Abstract
Background: MicroRNAs (miRNAs) are a group of small non-coding RNAs that play important roles in the pathogenesis of human diseases by negatively regulating gene expression. The aim of this study was to explore the effect of miR-204 on cell proliferation migration and invasion in T-cell acute lymphoblastic leukaemia (T-ALL). Method: miR-204 expression was determined in bone marrow samples from 32 leukemia patients and 32 healthy controls by quantitative real-time PCR (qRT-PCR). The effect of miR-204 on cell proliferation was evaluated by CCK8 assay, cell migration and invasion were evaluated by transwell migration and invasion assays, In addition, the regulation of SOX4 by miR-204 was evaluated by luciferase reporter assay and western blot. Results: our results revealed that miR-204 was low expressed in T-ALL. Cell proliferation assay showed that the cell proliferation ability was inhibited by miR-204 mimics. Moreover, migration and invasion assay suggested that overexpression of miR-204 could significantly suppressed the migration and invasion ability of T-ALL cells. Luciferase reporter assay confirmed that miR-204 directly bound to the 3’ untranslated region of SOX4, and western blot suggested that miR-204 inhibited the expression of SOX4 at the protein levels. Conclusions: Our findings indicated that miR-204 negatively regulates SOX4 and inhibited proliferation, migration and invasion of T-ALL cell lines. Thus, miR-204 might represent a potential therapeutic target for T-ALL intervention.
Keywords: miR-204, T-cell acute lymphoblastic leukaemia, SOX4
Introduction
Acute lymphoblastic leukemia (ALL) is a hematologic malignancy that affects both adults and children [1]. T cell ALL (T-ALL) represents about 15% of ALL cases in children and 25% of adult ALL cases [2]. The use of conventional cancer therapies has resulted in a complete remission rate of 85% and a high cure rate in childhood T-ALL, but adult T-ALL patients are at increased risk of both early BM recurrence and CNS relapse [3,4]. Despite recent advances in clinical and experimental oncology, the prognosis of T-ALL is still unfavorable, with a 5-year overall survival rate of approximately 45% for adult T-ALL patients [5]. Thus, it is necessary to develop novel diagnostic and therapeutic strategies to treat T-ALL patients.
MicroRNAs (miRNAs) are a class of small (19-25 nt), non-coding RNAs that regulate gene expression by base pairing with target mRNAs in the 3’ untranslated region (3’-UTR), leading to mRNA cleavage or translational repression [6]. In the recent years, Emerging evidence showed that miRNAs play essential roles in tumor cell biological processes, such as cell differentiation, proliferation, apoptosis, migration and invasion [7,8]. For example, Yang et al showed that miR-506 was downregulated in clear cell renal cell carcinoma and inhibited renal cancer cell growth and metastasis via suppressing FLOT1 expression [9]. Zheng et al suggested that miRNA-148a was decreased expression in gastric cancer and inhibited gastric cancer cell invasion and metastasis by downregulating ROCK1 [10]. Liu et al reported that miRNA-196a was overexpressed in non-small cell lung cancer and promoted non-small cell lung cancer cell proliferation and invasion through targeting HOXA5 [11]. However, to our knowledge the effect of miR-204 in T-ALL remains unclear.
In this study, our results revealed that miR-204 was downregulated in T-ALL. Ectopic expression of miR-204 inhibited T-ALL cells proliferation, migration, and invasion. Furthermore, we identified SOX4 as a direct target of miR-204 and showed that miR-204 function as a tumor suppressor by downregulating SOX4, indicating miR-204 may represent a potential therapeutic target for T-ALL intervention.
Materials and methods
Patients
This study included 32 patients with confirmed T-ALL. Their diagnosis was based on routine morphological evaluation, immunophenotyping and cytochemical smears using the French-American-British classification. Peripheral blood was obtained from patients and healthy volunteers. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki and with approval of the Clinical Research Ethics Board of the Xinxiang Central Hospital.
Cell culture and transfection
Human acute T-lymphoblastic leukemia cell lines Jurkat and MOLT-3 were purchased from the American Type Culture Collection (ATCC). All cells was cultured in RPMI 1640 media (Life Technologies) and supplemented with 10% fetal bovine serum (FBS). Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C.
