy [21]. In this study we found that HIF-1α expression is increased by hypoxia at the early phase of DR. Our observations are consistent with previous reports showing that the HIF-1 pathway is activated in the retina in early diabetes [7] and HIF1α plays an important role in regulating angiogenesis. These findings suggest that HIF-1α is involved in the formation of angiogenesis in DR. Further evidence to support that HIF-1α is correlated with diabetes, significantly increased expression of HIF-1α in a subgroup of eyes with PDR and HIF-1α is found more strongly in diabetic preretinal membranes [19,22].
Survivin, a member of the inhibitor of apoptosis protein (IAP) family, has been shown that tumor-specific expression of Survivin is caused by hypoxia. Recently research found that survivin is barely expressed in adult normal tissues but is usually overexpressed in most human cancers such as melanoma and cancers of lung, esophagus, stomach, intestine, pancreas and breast. The aberrant survivin expression is associated with tumor progression, angiogenesis, and poor survival rates in cancer patients.
Recently, it has been reported that HIF-1α contributes to tumor radioresistance by upregulating survivin expression under hypoxic conditions. In vivo, Hif-1α not only cooperates with survivin, but also prompts the expression of survivin [23,24]. In vitro, the expressions of HIF-1α and survivin in A549 cells were significantly increased under hypoxic conditions [25,26]. But in DR, the present pilot study is the first to examine their dynamic expression and relationship in the progression of DR. Our results show that the expression of HIF-1α and survivin is along with the change of capillaries morphology and the loss of cell. In DM3 group, the numbers of percytes began to decrease, capillary is irregular and dilated. The mRNA and protein expression of HIF-1α were obviously increased, but there was no expression of survivin. These changes of the significant decrease of pericytes and vessel occlusion occurred along with the expressions of HIF-1α. It suggested that retinal hypoxia exists, retinal NV occurs in disease in which the underlying disease process damages retinal vessels causing areas of vessel closure, lose of pericytes, deterioration of endothelial cells and retinal ischemia and hypoxia leading to up-regulation of HIF-1α which stimulate expression of a group of hypoxia-regulated genes that together stimulate the growth of new vessels, and eventually leading to development of proliferative diabetic retinopathy. Especially the increased levels of survivin are present in the DM6 group. Obliteration of small blood vessels and acellular capillaries were mainly detected in the DM6 group. Retinal neovascularization always seems to be preceded by such vessel closure and is consider being one of its consequences. Closure of retinal blood vessels is shown as an increased of acellular capillaries, capillary basement membrane tubes inadequate endothelial cells, and pericyte nuclei. Therefore, we further confirm that HIF-1α might be a transcriptional activator of survivin in the development of DR. At the same time, retinal ischemia and hypoxia leads to rapid up-regulation of survivin. We speculate that overexpression of survivin enhances cell proliferation, promotes retinal angiogenesis and involves in the progression of DR.
In summary, our studies results demonstrated that HIF-1α signaling was activated by hypoxia, contributing to survivin expression in the early stage of DR. Additionally, we show that overexpression of survivin is closely related to the formation of acellular vasculature leading to retinal ischemia and hypoxia. As such, HIF-1α may be an important transcription factor involved in regulation of survivin expression. Survivin has become an important progression marker of DR and targeting these factors might be an important implication in the development of novel therapeutic strategies aimed at blocking the NV of DR.
Acknowledgements
This research was supported by Doctoral Scientific Research Foundation of China (No. 20091115).
Disclosure of conflict of interest
None.
