Abstract
Objectives: We hypothesized that interferon-γ (IFN-γ) induces K17 over-expression in HaCaT cells by activating STAT3 and that Sh might inhibit the over-expression through interference of STAT3 signaling. Methods: In vitro culture of HaCaT cells treated with IFN-γ and measurement of K17 protein by enzyme linked immunosorbent assay. Results: The level of K17 protein (one kind of keratin protein) in the supernatant induced by IFN-γ was significantly reduced by Shikonin at various concentrations. Interference of STAT3 suppressed the effect of IFN-γ on K17 expression at both mRNA and protein levels. The over-expression of K17 in IFN-γ-induced HaCaT cells was significantly suppressed by 2 µg/L Shikonin. Interfering with STAT3 signaling with 2 µg/L Shikonin resulted in an intermediate level of IFN-γ-induced K17 protein in HaCaT cells. Conclusions: These data demonstrate that IFN-γ induces K17 protein over-expression of HaCaT cells by activating STAT3 and Shikonin may inhibit the over-expression partly through interference of STAT3.
Keywords: Shikonin, IFN-γ, K17, STAT3
Introduction
Psoriasis is an immunological skin disease characterized by epidermal hyperproliferation and chronic inflammation. Progress in the understanding of psoriasis has demonstrated it is a T cell mediated disease where the regulation of local and systemic cytokines plays an important role in its pathogenesis. Previous studies demonstrated that interleukin (IL)-17A is a key factor for the early onset of psoriasis and interferon (IFN)-γ is the central cytokine involved in the overall pathogenesis [1]. Cytoskeletal protein keratin 17 (K17) is over-expressed in psoriatic epidermis and considered to be a hallmark of psoriasis [2,3]. The up-regulation of K17 protein in HaCaT cell after exposure to IFN-γ indicates a close relationship between IFN-γ and K17 [4]. This effect was further supported by another study showing that IFN-γ strongly induced the expression of the K17 gene promoter [5]. Notably, K17 is the only keratin induced by IFN-γ [6].
Lithospermum erythrorhizon (LE) belongs to Boraginaceae perennial. The roots of LE have anti-inflammatory, anti-tumor, hepatoprotection, immune regulation, contraception, sterilization, and other antivirus effects [7]. Shikonin (Sh) is one of the main components of LE. In a previous study, we found that 50 µmol/L of Sh inhibited the over-expression of IL-17-induced vascular endothelial cell growth factor (VEGF), IL-6 and IL-23 in the supernatant of cultured HaCaT cells [8]. In another study, we revealed that 5, 7.5, 10, and 12.5 µg/ml of Sh decreased the level of IL-17 in peripheral blood mononuclear cells (PBMCs) from psoriasis patients in a dose-dependent manner. A dose of 12.5 µg/ml Sh also reduced IL-17 and IL-6 levels induced by IL-23 in PBMCs from psoriasis patients [9].
Recent studies showed that IFN-γ, IL-6 and IL-22 stimulation of podoplanin (PDPN) expression is signal transducers and activators of transcription (STAT) 1- and STAT3-dependent [10]. Studies also showed that K17 expression in HaCaT cells was primarily regulated by STAT-dependent signaling pathways [11,12]. A site within the promoter region of the K17 gene responds to IFN-γ via binding transcription factor STAT1 [11]. However, whether IFN-γ up-regulates K17 through STAT3 signaling pathways is unknown. Here, we hypothesized that IFN-γ induces K17 over-expression in HaCaT cells by activating STAT3 and that Sh might inhibit the over-expression through interference of STAT3 signaling. Our findings may contribute to the pathogenesis and treatment of psoriasis.
Methods
Cell culture and Sh treatment
HaCaT cells were cultured in DMEM (Dulbecco’s Modified Eagle Media) with 10% fetal bovine serumunder a humidified atmosphere containing 5% CO2 at 37°C. HaCaT cells at 1×105 cells were plated into each well of a 24-well plate. After 24 hours, cells were co-cultured with IFN-γ (250 U/ml) (5) and Sh (0, 2, 5 10, 12.5, 20 or 30 µg/L). Cyclosporine (10 µg/L) was used to replace Sh as a positive control because it inhibits HaCaT cell proliferation by induction apoptosis of HaCaT cells [13].