Jurkat and MOLT-3 cell lines were seeded in 24-well plates at 3×105 cells/wells and incubated overnight. Transfection of the miR-204 mimic or miR-NC was taken using Lipofectamine 2000 transfection reagent (Invitrogen). Transfection efficiency was monitored by qRT-PCR.
Cell proliferation assay
Cells were seeded into 96-well plates (5×103 cells per well). Cell viability was assessed by cell-counting kit-8 assay (CCK-8, Beyotime Institute of Biotechnology). The absorbance of each well was read on a spectrophotometer (Thermo) at 450 nm (A450). Three independent experiments were performed in quintuplicate.
Cell migration and invasion assays
For the determination of cell invasion, transwell chambers were coated with 30 μl Matrigel, then incubated at 37°C for 40 min. In transwell assays with and without Matrigel, cells were trypsinized and then seeded in chambers at the density of 5×104 cells/well at 48 h after transfection. These cells were cultured in RPMI 1640 medium with 2% serum. Meanwhile 500 μl of medium supplemented with 10% FBS was injected into the lower chambers. After harvest, the inserts were fixed and stained in a dye solution containing 0.1% crystal violet and 20% methanol. Cells adhering to the lower membrane of the inserts were imaged with microscope (Olympus).
Luciferase reporter assay
The 3’-UTR untranslated region of SOX4 was amplified by PCR and cloned downstream of the firefly luciferase gene in the pGL3 vector (Promega). The vector was named wild-type (Wt) 3’-UTR. Site-directed mutagenesis of the miR-204 binding site in SOX4 3’-UTR was performed using the Quick change site-directed mutagenesis kit (Stratagene) and named mutant (Mut) 3’-UTR. For reporter assays, Wt or Mut 3’-UTR vector and the control vector pRL-CMV (Promega) were cotransfected. The luciferase assay was performed by using the dual Luciferase reporter assay system (Promega) 48 h after transfection.
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted with Trizol (Invitrogen) according to the manufacturer’s instructions, and reverse-transcribed into cDNA with the Reverse Transcriptase M-MLV (TaKaRa). Real-time PCR was performed using a SYBR Premix Ex Taq™ kit (TaKaRa) on the iQ5 real-time PCR Detection System (Bio-Rad). PCR primers used were as follows: SOX4 forward, 5’-CTTGACATGATTAGCTGGCATGATT-3’ and reverse 5’-CCTGTGCAATATGCCGTGTAGA-3’, GAPDH forward, 5’-CCCACTCCTCCACCTTTGAC-3’ and reverse, 5’-ATGAGGTCCACCACCCTGTT-3’. For analysis of microRNA expression by qRT-PCR, reverse transcription and PCR were carried out using Bulge-LoopTM miRNA qPCR Primer Set for hsa-miR-204 (RiboBio), and U6 snRNA (RiboBio) according to the manufacturer’s instructions. Expression of SOX4, relative to GAPDH and miR-204, relative to U6, was determined using the 2-ΔΔCT method.
Western blot
Whole-cell proteins were collected with RIPA Buffer (Pierce) and separated by 12% SDS-PAGE before transfer. The PVDF membrane (Merck Millipore) was blocked with 5% non-fat milk and incubated with rabbit anti-SOX4 monoclonal antibody (Abcam). A β-Actin antibody (Abcam) was used as control for normalization. The results were obtained by Immobilon™ Western Chemiluminescent HRP Substrate (Merck Millipore).
Statistical analysis
Statistical analyses were performed using SPSS version 18.0. All data are presented as the mean ± SD from at least three independent experiments. Differences between groups were analyzed using Student’s t test or one-way ANOVA analysis. P value less than 0.05 was considered statistically significant.
Results
miR-204 is highly expressed in T-ALL
To investigate the potential link between miR-204 and T-ALL, in the present study, we first analyzed the expression of miR-204 in normal T cells and T-leukemic cells from healthy volunteers (NC) and T-ALL patients (T-ALL) by qRT-PCR. Our data showed that miR-204 was significantly upregulated in T-leukemic cells compared to the normal T cells samples (P<0.05, Figure 1). These data indicated that miR-204 might have an important role in T-cell leukemia progression.
Figure 1.

