Abstract
Notch signaling is a conserved and widely expressed signaling pathway, which mediates various physiological processes including tumorigenesis. This study aims to explore the potential role and mechanism of notch1 interacting with KRT6B in the progression of RCC. The results indicated that the mRNA and protein expression of notch1 and KRT6 were significantly increased in tumor tissues, and highly positive correlation existed between notch1 and KRT6. Moreover, the patients with high notch1 expression had a significantly poorer prognosis than those of low expression patients. In vitro, KRT6 loss-of-function could inhibit the expression of notch1 and induce renal carcinoma cell death. Eventually, we found that renin inhibitor, aliskiren, could inhibit cell proliferation and decrease the expression of notch1 and KRT6 as well as regulate apoptosis-related protein expression in 786-O and ACHN renal carcinoma cell lines. These results suggested that the upregulation of notch1 and KRT6B might be involved in the development, progression and prognosis of human RCC, and aliskiren could suppress renal carcinoma cell proliferation, at least partially, through downregulation the expression of notch1 and KRT6.
Keywords: Renal carcinoma, notch1, KRT6, aliskiren
Introduction
Renal cell carcinoma (RCC) is the third most common urological cancer and accounts for approximately 3% of cancers in adults as well as 85% of all primary malignant kidney tumors [1]. Moreover, epidemiologic evidence demonstrates that more than 61,000 patients are diagnosed, and over 13,000 patients die annually from RCC [2]. The scientific efforts and significant progress in understanding the basic cellular event in RCC, but the precise mechanisms underlying renal carcinogenesis are still unknown. Therefore, it is extremely urgent to explore the novel therapeutic targets and strategies.
Aliskiren is the latest drug that blocks the renin-agiontension system (RAS), and its primary indication is as an antihypertensive agent [3]. Moreover, aliskiren is widely used in the clinic to treat renal injury due to locally high RAS activity [4]. Intriguingly, the 5-year disease-specific survival rate is significantly higher among the RCC patients administered RAS inhibitors after surgery than among their counterparts [5,6]. In experimental animal model, blockade of RAS decreases tumor proliferation and metastatic capacity of RCC [7]. Previous studies suggest that notch signaling and keratin 6 (KRT6) play a crucial role in the transformation and neoplastic proliferation of human malignancy [8,9]. KRT6 driven ODC expression to hair follicle keratinocytes enhances stemness and tumorigenesis by negatively regulating Notch [8]. However, there is little evidence to suggest a potential role for KRT6 in RCC. Moreover, the interaction between Notch1 and KRT6 in RCC is unknown. While, given that renin is the rate-limiting enzyme of the RAS, whether the RAS inhibitor by inhibiting notch1/KRT6 axis could show beneficial effects on RCC is not known.
In the present study, we identified KRT6 as a novel mediator of dysregulated notch signaling and showed that activation of notch1 in renal carcinoma cell upregulated the expression of KRT6. We also performed a detailed experimental analysis to investigate the interaction between notch1 and KRT6 in aliskiren-induced cell apoptosis and to identify notch1/KRT6 axis which might be targeted for the treatment of highly aggressive RCC.
Materials and methods
Patients and specimens
Thirty RCC tissues and matched adjacent non-tumor tissues were collected from Shanghai Seventh People’s Hospital (Shanghai, China) between Jan 2007 and September 2013. All patients recruited in this study were not subjected to preoperative radiotherapy or chemotherapy and diagnosed with RCC based on histopathological evaluation. Clinicopathological characteristics analysis was shown in Tables 1 and 2. All collected tissue samples were immediately stored at liquid nitrogen until use. Human samples were obtained with written informed consent from all patients. The study was approved by the Ethics Committee of the Shanghai Seventh People’s Hospital (Shanghai, China).
Table 1.
Correlation clinicopathological factors and notch1 expression levels in RCC patients
| Variable | Number of patients | Notch1-Low | Notch1-High | P value |
|---|---|---|---|---|
| Gender | 0.634 | |||
| Male | 17 | 9 | 8 | |
| Female | 13 | 7 | 6 | |
| Age (years) | 0.332 | |||
| < 60 | 22 | 11 | 11 | |
| ≥ 60 | 8 | 5 | 3 | |
| Tumor size (cm) | ||||
| < 3 | 12 | 6 | 6 | 0.197 |
| ≥ 3 | 18 | 10 | 8 | |
| TNM stages | 0.001 | |||
| I-II | 14 | 11 | 3 | |
| III-IV | 16 | 5 | 11 | |
| Lymph nodes metastasis | 0.001 | |||
| Negative (N) | 15 | 12 | 3 | |
| Positive (P) | 15 | 4 | 11 |
Table 2.