MTS for cell viability detection
The Cell Titer 96® Aqueous One Solution Reagent was used for MTS (Promega, America). The reagent was placed at room temperature for approximately 90 minutes until completely thawed, then 20 μl was dispensed into each well of a 96-well plate containing HaCaT cells and 100 μl culture medium. The plate was incubated at 37°C for 3 hours in a humidified atmosphere containing 5% CO2. The absorbance at 490 nm was recorded using a 96-well plate reader.
K17 expression using enzyme linked immunosorbent assay
HaCaT cells were seeded into 96-well plates precoated with 10 µg/ml poly-L-lysine with a density of 1.5×104 cells per well, and then cultured at 37°C for 24 hours. After addition of IFN-γ as described above, the expression of K17 was determined by cell-based enzyme linked immunosorbent assay (ELISA). In brief, after adherence of cells, they were stimulated by IFN-γ 250 U/ml at 37°C for 48 hours. After washing, the cells were blocked with blocking buffer for 1 hour at room temperature and then incubated with Biotin-antibody working solution (Cusabio-Barksdale Delaware state US) at 37°C for 1 hour. Next, the cells were washed and incubated with horseradish peroxidase (HRP)-avidin working solution (Cusabio-Barksdale Delaware State) at 37°C for 1 hour. Subsequently, the cells were incubated with 90 µl of TMB Substrate for 10 minutes at room temperature. The reaction was stopped with 50 µl of stop solution and the absorbance was measured at an optical reference wavelength of 450 nm with an ELISA reader.
Transfection of siRNA
Concentrations of 50-100 μM of AllStars negative control siRNA with green fluorescent protein (GFP) and STAT3-specific siRNAs were transfected into HaCaT cells using HiPerFect Transfection Reagent according to the manufacturer’s protocol (Qiagen, Hilden, Germany).STAT3 target sequence was: CAGGCTGGTAATTTATATAAT.
RT-PCR-for K17 mRNA detection
Total cellular RNA was isolated using an RNeasy Mini Kit (Qiagen, Hilden, Germany) per the manufacturer’s instructions, and cDNA was synthesized with QuantiTect Rev., Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. This was used as the template for quantitative PCR. Subsequently, Real-time PCR was performed using the Quantifast SYBR green PCR kit (Qiagen Hilden, Germany). The reaction components were 0.5 ml of forward and reverse primers for K17 and GAPDH gene as an internal control. Primers sets were: 5’-ccacccagaagactgtggat-3’, and 5’-ttctagacggcaggtcaggt-3’ for the amplification of GAPDH and QuantiTect Primer Assays (Qiagen) were used for KRT17 (Hs_KRT17_ 1_SG, QT00001680), respectively. The cycling conditions were as follows: 95°C for 2 minutes, followed by 45 cycles of denaturation at 95°C for 5 seconds, annealing at 60°C for 10 seconds, and extension at 72°C for 15 seconds. All reactions were run in triplicate in at least three independent experiments.
Flow cytometry for K17 protein detection
Cells were resuspended at approximately 1-5×106 cells/ml in ice cold PBS. The cells were sequentially incubated with primary and secondary antibodies (Abcam, USA). Appropriate isotype specific negative controls were used in all staining. Cell suspensions were immediately stored at 4°C in the dark.
Results
Effects of Sh on K17 expression in the supernatant of HaCaT cells induced by IFN-γ
The level of K17 protein in the supernatant from HaCaT cells treated with IFN-γ was high, but significantly decreased (P<0.01) when Shikonin was added at various concentrations (Figure 1). Thus, Sh inhibited the secretion of K17. For Sh, the optimal concentration that did not influence the survival rate of HaCaT cells was 2 µg/ml (Figure 2).
