Abstract
This study aims to explore the relationship between Sirt3 expression and lipid accumulation in macrophages by inducing mitochondrial IDH2 deacetylation. In this study, Sirt3 interference and overexpression lentiviral vectors were constructed. Macrophages collected from C57BL/6J mice by peritoneal lavage were used to construct Sirt3 gene interference and overexpression models, and cultured in medium containing 1 mg/ml ox-LDL for 72 h to observe the enrichment of ox-LDL. Reverse transcription PCR was used to detect the expression of Sirt3 mRNA, western blot to detect Sirt3 and acetylated IDH2 proteins, and Nile Red staining and flow cytometry to detect intracellular lipids in macrophages. The results indicated that as compared to Sirt3 overexpressed and normal groups, the acetylation of IDH2 and accumulation of ox-LDL were significantly higher in the Sirt3 inhibited group. In conclusion, the expression of Sirt3 can inhibit lipid accumulation in macrophages by inducing mitochondrial IDH2 deacetylation.
Keywords: Macrophages, mitochondria, Sirt3, IDH2
Introduction
Macrophages regulate lipid metabolism and inflammation by taking up large amounts of oxidized low-density lipoprotein cholesterol (ox-LDL) leading to intracellular lipid accumulation and formation of foam cells [1]. Monocytes in peripheral blood are induced by various cytokines inside blood vessel walls to differentiate into macrophages that are released to deal with the redundant lipid from ruptured foam cells. When more foam cells are formed and ruptured, this vicious cycle results in the formation of fatty necrotic matter, consisting of lipids, cholesterol crystals and cell debris [2]. Therefore, enrichment and metabolic disorders of lipids in macrophages is the major focus of atherosclerotic disease research and intervention.
Mitochondrial cholesterol transport is the key step to control the transport of cholesterol out of the macrophages [3], and is associated with the ATP combined transporters (ABC) [4,5], which are critical molecules for cholesterol discharge [6-9], whose function is dependent on isocitrate dehydrogenase 2 (IDH2) of the Krebs cycle [10,11]. Therefore, effective supply of energy is key for macrophage lipid processing. Additionally, the types of mitochondrial protein acetylation modifications include almost all the major metabolic pathways involved in mitochondrial proteins [12]. Therefore, we hypothesize that acetylation of IDH2 may affect the Krebs cycle.
Sirt3, a member of sirtuins (SIRTs), is a major regulatory enzyme in mitochondrial protein acetylation [13,14]. Studies have shown that Sirt3 plays a role in the nucleus or the mitochondria based on cellular stress [15-17]. The regulation of Sirt3 gene in the liver, heart and skeletal muscle cells has been established [18-20]. However, its role in lipid accumulation in macrophages through inhibition of IDH2 acetylation remains unclear, and was examined in this paper.
Materials and methods
Animals
Male and female C57BL/6J mice were purchased from the Animal Center of Chongqing Medical University, and individually housed. Details of the methods used to house and feed the mice have been previously described [21].
Preparation of cells
The macrophages were isolated from C57BL/6J mice by intraperitoneal lavage and cultured in 37°C, 5% CO2 incubator for more than 24 hours to adapt to the environment. The cells were centrifuged and seeded at 3-5 × 104 cells/well in 24-well culture plates in RPMI1640 (Gibco, USA) with 100 U/ml each of penicillin and streptomycin (Huabeizhiyao, China) in 37°C, 5% CO2 incubator.
Construction of Sirt3 siRNA lentiviral vectors for interference and overexpression
The Sirt3 sequence (NM_022433.2) of C57BL/6J mouse was obtained from Gene Bank, and the target primer sequences for interference and overexpression were designed based on a previous study [22]. After ligation with GPH, the products were transfected into DH5α cells, and then identified by PCR and enzyme digestion. The positive clones were stored after sequence analysis.
Preparation of macrophages integrated with Sirt3 siRNA
After seeding the macrophages in 24-well plates for 24 hours, the Sirt3 siRNA lentiviral vectors were added to the wells for integration into the cells. Macrophages integrated with GPH lentiviral vectors only were used as the control group. After 24 hours, fresh culture medium containing 1 mg/ml ox-LDL (Sigma, USA) was added and the cells were cultured for 72 hours.