miR-204 expression is downregulated in T-ALL. miR-204 expression in T-ALL patients (T-ALL) (n=32) and healthy volunteers (NC) (n=32) relative to U6 detected by using qRT-PCR. *P<0.05.
miR-204 inhibits T-leukemic cell proliferation, migration and invasion
To investigate the function of miR-204 in the T-ALL progression, Jurkat and MOLT-3 cells were transfected with miR-204 mimics, The effect of miR-204 mimics was confirmed by qRT-PCR (P<0.05, Figure 2A). Cell proliferation assay showed that overexpression of miR-204 significantly inhibited the proliferation of the Jurkat and MOLT-3 cells (P<0.05, Figure 2B). Moreover, migration and invasion assay indicated that overexpression of miR-204 could significantly suppress the migration and invasion capability of Jurkat and MOLT-3 cells compared with the miR-NC group (P<0.05, Figure 2C and 2D).
Figure 2.
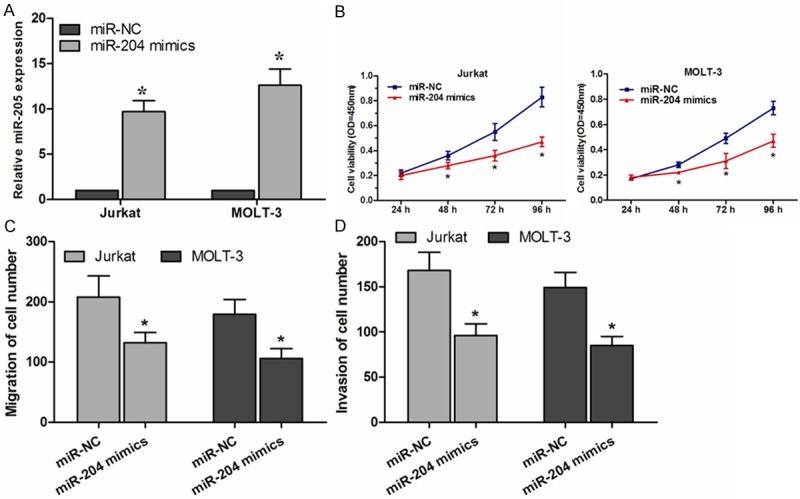
miR-204 inhibits cell proliferation, migration and invasion in vitro. A. qRT-PCR assay confirmed that miR-204 was overexpressed in miR-204 mimic transfected cells, compared with those transfected with miR-NC. B. miR-204 inhibited Jurkat and MOLT-3 cell proliferation, as determined by CCK-8 assay. C. miR-204 suppressed migration ability of Jurkat and MOLT-3 cells, as determined by transwell migration assay. D. miR-204 inhibited invasion capability of Jurkat and MOLT-3 cells, as determined by transwell invasion assay. *P<0.05.
miR-204 targets and negatively regulates SOX4 in T-ALL cells
Next, we explored the molecular mechanism through which miR-204 regulated T-leukemic cell progression. In the present study, TargetScan were used to search for potential miR-204 target genes. Among the mRNAs containing miR-204 recognition sites in their 3’-UTRs, we focused on SOX4, a protein involved in tumorigenesis and progression of acute myeloid leukemia [12,13]. To verify that SOX4 is a direct target of miR-204, SOX4 wild-type (Wt) or mutant 3’-UTR was subcloned into a luciferase reporter vector and co-transfected with miR-204 mimics or scrambled control into Jurkat cells (Figure 3A). MiR-204 significantly inhibited the luciferase activity of the SOX4 Wt 3’-UTR but not that of the mutant in Jurkat cells (Figure 3B). Furthermore, overexpression of miR-204 significantly inhibited SOX4 protein expression levels (P<0.05, Figure 3C). These evidences suggested that SOX4 is a target gene of miR-204 in T-ALL cells.
Figure 3.