Figure 1.
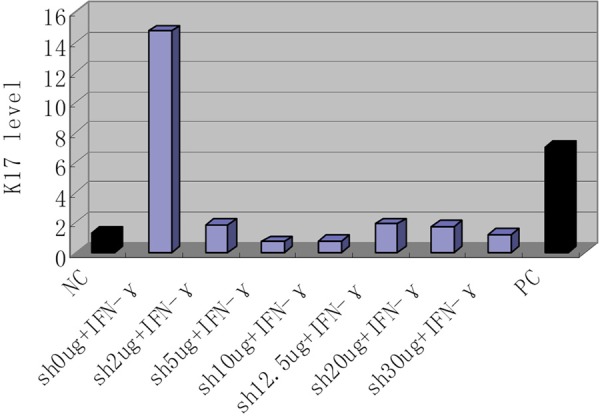
K17 expression in IFN-γ-induced and Shikonin-treated HaCaT cells. NC, negative control (no IFN-γ, no Shikonin); Sh, Shikonin; IFN, IFN-γ; PC, positive control (cyclosporineA10 µg/L + IFN-γ).
Figure 2.
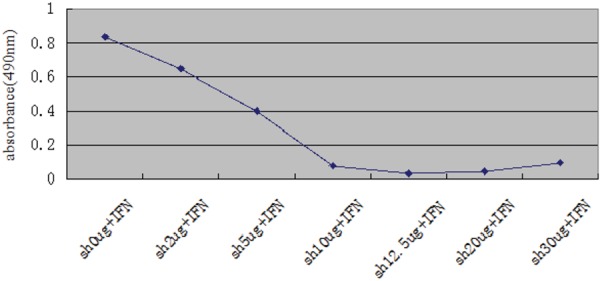
HaCaT cells activated by IFN-γ and treated with Shikonin. MTS assay measured Cell activity from HaCaT cells. Sh, Shikonin; IFN, IFN-γ.
Expression of K17 and the effects of Sh on K17 expression in IFN-γ-induced HaCaT cells
Both RT-PCR (Figure 3) and flow cytometry (Figure 4) results showed significant differences between the IFN-γ only group and other four groups (IFN-γ+stst3-siRNA, IFN-γ+Sh, IFN-γ+Sh+stat3-siRNA and blank) (P<0.01). The K17 level was highest in the IFN-γ only group, and the lowest was in the IFN-γ+Sh group, indicating the high level of K17 induced by IFN-γ was significantly suppressed by Sh. The K17 expression of IFN-γ+stat3-siRNA group was lower than that of the IFN-γ only group, but higher than that of the negative control (P<0.01) suggesting STAT3 was in part involved in the inhibition of IFN-γ. When IFN-γ treated cells were incubated with Sh in the presence of STAT3-specific siRNA, K17 expression was higher than in the IFN-γ+Sh group and lower than the IFN-γ+stat3-siRNA group (P<0.01), indicating STAT3 was a target of Sh although other targets may exist.
Figure 3.
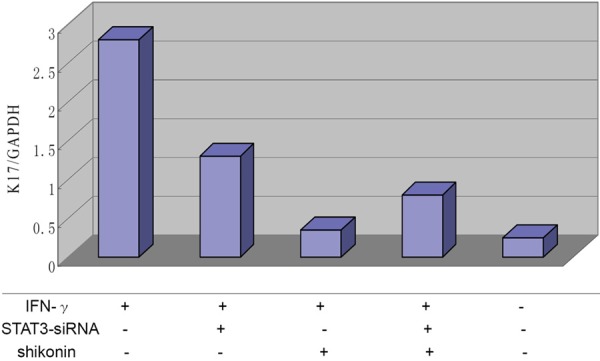
K17 mRNA expression in IFN-γ-induced, Shikonin treated HaCaT cells. RT PCR analysis of K17 mRNA expression from HaCaT cells.
Figure 4.