Univariate and multivariate regression analyses of parameters associated with prognosis of RCC patients
| Characteristics | Subset | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
|
| |||||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender | Male/Female | 1.126 (0.741-1.894) | 0.663 | ||
| Age | < 60/≥ 60 | 1.248 (0.874-2.047) | 0.449 | ||
| Tumor size (cm) | < 3/≥ 3 | 1.337 (0.834-1.985) | 0.462 | ||
| TNM stages | I-II/III-IV | 2.792 (1.645-5.069) | 0.001 | 2.143 (1.152-3.569) | 0.017 |
| Lymph nodes metastasis | N/P | 3.627 (1.942-6.241) | 0.001 | 2.425 (1.372-4.225) | 0.002 |
| Notch1 | High/Low | 2.779 (1.538-4.927) | 0.001 | 1.936 (1.062-3.258) | 0.029 |
Cell culture
The 786-O and ACHN renal carcinoma cell were obtained from the Chinese Academy of Sciences (Institute of Shanghai Cell Biology and Chinese Type Culture Collection, China), and maintained in DMEM (Dulbecco’s modified Eagle’s medium; Invitrogen), supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 100 units/ml penicillin, and 100 mg/ml streptomycin (Invitrogen) at 37°C in a humidified, 5% CO2, 95% air atmosphere. The medium was replenished every day.
Cell viability detection by CCK8
The 786-O and ACHN renal carcinoma cell (1.0 × 105/well) were plated and treated in 96-well plates (three wells per group) with aliskiren (0-100 μM) for 24 or 48 h, respectively. 10 μL of Cell Counting Kit-8 (CCK8, Dojindo) was added to the cells, and the viability of the cells was measured at 490 nm using an ELISA reader (BioTek, Winooski, VT, USA) according to the manufacturer’s instructions.
Immunofluorescence staining
The 786-O cells were incubated in 6-well plates and washed with PBS, fixed with 4% paraformaldehyde and permeabilised in 0.1% Triton X-100, then blocked non-specific binding sites with 1/100 diluted goat serum and incubated overnight at 4°C with anti-notch1 primary antibody (Santa Cruz Biotechnology, USA). After washed twice with PBS, cells were incubated for 1 h at 37°C with secondary antibody (Santa Cruz Biotechnology, USA). Cells were washed again and stained nuclear with DAPI. Samples were visualized by fluorescence microscopy (Carl Zeiss, Germany).
Gene silencing by small interfering RNA
For small interfering RNA (siRNA) experiments, RNA primers complementary to notch1 was designed and synthesized by the Shanghai Invitrogen Biotechnology Company. 786-O cells were transfected with the annealed RNA primer pair using Lipofectamine 2000 (Invitrogen Inc, USA) in accordance with the instructions provided by the manufacturer. The small interfering RNA with the following primers: KRT6, Forward 5’-GUCUCUGAGAGCGACAAGGCGUGACA-3’ and Reverse 5’-UGCAGACGUUGUCGCCGUGCACACUA-3’.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted by Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reaction mixture (20 μl) containing 2 μg of total RNA was reversely transcribed to cDNA by using PrimeScript RT-polymerase (Takara, Dalian, China). Quantitative PCR was performed on the cDNA using specific primers (Sangon, Shanghai, China). The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 9700). The cycling conditions were 30 s polymerase activation at 95°C followed by 40 cycles at 95°C for 5 s and 60°C for 30 s. PCR with the following primers: notch1, Forward 5’-GTCTCCATTGCTAGCCAC-3’ and Reverse 5’-ATGCAGCTGCAGGTCTTAAGAG-3’; KRT6, For-ward 5’-CCGACGATTCGGTACACCAT-3’ and Reverse 5’-GCGCAGGAGCTGCAGAGTTGTG-3’; GAPDH, Forward 5’-ACAGGGGAGGTGATAGCATT-3’ and Reverse 5’-GACCAAAAGCCTTCATACATCTC-3’. Glyceraldehyde-phosphate dehydrogenase (GAPDH) as an internal control was used to normalize the data to determine the relative expression of the target genes.