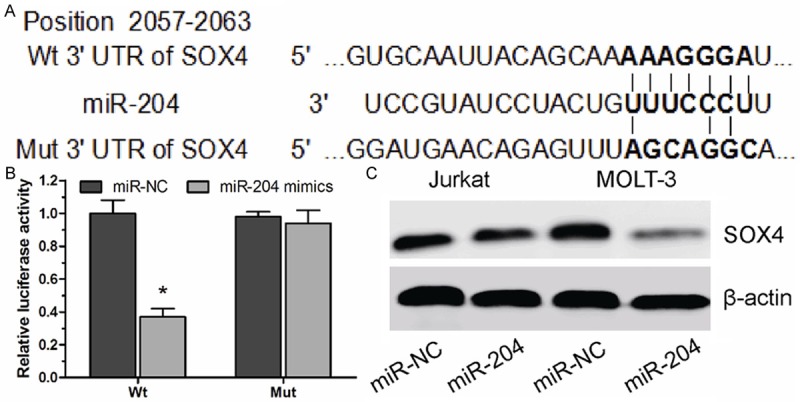
SOX4 is directly regulated by miR-204. A. Predicted binding of miR-204 to 3’-UTRs of SOX4. B. Luciferase assays in Jurkat cells with wild-type (Wt) or mutant (Mut) SOX4 3’-UTR vectors and miR-204 mimic or miR-NC. C. Western blot for SOX4 in Jurkat and MOLT-3 cells after transfection with miR-204 mimics or miR-NC. β-actin is a loading control. *P<0.05.
Knockdown of SOX4 promotes cell proliferation, migration and invasion
Our results suggested that miR-204 could inhibit T-ALL cells proliferation, migration and invasion, a specific siRNA introduced into T-ALL cells to determine whether downregulation of SOX4 had a phenocopy of over-expression of miR-204. When si-SOX4 was used, the expression of SOX4 in Jurkat and MOLT-3 cells was significantly decreased determined by qRT-PCR (P<0.05, Figure 4A). Furthermore, Decreased expression of SOX4 suppressed Jurkat and MOLT-3 cell proliferation (P<0.05, Figure 4B). Cell migration and invasion capability were also decreased in Jurkat and MOLT-3 cells transfected with si-SOX4 compared with si-NC group (P<0.05, Figure 4C, 4D). These data indicated that knockdown SOX4 expression could mimic the effect of miR-204 in T-ALL cells. Negative regulation of SOX4 by miR-204 was, at least in part, responsible for miR-204-induced T-ALL cell progression.
Figure 4.
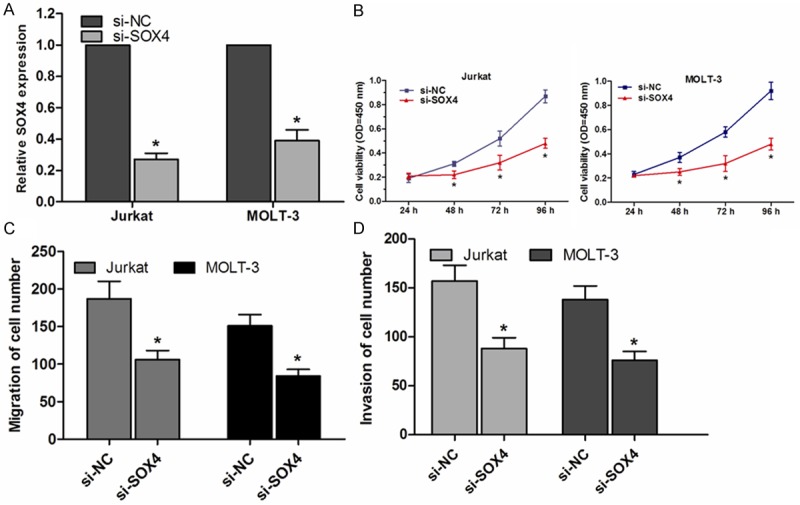
Knockdown of SOX4 inhibits cell proliferation, migration and invasion in vitro. A. qRT-PCR assay confirmed that SOX4 was downregulated in si-SOX4 transfected cells, compared with those transfected with si-NC. B. Decreased expression of SOX4 inhibited Jurkat and MOLT-3 cell proliferation, as determined by CCK-8 assay. C. Knockdown SOX4 expression suppressed migration ability of Jurkat and MOLT-3 cells, as determined by transwell migration assay. D. Downregulated SOX4 expression inhibited invasion capability of Jurkat and MOLT-3 cells, as determined by transwell invasion assay. *P<0.05.