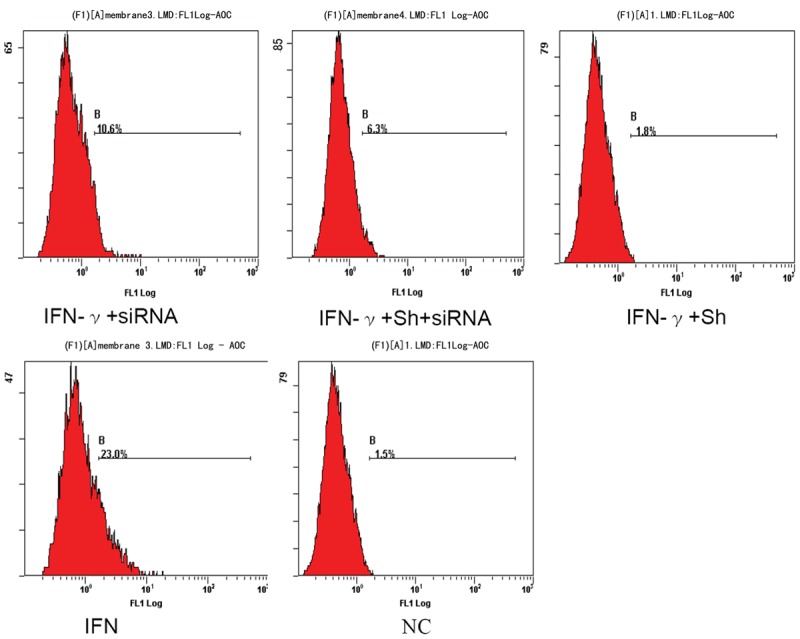
K17 protein expression in IFN-γ-induced, Shikonin treated HaCaT cells. Flow cytometry to measure K17 protein expression.NC, negative control (no IFN-γ, no Shikonin); Sh, Shikonin; IFN, IFN-γ.
Discussion
Many chemokines concomitant with an influx of T cells and inflammatory dendritic cells (DCs) can be up-regulated by IFN-γ in the skin. A study showed that IFN-γ-enhanced IL-23 and IL-1 production by DCs and subsequently induced Th17 cells [12]. IFN-γ has a synergetic effect with other psoriasis-related inflammatory cytokines such as IL-17, IL-22, and this synergy may be an important process in the pathogenesis of psoriasis [14,15]. These facts support the notion that IFN-γ is a key pathogenic cytokine that can induce many features of the inflammatory cascade [16]. K17 is the only keratin reported to be induced by IFN-γ. It is highly expressed in psoriatic lesions, but is not expressed in the healthy epidermis. Our research confirmed that IFN-γ, via its induction of K17 expression, plays an important role in the regulation of keratinocytes.
STAT was first identified as a member of the cytosolic protein family during the study of interferon inducible gene transcription. STAT3 is one of the most important members of the family and mediates a variety of cytokine and growth factor signal transduction pathways to the nucleus to induce the transcription of target genes, thereby regulating cell function. It is also closely related to tumor occurrence, development and apoptosis [17,18]. The abnormality of growth factor signaling plays an important role in the constitutive activation of STAT3, [19] the main contact between keratinized cells and immune cells [20]. STAT3 is involved in Th17 signaling and STAT3 was highly expressed in keratinocytes in psoriatic lesions [21]. Thus, STAT3 is considered to be closely associated with psoriasis.
Studies confirmed that IFN-γ and IL-17 upregulate K17 expression by activating STAT1 [10] and STAT1/3, respectively [21]. Another study showed that IFN-γ, IL-6, and IL-22 induced PDPN-expression of keratinocytes, and the upregulation was both STAT1- and STAT3-dependent [22]. In our experiment using STAT3 siRNA, K17 mRNA and protein expression induced by IFN-γ was significantly decreased; indicating IFN-γ induces K17 expression in HaCaT cells through STAT3.