Flow cytometry detection
The concentration of ox-LDL in macrophages was analyzed using Nile red dye. Macrophages transfected with Sirt3 lentiviral vector were digested with trypsin (Sigma, USA), washed twice with PBS (Zhongshan, China), and 100 μl PBS and 10 μl Nile red dye (R&D, USA) were added. The cells were kept in a water bath at 37°C for 5 min, and then detected by flow cytometry.
Sirt3 mRNA detection
Sirt3 RNA was collected using TRIzol (ROCHE, Germany) from macrophages transfected with Sirt3 lentiviral vector and cultured in medium containing 1 mg/ml ox-LDL for 72 hours. The first-strand cDNA was synthesized using M-MuLV reverse transcriptase (dNTP Mixture M-MuLV Reverse Transcriptase, Promega), DEPC (Sigma, USA), and amplified by PCR (Taq, DNaseI, RNase free, TaKaRa). 8 µl of each PCR product was used for electrophoresis, with β-actin as the internal gene. The primers for Sirt3 were: GCTGCTTCTGCGGCTCTATAC and GAAGGACCTTC GACAGACCGT and for β-actin were: ATATCGCTGCGCTGGTCGTC and GCT CTCCCTCACGCCATCCT.
Analysis of Sirt3 protein and acetylated IDH2
Antibodies used for western blotting included anti-IDH2 and anti-Sirt3 (R&D, USA). RIPA buffer contained 1 ml of 1.5 M NaCl (Guoyao Group), 5 ml of 100 mM Tris-HCl (pH 7.4) (Amresco, USA), 100 µl of 10% SDS (Bioins, Shanghai), 100 µl of 500 mM DTT (Amresco, USA), 100 µl of NP-40, complete mini protease inhibitors (Roche, Germany), and H2O2. For immunoprecipitation, macrophage mitochondrial lysates were incubated with anti-IDH2 and anti-Sirt3 antibodies overnight at 4°C. After resins were washed, samples were boiled with SDS loading buffer and subjected to western blotting [23] using GAPDH as the internal control.
The LabworksTM Analysis Software GDS8000 and UVP gel image processing system were used to quantitatively analyze the electrophoretic bands.
Data processing and statistical analysis
All data were analyzed with SPSS (version 17) statistical package, and presented as mean ± SD. Two paired groups were compared using the t-test. P < 0.05 was considered to be statistically significant.
Results
Establishment of lentiviral vecrtor
Sirt3 interference lentiviral vector GPH-SIRT3-SH2 and overexpression lentiviral vector LV5-Sirt3 were successfully constructed with a titer of 3 × 108 TU/ml.
Ox-LDL triggers the adherence changes of Sirt3 treated cells
The lentiviral vectors were transfected into different groups of macrophages. The immunofluorescence images of macrophages are shown in Figure 1. The macrophages transfected with Sirt3 interference or overexpression lentiviral vectors cultured in medium containing ox-LDL, showed decreased adherence, which was more prominent in the latter than the former group (Figure 1B and 1D).
Figure 1.
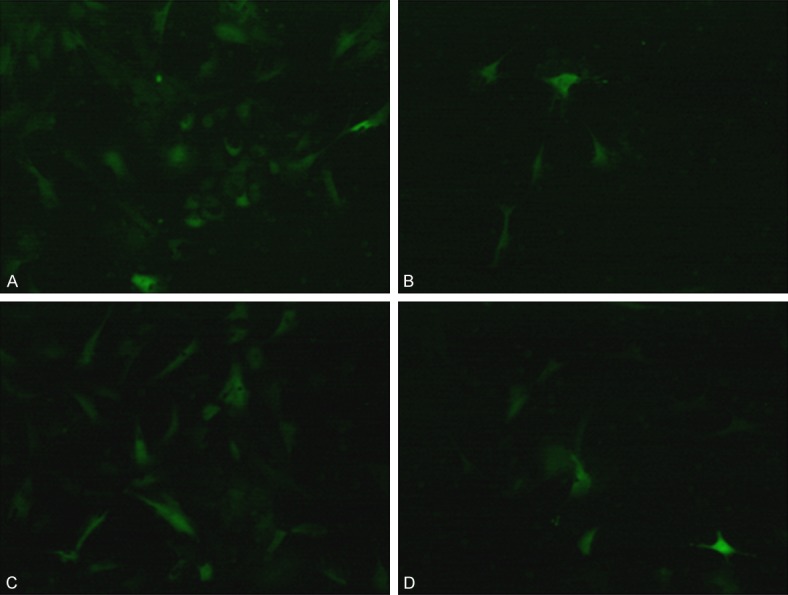
Images of macrophages and foam cells after transfection with Sirt3 interference and overexpression lentiviral vectors (100 ×). A. Sirt3 interference; B. Sirt3 interference cultured with ox-LDL; C. Sirt3 overexpression; D. Sirt3 overexpression cultured with ox-LDL.