Discussion
T-cell acute lymphoblastic leukemia (T-ALL) is a significant health burden world-wide [14]. Despite the significant advancements in treatment options, improvements in T-ALL patient survival have been limited owing to limited capacity for optimal therapeutic decision-making [15]. In recent years, numerous miRNAs have been identified to be major transcriptional regulators involved in many biological processes such as cell differentiation and carcinogenesis. The aberrant expression of miR-204 is a frequent event in various cancers, indicating that miR-204 play a critical role in tumorigenesis. For example, Wu et al found that miRNA-204 was downregulated in retinoblastoma and inhibited retinoblastoma cell proliferation and metastasis by suppressing CyclinD2 and MMP-9 expression [16]. Shi et al revealed that miRNA-204 inhibited proliferation, migration, invasion and epithelial-mesenchymal transition in osteosarcoma cells via targeting Sirtuin 1 [17]. Xia et al showed that miR-204 functions as a tumor suppressor by regulating SIX1 in non-small cell lung cancer [18]. However, the expression level and downstream target genes of miR-204 as well as its biological roles in T-ALL are still unknown.
The sex determining region Y-box 4 (SOX4) gene is a developmental transcription factor which belongs to the C subgroup of SRY-related HMG box (SOX) transcription factor family [19]. Recent studies suggested that SOX4 played critical roles in tumor development and progression [20]. For example, Song et al showed that SOX4 expression was significantly increased in breast cancer and positively correlated with the malignant status. Furthermore, they suggested that SOX4 was an independent poor prognostic factor for breast cancer patients [21]. Yang et al found that estrogen induced androgen-repressed SOX4 expression to promote progression of prostate cancer cells [22]. Kang et al demonstrated that miR-129-2 could suppress proliferation and migration of esophageal carcinoma cells through downregulation of SOX4 expression [23].
In our study, we found miR-204 was significantly decreased in T-leukemic cells compared to the T-cell samples. Then, we showed that ectopic expression of miR-204 could inhibit cell proliferation, migration and invasion of T-ALL cells, indicating that miR-204 may act as a tumor suppressor in the regulation of cell growth and metastasis in T-ALL cells. However, the underlying mechanisms were still unknown. In further study, we showed that SOX4 was a direct target of miR-204 and restoration of miR-204 could decrease SOX4 expression in T-ALL cells. Moreover, knockdown SOX4 expression by siRNA has the similar effect of miR-204 in T-ALL cells. Taken together, these data demonstrated that miR-204 impacted on T-ALL cells partially by inactivation of SOX4.
In conclusion, our findings showed that miR-204 was decreased in T-ALL, and its ectopic expression suppressed cell proliferation, migration and invasion. The tumor-suppressor function of miR-204 was mediated by the downregulation of its downstream target gene SOX4. These data suggested that miR-204 could be a potential therapeutic target for the treatment of T-ALL.
Disclosure of conflict of interest
None.
References
- 1.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 2.Onciu M. Acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:655–674. doi: 10.1016/j.hoc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, Sandlund JT, Hudson M, Relling M, Evans WE, Pui CH. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005;103:368–376. doi: 10.1002/cncr.20743. [DOI] [PubMed] [Google Scholar]
- 4.Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, Ebell W, Escherich G, Schrappe M, Klingebiel T, Fengler R, Henze G, von Stackelberg A. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J. Clin. Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 5.Asnafi V, Buzyn A, Le Noir S, Baleydier F, Simon A, Beldjord K, Reman O, Witz F, Fagot T, Tavernier E, Turlure P, Leguay T, Huguet F, Vernant JP, Daniel F, Béné MC, Ifrah N, Thomas X, Dombret H, Macintyre E. NOTCH1/FBXW7 mutation identifies a large subgroup with favorable outcome in adult T-cell acute lymphoblastic leukemia (T-ALL): a Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) study. Blood. 2009;113:3918–3924. doi: 10.1182/blood-2008-10-184069. [DOI] [PubMed] [Google Scholar]