The naphthoquinone pigment Sh is the most important pharmacologically active substance in the dried root of LE [23]. Sh can be categorized as a mitocan, a class of compounds that act by interfering with energy-generating mitochondrial processes, which in turn leads to reactive oxygen species accumulation, mitochondrial destabilization, and induction of apoptosis [24]. One study showed that Sh and its derivatives could inhibit VEGF-induced proliferation [25] and that Sh may be a promising agent for the treatment of psoriasis [26]. In the present study, we examined the inhibition role of Sh on IFN-γ-induced K17 expression and its mechanism of action. In the IFN-γ+Sh group, the expression of K17 was significantly decreased comparing to the IFN-γ only group, indicating Sh suppressed IFN-γ induced K17 expression. When IFN-γ and STAT3-siRNA were added to the cells, K17 expression decreased approximately 70%, indicating the IFNγ induction effect was partially dependent on STAT3. In the IFN-γ+Sh+siRNA group, K17 expression was further decreased compared to the IFN-γ+ siRNA group, but higher than of the IFN-γ+Sh group. These results suggest STAT3 could be one of the targets of the therapeutic mechanism of Sh. Further investigation is warranted.
An improved understanding of the complex interplay between cytokines, their molecular signaling pathways in affected cells, and the resulting changes in these affected cells will allow a better understanding of the pathomechanisms involved in proliferative diseases including psoriasis. IFN-γ is an important cytokine in the autoimmune loop of K17, T cells and cytokines of psoriasis. The present study demonstrated that K17 expression is induced by IFN-γ stimulation in HaCaT cells via STAT3. Sh suppressed this pathway by inhibiting STAT3 signaling. Our findings may help to elucidate further the molecular and cellular mechanisms underlying the pathogenesis of psoriasis, and to provide new treatment strategies for this chronic disease.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (30872277) and Program for Liaoning Excellent Talents in University (LR2012026).
Disclosure of conflict of interest
None.
References
- 1.Martin DA, Towne JE, Kricorian G, Klekotka P, Gudjonsson JE, Krueger JG, Russell CB. The Emerging Role of Interleukin-17 in the Pathogenesis of Psoriasis: Preclinical and Clinical. J Invest Dermatol. 2012;133:17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnekoh B, Wevers A, Geisel J, Rasokat H, Mahrle G. Antiproliferative potential of zidovudine in human keratinocyte cultures. J Am Acad Dermatol. 1991;25:483–90. doi: 10.1016/0190-9622(91)70228-t. [DOI] [PubMed] [Google Scholar]
- 4.de Jong EM, van Vlijmen IM, van Erp PE, Ramaekers FC, Troyanovski SM, van de Kerkhof PC. Keratin 17: a useful marker in anti-psoriatic therapies. Arch Dermatol Res. 1991;283:480–82. doi: 10.1007/BF00371788. [DOI] [PubMed] [Google Scholar]
- 5.Bonnekoh B, Huerkamp C, Wevers A, Geisel J, Sebök B, Bange FC, Greenhalgh DA, Böttger EC, Krieg T, Mahrle G. Up-regulation of keratin17 expression in human HaCaT keratinocytes by interferon- gamma. J Invest Dermatol. 1995;104:58–61. doi: 10.1111/1523-1747.ep12613492. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi H, Takahashi M, Takahashi H, Ishida-Yamamoto A, Hashimoto Y, Sato K, Tateno M, Iizuka H. CD4+T-cells from peripheral blood of a patient with psoriasis recognize keratin 14 peptide but not ‘homologous’ streptococcal M-protein epitope. J Dermatol Sci. 2002;30:240–247. doi: 10.1016/s0923-1811(02)00111-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen HM, Wang PH, Aravindaram K, Chen YH, Yu HH, Yang WC, Yang NS. Shikonin enhances efficacy of a gene-based cancer vaccine via induction of RANTES. J Biomed Sci. 2012;19:42. doi: 10.1186/1423-0127-19-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min H, Geng L, Qu HM, editors. Shikonin inhibits the secretion of VEGF, IL-6 and IL-23 in IL-17-induced HaCaT cell; Skin venereal disease academic conference proceedings of 2010 National Traditional Chinese Medicine and Western Medicine Combined; 2010. p. 168. [Google Scholar]