Western blotting
Protein extracted from tissues or cells were separated by 10% SDS-PAGE and transferred to PVDF membranes (Millipore, Germany). Membranes were blocked and then incubated with primary antibodies specific for notch1, KRT6, BAX, Bcl-2 and caspase8 (Santa Cruz Biotechnology, CA, USA). β-actin was used as protein loading control .The membranes were next incubated with the appropriate HRP (horseradish peroxidase)-conjugated antibody visualized and detected by chemiluminescence (Thermo, USA).
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software. Data were analyzed using independent two-tailed t test. Categorical data were analyzed using the two-side chi-square test. Overall survival was estimated by using Kaplan-Meier method, and univariate analysis was conducted by log-rank test. The Cox proportional hazards model was used in the multivariate analysis. Values of P < 0.05 were considered statistically significant.
Results
Identification of tumor tissues-enriched notch1 and KRT6B implicated in RCC patients
To demonstrate the pathogenesis of RCC, the RT-PCR and western blotting analysis were performed to determine the levels of notch1 and KRT6B in tumor tissues and corresponding nontumourous specimens from RCC patients. The mRNA and protein expression of notch1 and KRT6 were significantly higher in tumor tissues than those of corresponding nontumor adjacent tissues (Figure 1A-C). To assess the correlation of notch1 with KRT6B, the protein expression levels of notch1 and KRT6B in 32 RCC tumor tissues was measured. As shown in Figure 1D, the measurements obtained from tumor tissues were strongly correlated between notch1 and KRT6B (r = 0.848, P < 0.01). In addition, to understand the prognostic significance of notch1 upregulation in RCC, we analyzed the relationship between notch1 expression in RCC and patient survival and found that the patients with high notch1 expression had a significantly poorer prognosis than those of low expression patients (Figure 1E; Table 2, P < 0.001). In general, these results suggested that the upregulation of notch1 and KRT6B might be involved in the development, progression and prognosis of the majority of human RCC.
Figure 1.
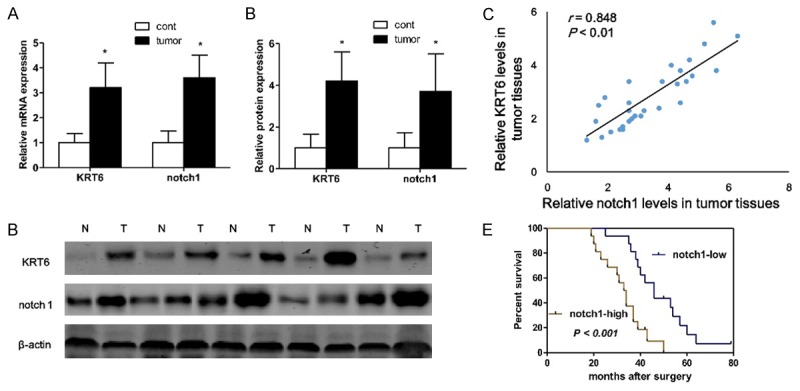
Identification of tumor tissues-enriched notch1 and KRT6B implicated in RCC patients. Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) (A) and western blotting (B) are used to detect the expression of notch1 and KRT6 in the tumor tissues and corresponding nontumourous specimens from RCC patients, and densitometric quantification for western blotting (C). Linear correlation plot of protein expression between notch1 and KRT6 in tumor tissues from RCC patients (D, n = 32). Kaplan-Meier survival curve and log-rank test are used to evaluate whether notch1 expression level is associated with overall survival rate. Patients are segregated into notch1-high group and notch1-low according to the median of notch1 expression in tumor tissues from RCC patients (E). *P < 0.05, versus normal control group. Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) is used to detect the expression of UCA1 (n = 40).
KRT6 regulates the expression of notch1 in renal carcinoma cell
To inhibit the function of notch1, 786-O cells were transfected with small interfering RNA (siRNA). Western blotting analysis demonstrated that the expression of KRT6 was dramatically inhibited by siRNA-KRT6 transfection (Figure 2A). Therefore, our data suggested that the siRNA experiments were successfully performed. As shown in Figure 2B, a dramatic decrease in notch1 expression was clearly confirmed by immunofluorescence staining in siRNA-KRT6 transfected renal carcinoma cells. In addition, western blotting showed that the protein expression of notch1 was significantly increased in siRNA-KRT6 treatment group compared to the control group (Figure 2C). Finally, we investigated the cell viability of 786-O cell lines transfected with siRNA-KRT6, the results indicated that siRNA-KRT6 transfection induced cell death in a time-dependent manner (Figure 2D).
Figure 2.