- 6.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 7.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 8.Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends Mol Med. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Yang FQ, Zhang HM, Chen SJ, Yan Y, Zheng JH. MiR-506 Is Down-Regulated in Clear Cell Renal Cell Carcinoma and Inhibits Cell Growth and Metastasis via Targeting FLOT1. PLoS One. 2015;10:e0120258. doi: 10.1371/journal.pone.0120258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Tian Q, Wu J, Ye Y, Wang Q, Long Z, Zhou Y, Du C, He X, Shi Y. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 11.Liu XH, Lu KH, Wang KM, Sun M, Zhang EB, Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, De W, Wang ZX. MicroRNA-196a promotes non-small cell lung cancer cell proliferation and invasion through targeting HOXA5. BMC Cancer. 2012;12:348. doi: 10.1186/1471-2407-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung TK, Leung AYH, So CWE. Sox4you: A New Player in C/EBPα Leukemia. Cancer Cell. 2013;24:557–559. doi: 10.1016/j.ccr.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Sandoval S, Kraus C, Cho EC, Cho M, Bies J, Manara E, Accordi B, Landaw EM, Wolff L, Pigazzi M, Sakamoto KM. Sox4 cooperates with CREB in myeloid transformation. Blood. 2012;120:155–165. doi: 10.1182/blood-2011-05-357418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiaretti S, Foà R. T-cell acute lymphoblastic leukemia. Haematologica. 2009;94:160–162. doi: 10.3324/haematol.2008.004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Vlierberghe P, Palomero T, Khiabanian H, Van der Meulen J, Castillo M, Van Roy N, De Moerloose B, Philippé J, González-García S, Toribio ML, Taghon T, Zuurbier L, Cauwelier B, Harrison CJ, Schwab C, Pisecker M, Strehl S, Langerak AW, Gecz J, Sonneveld E, Pieters R, Paietta E, Rowe JM, Wiernik PH, Benoit Y, Soulier J, Poppe B, Yao X, Cordon-Cardo C, Meijerink J, Rabadan R, Speleman F, Ferrando A. PHF6 mutations in T-cell acute lymphoblastic leukemia. Nat Genet. 2010;42:338–342. doi: 10.1038/ng.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu XJ, Zeng Y, Wu S, Zhong J, Wang Y, Xu J. MiR-204, down-regulated in retinoblastoma, regulates proliferation and invasion of human retinoblastoma cells by targeting CyclinD2 and MMP-9. FEBS Lett. 2015;589:645–650. doi: 10.1016/j.febslet.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y, Huang J, Zhou J, Liu Y, Fu X, Li Y, Yin G, Wen J. MicroRNA-204 inhibits proliferation, migration, invasion and epithelial-mesenchymal transition in osteosarcoma cells via targeting Sirtuin 1. Oncol Rep. 2015;34:399–406. doi: 10.3892/or.2015.3986. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Zhu Y, Ma T, Pan C, Wang J, He Z, Li Z, Qi X, Chen Y. miR-204 functions as a tumor suppressor by regulating SIX1 in NSCLC. FEBS Lett. 2014;588:3703–3712. doi: 10.1016/j.febslet.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 19.van de Wetering M, Oosterwegel M, van Norren K, Clevers H. Sox-4, an Sry-like HMG box protein, is a transcriptional activator in lymphocytes. EMBO J. 1993;12:3847. doi: 10.1002/j.1460-2075.1993.tb06063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina PP, Castillo SD, Blanco S, Sanz-Garcia M, Largo C, Alvarez S, Yokota J, Gonzalez-Neira A, Benitez J, Clevers HC, Cigudosa JC, Lazo PA, Sanchez-Cespedes M. The SRY-HMG box gene, SOX4, is a target of gene amplification at chromosome 6p in lung cancer. Hum Mol Genet. 2009;18:1343–1352. doi: 10.1093/hmg/ddp034. [DOI] [PubMed] [Google Scholar]
- 21.Song GD, Sun Y, Shen H, Li W. SOX4 overexpression is a novel biomarker of malignant status and poor prognosis in breast cancer patients. Tumor Biol. 2015;36:4167–73. doi: 10.1007/s13277-015-3051-9. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Wang J, Wang L, Shen C, Su B, Qi M, Hu J, Gao W, Tan W, Han B. Estrogen induces androgen-repressed SOX4 expression to promote progression of prostate cancer cells. Prostate. 2015;75:1363–75. doi: 10.1002/pros.23017. [DOI] [PubMed] [Google Scholar]
- 23.Kang M, Li Y, Liu W, Wang R, Tang A, Hao H, Liu Z, Ou H. miR-129-2 suppresses proliferation and migration of esophageal carcinoma cells through downregulation of SOX4 expression. Int J Mol Med. 2013;32:51–58. doi: 10.3892/ijmm.2013.1384. [DOI] [PubMed] [Google Scholar]