- 9.Qu HM, Min H, Geng L, editors. Shikonin inhibits IL-6 and IL-17 production induced by IL-23 in psoriasic peripheral blood mononuclear cells; Skin venereal disease academic conference proceedings of 2010 National Traditional Chinese medicine and Western medicine combined, China; 2010. p. 279. [Google Scholar]
- 10.Honma M, Minami-Hori M, Takahashi H, Iizuka H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: regulation by TGF-β and STAT-3 activating cytokines, IFN-γ, IL-6, and IL-22. J Dermatol Sci. 2012;65:134–40. doi: 10.1016/j.jdermsci.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Jiang CK, Flanagan S, Ohtsuki M, Shuai K, Freedberg IM, Blumenberg M. Disease-activated transcription factor: allergic reactions in human skin cause nuclear translocation of STAT-91 and induce synthesis of keratin K17. Mol Cell Biol. 1994;14:4759–69. doi: 10.1128/mcb.14.7.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, Elder JT, Zou W. Induction of IL-17+Tcell traffi cking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–41. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge Y, Xu Y, Sun W, Man Z, Zhu L, Xia X, Zhao L, Zhao Y, Wang X. The molecular mechanisms of the effect of Dexamethasone and Cyclosporin A on TLR4 /NF-κB signaling pathway activation in oral lichen planus. Gene. 2012;508:157–64. doi: 10.1016/j.gene.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinfl ammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–7. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 15.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, de Waal Malefyt R. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–87. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Huang LM, Suárez-Fariñas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, Sullivan-Whalen M, Gilleaudeau P, Krueger JG, Haider AS, Lowes MA. A single intradermal injection of IFN-γ induces an infl ammatory state in both non-lesional psoriatic and healthy skin. J Invest Dermatol. 2012;132:1177–87. doi: 10.1038/jid.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Jove R. The Stats of cancer-new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 18.Matsutani Y, Yamauchi A, Takahashi R. Inverse correlation of thioredoxin expression with estrogen receptor-and p53-dependent tumor growth in breast cancer tissues. Clinical Cancer Res. 2001;7:3430–6. [PubMed] [Google Scholar]
- 19.Bromberg J. Stat proteins and oncogenesis. Clin Invest. 2002;109:1139–42. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–9. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 21.Shi X, Jin L, Dang E, Chang T, Feng Z, Liu Y, Wang G. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J Invest Dermatol. 2011;131:2401–8. doi: 10.1038/jid.2011.222. [DOI] [PubMed] [Google Scholar]
- 22.Ralph SJ, Neuzil J. Mitochondria as targets for cancer therapy. Mol Nutr Food Res. 2009;53:9–28. doi: 10.1002/mnfr.200800044. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 24.Guizzunti G, Theodorakis EA, Yu AL, Zurzolo C, Batova A. Cluvenone induces apoptosis via a direct target in mitochondria: a possible mechanism to circumvent chemo-resistance? Invest New Drugs. 2012;30:1841–8. doi: 10.1007/s10637-011-9745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ, Lee HJ, Magesh V, Nam D, Lee EO, Ahn KS, Jung MH, Ahn KS, Kim DK, Kim JY, Kim SH. Shikonin, acetylsh, and isobutyroylsh inhibit VEGF-induced angiogenesis and suppress tumor growth in Lewis lung carcinoma-bearing mice. Yakugaku Zasshi. 2008;128:1681–8. doi: 10.1248/yakushi.128.1681. [DOI] [PubMed] [Google Scholar]
- 26.Mao X, Yu CR, Li WH, Li WX. Induction of apoptosis by Shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008;18:879–88. doi: 10.1038/cr.2008.86. [DOI] [PubMed] [Google Scholar]