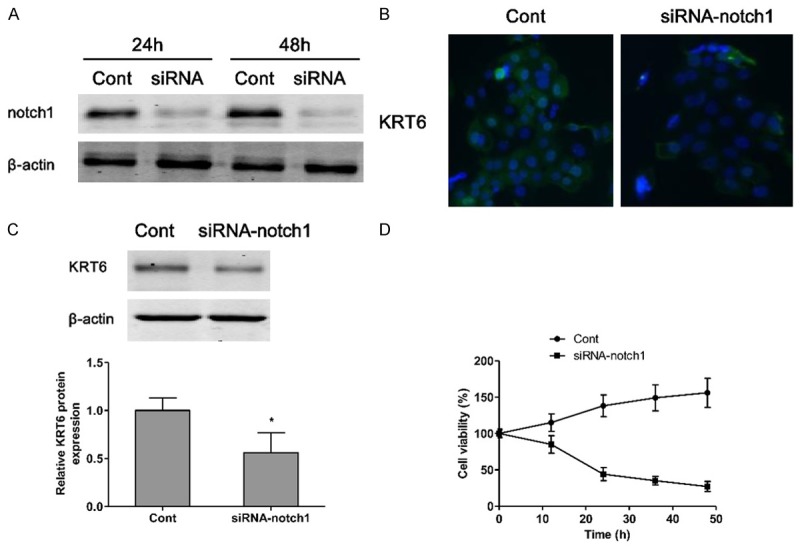
KRT6 regulates the expression of notch1 in renal carcinoma cell. The small interfering RNA is transfected into 786-O cells for 24 h or 48 h suppressing the expression of KRT6, which is measured by western blotting (A), Cont, si-RNA-Control; siRNA, siRNA-KRT6. Notch1 is visualized by fluorescent microscopy (B). The protein expression of notch1 is measured by western blotting (C). The cell viability of siRNA-KRT6 786-O cells is evaluated by CCK-8 (D). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05 versus control group.
Aliskiren inhibits cell proliferation
786-O and ACHN renal carcinoma cell viability were measured when cells were exposed to various concentrations of aliskiren (0-100 μM) for 24 and 48 h. The results showed that the growth of 786-O and ACHN renal carcinoma cell were inhibited with aliskiren (Figure 3A and 3B). The viabilities of 786-O cells treated with aliskiren were significantly lower than those of ACHN cells (Figure 3A and 3B). As shown in Figure 3A and 3B, the concentrations at which aliskiren inhibited 786-O cells growth by 50% (IC50) was 80 μM at 24 h or 20 μM at 48 h. The IC50 of growth inhibition of aliskiren for ACHN was above 100 μM at 24 h or 80 μM at 48 h. Moreover, treatment of 786-O and ACHN with aliskiren induced cell growth inhibition in a dose-dependent manner by using CCK8 assay. Interestingly, the mRNA and protein of notch1 and KRT6 were significantly inhibited both 786-O and ACHN cells by aliskiren with a dose-dependent manner (Figure 3B-D). In addition, western blotting results showed that treatment of 786-O and ACHN cells with increasing doses of aliskiren could significantly increase the pro-apoptotic protein BAX and caspase8 levels as well as decrease the anti-apoptotic protein Bcl-2 levels (Figure 4A and 4B).
Figure 3.
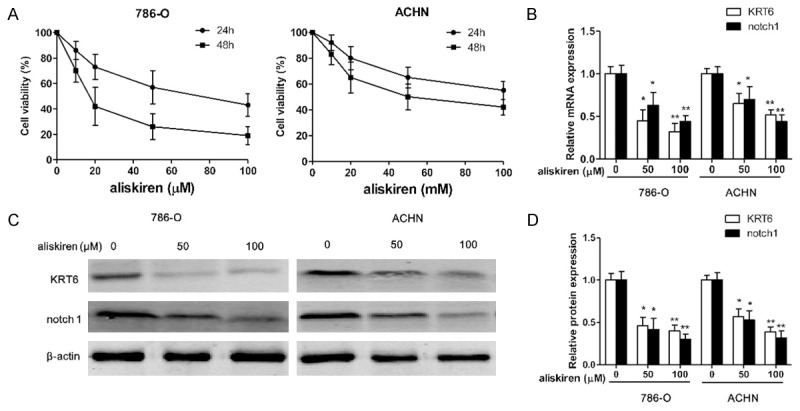
Aliskiren inhibits cell proliferation. The 786-O and ACHN cells are incubated with various concentrations of aliskiren (0-100 μM) for 24 h and 48 h respectively, and the cell viability is examined by CCK8 assay (A). The mRNA (B) and protein (C) expression of notch1 and KRT6 are measured by RT-PCR and western blotting respectively, and protein bands are quantified (D). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, **P < 0.01 versus control group.