Sirt3 inhibition induces the lipids accumulation
In order to understand this phenomenon, the intracellular lipids were detected by flow cytometry after Nile Red staining (Figure 2), which showed more lipids in Sirt3 inhibited macrophages than in wild type and Sirt3 overexpressed cells.
Figure 2.
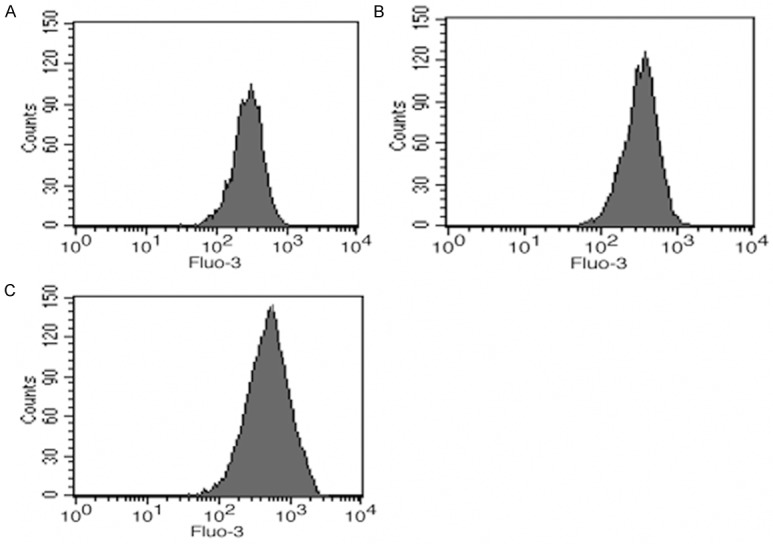
Intracellular lipid counts in macrophages. A. Wild type cultured with ox-LDL; B. Sirt3 overexpression cultured with ox-LDL; C. Sirt3 interference cultured with ox-LDL.
Sit3 inhibition increases the IDH2 acetylation and lipid accumulation
The Sirt3 protein expression and IDH2 acetylation were detected by western blot (Figures 3 and 4). The results showed that IDH2 acetylation and lipid accumulation in macrophages increased after inhibition of Sirt3 expression.
Figure 3.
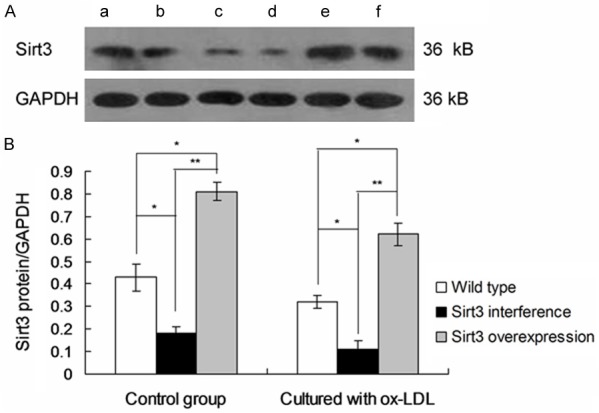
Observation of Sirt3 protein expression in macrophages by using Western blots. a. Wild type; b. Wild type cultured with ox-LDL; c. Sirt3 interference; d. Sirt3 interference cultured with ox-LDL; e. Sirt3 overexpression; f. Sirt3 overexpression cultured with ox-LDL. *P < 0.05, **P < 0.01 represent the comparison between group illustrated in images.