References
- 1.Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012;57:347–70. doi: 10.1016/j.survophthal.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Dorrell M, Unsitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neovascularization: basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52:S3–19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Sear JE. Anti-vascular endothelial growth factor and retinopathy of prematurity. Br J Ophthalmol. 2008;92:1437–1438. doi: 10.1136/bjo.2008.141481. [DOI] [PubMed] [Google Scholar]
- 4.Kiani AA, Kazemi A, Halabian A, Mohammadipour M, Jahanian-Najafabadi A, Roudkenar MH. HIF-1α confers resistance to induced stress in bone marrow-derived mesenchymal stem cells. Arch Med Res. 2013;44:185–93. doi: 10.1016/j.arcmed.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kitajima Y, Miyazaki K. The Critical Impact of HIF-1α on Gastric Cancer. Cancers (Basel) 2013;5:15–26. doi: 10.3390/cancers5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gayed BA, O’Malley J, Pilch KJ, Wang Z. Digoxin inhibits blood vessel density and HIF-1a expression in castration-resistant C4-2 xenograft prostate tumors. Clin Transl Sci. 2012;5:39–42. doi: 10.1111/j.1752-8062.2011.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Wu Q, Liu B, Yao Y, Chen Y, Zhang H, Wang C, Cao J, Ge S. Detection and validation of circulating endothelial cells, a blood-based diagnostic marker of acute myocardial infarction. PLoS One. 2013;8:e58478. doi: 10.1371/journal.pone.0058478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forooghian F, Das B. Anti-angiogenic effects of ribonucleic acid interference targeting vascular endothelial growth factor and hypoxia-inducible factor-1alpha. Am J Ophthalmol. 2007;144:761–8. doi: 10.1016/j.ajo.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Goda N, Dozier SJ, Johnson RS. HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid Redox Signal. 2003;5:467–73. doi: 10.1089/152308603768295212. [DOI] [PubMed] [Google Scholar]
- 10.Boidot R, Végran F, Lizard-Nacol S. Transcriptional regulation of the survivin gene. Mol Biol Rep. 2014;41:233–40. doi: 10.1007/s11033-013-2856-0. [DOI] [PubMed] [Google Scholar]
- 11.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 12.Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG. Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PLoS One. 2013;8:e71930. doi: 10.1371/journal.pone.0071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tong E, Xu Y, Li G, Zou K, Zou L. The effects of β-elemene on the expression of mTOR, HIF-1α, survivin in lung adenocarcinoma A549 cell. Afr J Tradit Complement Altern Med. 2013;10:18–23. doi: 10.4314/ajtcam.v10i4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai H, Ge S, Lu J, Qian G, Xu R. Hypoxia inducible factor-1α-mediated activation of survivin in cervical cancer cells. J Obstet Gynaecol Res. 2013;39:555–63. doi: 10.1111/j.1447-0756.2012.01995.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Gorbey S, Pfister F, Höger S, Dorn-Beineke A, Krügel K, Berrone E, Wu L, Korff T, Lin J, Busch S, Reichenbach A, Feng Y, Hammes HP. Long-term treatment with suberythropoietic Epo is vaso- and neuroprotective in experimental diabetic retinopathy. Cell Physiol Biochem. 2011;27:769–82. doi: 10.1159/000330085. [DOI] [PubMed] [Google Scholar]
- 16.Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38:1203–1206. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- 17.Campochiaro PA. Ocular neovascularization. J Mol Med (Berl) 2013;91:311–21. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ameri A, Liu H, Liu R, Ha Y, Paulucci-Holthauzen AA, Hu S, Motamedi M, Godley BF, Tilton RG, Zhang W. TWEAK/Fn14 pathway is a novel mediator of retinal neovascularization. Invest Ophthalmol Vis Sci. 2014;55:801–13. doi: 10.1167/iovs.13-12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita M, Ohneda O, Yamashita T, Takahashi S, Suzuki N, Nakajima O, Kawauchi S, Ema M, Shibahara S, Udono T, Tomita K, Tamai M, Sogawa K, Yamamoto M, Fujii-Kuriyama Y. HLF/HIF-2α is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Chen Y, Zhang B, Shi SB, Weng W, Chen Z, Guo N, Hua Y, Zhu L. Hypoxia-inducible factor-1α polymorphisms and risk of cancer metastasis: a meta-analysis. PLoS One. 2013;8:e70961. doi: 10.1371/journal.pone.0070961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim JI, Spee C, Hinton DR. A comparison of hypoxia-inducible factor-α in surgically excised neovascular membranes of patients withdiabetes compared with idiopathic epiretinal membranes in nondiabetic patients. Retina. 2010;3:1472–8. doi: 10.1097/IAE.0b013e3181d6df09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loukovaara S, Koivunen P, Inglés M, Escobar J, Vento M, Andersson S. Elevated protein carbonyl and HIF-1α levels in eyes with proliferative diabetic retinopathy. Acta Ophthalmol. 2014;92:323–7. doi: 10.1111/aos.12186. [DOI] [PubMed] [Google Scholar]
- 23.Li DW, Zhou L, Jin B, Xie J, Dong P. Expression and significance of hypoxia-inducible factor-1α and survivin in laryngeal carcinoma tissue and cells. Otolaryngol Head Neck Surg. 2013;148:75–81. doi: 10.1177/0194599812464759. [DOI] [PubMed] [Google Scholar]
- 24.Wu XY, Fu ZX, Wang XH. Effect of hypoxia-inducible factor 1-α on Survivin in colorectal cancer. Mol Med Rep. 2010;3:409–15. doi: 10.3892/mmr_00000273. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Chen YQ, Shen YB, Shu HM, Wang XJ, Zhao CL, Chen CJ. HIF-1α knockdown by miRNA decreases survivin expression and inhibits A549 cell growth in vitro and in vivo. Int J Mol Med. 2013;32:271–80. doi: 10.3892/ijmm.2013.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Li D, Liu H, Xu H, Zheng H, Qian F, Li W, Zhao C, Wang Z, Wang X. Notch-1 signaling facilitates survivin expression in human non-small cell lung cancer cells. Cancer Biol Ther. 2011;11:14–21. doi: 10.4161/cbt.11.1.13730. [DOI] [PubMed] [Google Scholar]