Figure 4.
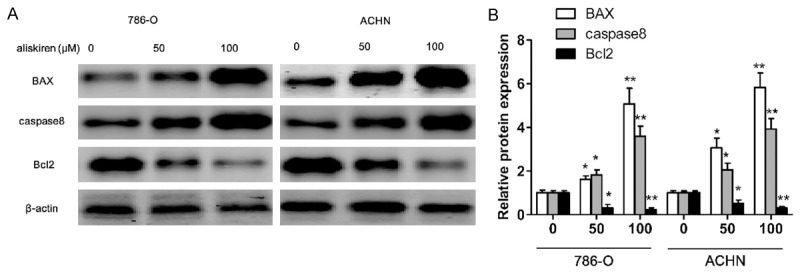
Aliskiren regulates apoptosis-related protein expression. The 786-O and ACHN cells are incubated with aliskiren (0, 50, 100 μM) for 48 h, the expression levels of BAX, Caspase8 and Bcl-2 are determined by western blotting (A), and protein bands are quantified in 786-O and ACHN cells (B). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, **P < 0.01 versus control group.
Discussion
It is known that notch signaling pathways play a role in multiple cancers [10]. Previous study demonstrates that high-level expression of notch signaling is associated with the metastasis of T1 stage RCC by stimulating the proliferation and migration of tumor cells [9], however, the exact role of notch signaling in conclusion, high-level expression of notch signaling is associated with the metastasis of T1 stage ccRCC by stimulating the proliferation and migration of tumor cells, kidney cancer is not clear yet, so we intended to explore the function of notch signaling in the RCC. In the present study we made several important observations. First, we studied the basic expression level of notch1 and KRT6 in 32 tumor tissues and adjacent nontumorous kidney tissue counterparts. Compared to corresponding nontumor adjacent tissues, notch1 and KRT6 expression were significantly elevated both in mRNA and protein expression. Next, the correlation of notch1 with KRT6B was confirmed, and the linear correlation plot of protein expression between notch1 and KRT6 in tumor tissues from RCC patients was highly positive correlation. Furthermore, we analyzed the relationship between notch1 expression in RCC and patient survival and found that the patients with high notch1 expression had a significantly poorer prognosis than those of low expression patients. These results suggested that notch1 signaling provided a new angle for the treatment of renal cancer in clinic.
Recent studies have indicated that RAS inhibitors may have a protective effect against angiogenesis and prevent carcinogenesis [7,11,12]. In murine orthotopic model of RCC, losartan and captopril alone or in combination treatment can significantly reduce tumor growth and the number of lung metastases [7]. Captopril and losartan also suppress vascular endothelial growth factor-A expression in lung carcinoma cells under hypoxia [13]. Intriguingly, (Pro) renin receptor is crucial for Wnt/β-catenin-dependent genesis of pancreatic ductal adenocarcinoma [14]. Based on the above research results, we postulated that aliskiren, as a renin inhibitor, might possess the anti-cancer ability in RCC. In our study, we found that the growth of renal carcinoma cell was inhibited with aliskiren treatment, and the underlying mechanism was mediated, at least partially, through downregulation the expression of notch1 and KRT6. Recent studies have identified a high expression of KRT6 in triple-negative breast cancer [15] and urothelial cancer [16]. Moreover, KRT6 drives ornithine decarboxylase (ODC) expression to hair follicle keratinocytes enhances stemness and tumorigenesis by negatively regulating notch signaling [8]. In contrast to that KRT6 loss-of-function inhibited the expression of notch1 and renal carcinoma cell proliferation. These studies suggested that KRT6 down-expression might be involved in the proliferation of renal carcinoma by down-regulating notch1 expression.
In conclusion, upregulation of notch1 and KRT6B might be involved in the development, progression and prognosis of the majority of human RCC, and aliskiren might block the growth of renal carcinoma cell through suppression KRT6 as well as downregulation the expression of notch1.
Disclosure of conflict of interest
None.