Figure 4.
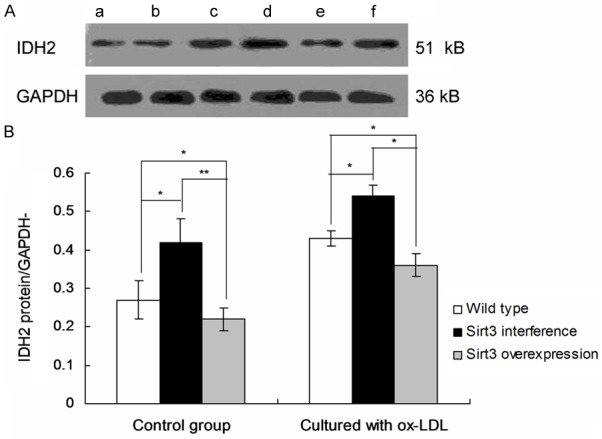
Observation of IDH2 acetylation in macrophages by using Western blots. a. Wild type; b. Wild type cultured with ox-LDL; c. Sirt3 interference; d. Sirt3 interference cultured with ox-LDL; e. Sirt3 overexpression; f. Sirt3 overexpression cultured with ox-LDL. *P < 0.05, **P < 0.01 represent the comparison between group illustrated in images.
Discussion
In this study, Sirt3 interference and overexpression lentiviral vectors were constructed and transfected into macrophages to study the influence of changes in Sirt3 expression on lipid accumulation and metabolism. Macrophages transfected with Sirt3 lentiviral vectors were cultured with ox-LDL to examine its intake and accumulation, and the generation of foam cells.
Immunofluorescence and flow cytometry confirmed that inhibition of Sirt3 expression increased the accumulation of ox-LDL in macrophages, while overexpression of Sirt3 increased the survival of macrophages. Accumulation of liposomes is an important means by which macrophages transform into foam cells [24-28]. Many foam cells formed by macrophages due to excessive cholesterol intake are seen in artery intima, which is a pathological sign of atherosclerosis [29].
Studies have found that Sirt3 is the strongest mitochondrial protein deacetylation enzyme due to its high deacetylation ability [30,31], and IDH2 is its substrate in the TCA cycle [3-34], but whether this relationship exists in macrophages remains unknown. Our results found that mitochondrial acetylated IDH2 was significantly decreased when Sirt3 protein was overexpressed. After culturing with ox-LDL for 72 hours, the intracellular lipids and foam cells formation were lower in macrophages with Sirt3 overexpression than in macrophages with Sirt3 interference. Thus, changes in Sirt3 expression can regulate the accumulation of lipids and the formation of foam cells by altering the acetylation status of mitochondrial proteins in macrophages.
In conclusion, Sirt3 can inhibit lipid accumulation in macrophages by inducing mitochondrial IDH2 deacetylation.
Acknowledgements
This study was granted by the Bureau of science and technology major science and technology projects of Yibin City (Grant No. 2014SF015). We are grateful to the staff of the Department of Laboratory Medicine and the Molecular Diagnosis Laboratory at the No. 2 People’s Hospital of Yibin for technical assistance in biochemical analyses and PCR detection, respectively. This study did not involve any legal or ethical disputes.