References
- 1.Slaby O, Jancovicova J, Lakomy R, Svoboda M, Poprach A, Fabian P, Kren L, Michalek J, Vyzula R. Expression of miRNA-106b in conventional renal cell carcinoma is a potential marker for prediction of early metastasis after nephrectomy. J Exp Clin Cancer Res. 2010;29:90. doi: 10.1186/1756-9966-29-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho TH, Liu XD, Huang Y, Warneke CL, Johnson MM, Hoang A, Tamboli P, Wang F, Jonasch E. The impact of FGFR1 and FRS2alpha expression on sorafenib treatment in metastatic renal cell carcinoma. BMC Cancer. 2015;15:304. doi: 10.1186/s12885-015-1302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JT, Chen KP, Guan T, Zhang S. Effect of aliskiren on cardiovascular outcomes in patients with prehypertension: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2015;9:1963–1971. doi: 10.2147/DDDT.S75111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Qiu L, Howard A, Solis N, Li C, Wang X, Kopp JB, Levi M. Protective effects of aliskiren and valsartan in mice with diabetic nephropathy. J Renin Angiotensin Aldosterone Syst. 2014;15:384–395. doi: 10.1177/1470320313507123. [DOI] [PubMed] [Google Scholar]
- 5.Miyajima A, Yazawa S, Kosaka T, Tanaka N, Shirotake S, Mizuno R, Kikuchi E, Oya M. Prognostic Impact of Renin-Angiotensin System Blockade on Renal Cell Carcinoma After Surgery. Ann Surg Oncol. 2015;22:3751–3759. doi: 10.1245/s10434-015-4436-0. [DOI] [PubMed] [Google Scholar]
- 6.McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik OP, Sabbisetti VS, Bhatt RS, Simantov R, Choueiri TK. Angiotensin System Inhibitors and Survival Outcomes in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2015;21:2471–9. doi: 10.1158/1078-0432.CCR-14-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araujo WF, Naves MA, Ravanini JN, Schor N, Teixeira VP. Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol Oncol. 2015;33:389, e1–7. doi: 10.1016/j.urolonc.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Arumugam A, Weng Z, Chaudhary SC, Afaq F, Elmets CA, Athar M. Keratin-6 driven ODC expression to hair follicle keratinocytes enhances stemness and tumorigenesis by negatively regulating Notch. Biochem Biophys Res Commun. 2014;451:394–401. doi: 10.1016/j.bbrc.2014.07.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai Q, Ma X, Huang Q, Liu S, Shi T, Zhang C, Zhu M, Zhang Y, Wang B, Ni D, Li H, Zheng T, Zhang X. High-level expression of Notch1 increased the risk of metastasis in T1 stage clear cell renal cell carcinoma. PLoS One. 2012;7:e35022. doi: 10.1371/journal.pone.0035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 11.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JW. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 12.de Araujo Junior RF, Leitao Oliveira AL, de Melo Silveira RF, de Oliveira Rocha HA, de Franca Cavalcanti P, de Araujo AA. Telmisartan induces apoptosis and regulates Bcl-2 in human renal cancer cells. Exp Biol Med (Maywood) 2015;240:34–44. doi: 10.1177/1535370214546267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan L, Feng Y, Wan HY, Ni L, Qian YR, Guo Y, Xiang Y, Li QY. Hypoxia induces dysregulation of local renin-angiotensin system in mouse Lewis lung carcinoma cells. Genet Mol Res. 2014;13:10562–10573. doi: 10.4238/2014.December.12.19. [DOI] [PubMed] [Google Scholar]
- 14.Shibayama Y, Fujimori T, Nguyen G, Hirose T, Totsune K, Ichihara A, Kitada K, Nakano D, Kobori H, Kohno M, Masaki T, Suzuki Y, Yachida S, Nishiyama A. (Pro)renin receptor is crucial for Wnt/beta-catenin-dependent genesis of pancreatic ductal adenocarcinoma. Sci Rep. 2015;5:8854. doi: 10.1038/srep08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inanc M, Ozkan M, Karaca H, Berk V, Bozkurt O, Duran AO, Ozaslan E, Akgun H, Tekelioglu F, Elmali F. Cytokeratin 5/6, c-Met expressions, and PTEN loss prognostic indicators in triple-negative breast cancer. Med Oncol. 2014;31:801. doi: 10.1007/s12032-013-0801-7. [DOI] [PubMed] [Google Scholar]
- 16.Fichtenbaum EJ, Marsh WL Jr, Zynger DL. CK5, CK5/6, and double-stains CK7/CK5 and p53/CK5 discriminate in situ vs invasive urothelial cancer in the prostate. Am J Clin Pathol. 2012;138:190–197. doi: 10.1309/AJCP5ZC4GQVNWTYR. [DOI] [PubMed] [Google Scholar]