Disclosure of conflict of interest
None.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Glass C, Witztum J. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Taylor JM, Borthwick F, Bartholomew C, Graham A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein A-I. Cardiovasc Res. 2010;86:526–534. doi: 10.1093/cvr/cvq015. [DOI] [PubMed] [Google Scholar]
- 4.Pennings M, Meurs I, Ye D, Out R, Hoekstra M, Van Berkel TJ, Van Eck M. Regulation of cholesterol homeostasis in macrophages and consequences for atherosclerotic lesion development. FEBS Lett. 2006;580:5588–5596. doi: 10.1016/j.febslet.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Allen AM, Graham A. Mitochondrial function is involved in regulation of cholesterol efflux to apolipoprotein (apo) A-I from murine RAW 264.7 macrophages. Lipids Health Dis. 2012;11:169. doi: 10.1186/1476-511X-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Eck M, Pennings M, Hoekstra M. Scavenger receptor BI and ATP-binding cassette transporter A1 in reverse cholesterol transport and atherosclerosis. Curr Opin Lipidol. 2005;16:307–315. doi: 10.1097/01.mol.0000169351.28019.04. [DOI] [PubMed] [Google Scholar]
- 7.Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol. 2000;20:7311–7318. doi: 10.1128/mcb.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong J, Liu BB, Wu SD, Wang Y, Jiang QQ, Guo EL. Enhancement of interaction of BSEP and HAX-1 on the canalicular membrane of hepatocytes in a mouse model of cholesterol cholelithiasis. Int J Clin Exp Pathol. 2014;7:1644–1650. [PMC free article] [PubMed] [Google Scholar]
- 9.Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Wiltzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S, Xu W, Jiang W. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K, Takeya M, Sakashita N. Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc. 2002;35:179–203. doi: 10.1007/s007950200023. [DOI] [PubMed] [Google Scholar]
- 14.James AM, Murhy MP. How mitochondrial damage affects cell function. J Biomed Sci. 2002;9:475–487. doi: 10.1159/000064721. [DOI] [PubMed] [Google Scholar]
- 15.Ie YW, Kaminski PM, Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ Res. 1998;82:891–897. doi: 10.1161/01.res.82.8.891. [DOI] [PubMed] [Google Scholar]
- 16.Bai J, Cederbaum AI. Mitochondrial catalase and oxidative injury. Biol Signals Recept. 2001;10:189–199. doi: 10.1159/000046887. [DOI] [PubMed] [Google Scholar]
- 17.Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol. 2013;48:634–639. doi: 10.1016/j.exger.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Roucou X, Antonsson B, Martinou JC. Involvement of mitochondria in apoptosis. Cardiol Clin. 2001;19:45–55. doi: 10.1016/s0733-8651(05)70194-6. [DOI] [PubMed] [Google Scholar]
- 19.Yao PM, Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem. 2001;276:42468–42476. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
- 20.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos againstoxidative stress–induced p53-mediated developmental arrest. J Clin Invest. 2010;120:2817–2828. doi: 10.1172/JCI42020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith BC, Hallows WC, Denu JM. A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal Biochem. 2009;394:101–109. doi: 10.1016/j.ab.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naito M, Suzuki H, Mori T, Matsumoto A, Kodama T, Takahashi K. Coexpression of type I and type II human macrophage scavenger receptors in macrophages of various organs and foam cells in atherosclerotic lesions. Am J Pathol. 1992;141:591–599. [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto A, Naito M, Itakura H, Ikemoto S, Asaoka H, Hayakawa I, Kanamori H, Aburatani H, Takaku F, Suzuki H. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990;87:9133–9137. doi: 10.1073/pnas.87.23.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiltunen TP, Luoma JS, Nikkari T, Yla-Herttuala S. Expression of LDL receptor, VLDL receptor, LDL receptor-related protein, and scavenger receptor in rabbit atherosclerotic lesions: marked induction of scavenger receptor and VLDL receptor expression during lesion development. Circulation. 1998;97:1079–1086. doi: 10.1161/01.cir.97.11.1079. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 28.Lougheed M, Lum CM, Ling W, Suzuki H, Kodama T, Steinbrecher U. High affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class A type I/II. J Biol Chem. 1997;272:12938–12944. doi: 10.1074/jbc.272.20.12938. [DOI] [PubMed] [Google Scholar]
- 29.Ross R. Atherosclerosis-an inflammatory disease. New Engl J Med. 1990;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 30.Taylor JM, Borthwick F, Bartholomew C, Graham A. Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI. Cardiovasc Res. 2010;86:526–534. doi: 10.1093/cvr/cvq015. [DOI] [PubMed] [Google Scholar]
- 31.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382:790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 33.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu M, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie Q, Guo P, Guo L, Lan J, Lin Y, Guo F, Zhou S, Ge J, Mao Q, Li X, Qiu Y. Overexpression of isocitrate dehydrogenase-1R132H enhances the proliferation of A172 glioma cells via aerobic glycolysis. Mol Med Rep. 2015;11:3715–3721. doi: 10.3892/mmr.2015.3187. [DOI] [PubMed] [Google Scholar]


