Abstract
Objective: To investigate the effects of rapamycin (RAPA) on the tumor growth of lung cancer in the mice bearing A549 and the mechanisms. Methods: 60 mice with A549 lung cancer models established were randomly divided into model group, low RAPA dose group and high RAPA dose group. The low dose group underwent intraperitoneal injection of 1.5 mg/kg RAPA, while the high dose group underwent intraperitoneal injection of 4.5 mg/kg RAPA, and the control group was given the same volume of PBS. 21 d after the administration, the changes of the tumor growth and survival rates of three groups were observed. RT-PCR and Western blot were utilized to analyze Caspase-3 mRNA and protein levels in the tumor tissues of the mice, and TUNEL staining method was used to analyze the cellular apoptosis of tumor tissues. Results: Compared with the model group, the low and high dose groups significantly inhibit tumor growth and have remarkably higher survival rates (P<0.05). The high dose group has obviously better effects on inhibiting tumors and a higher survival rate than low dose group (P<0.05). Compared with the model group, the low and high dose groups have significantly increased Caspase-3 mRNA and protein levels in tumor tissues (P<0.05), and higher cellular apoptosis rates in tumor tissues (P<0.05); Caspase-3 mRNA and protein levels and apoptosis rates of the mice’s tumor tissues of high dose group are markedly higher than those of low dose group (P<0.05). Conclusions: RAPA can significantly increase the expression of Caspase-3 in tumor tissues and promote the apoptosis of tumor tissue cells, and thus achieve good anti-tumor effects.
Keywords: Rapamycin, tumor growth, Caspase-3
Introduction
Lung cancer is the most common malignant tumor in the world and has the highest fatality rate. Even after clinical treatment, five-year survival rate is still low. As one of the clinically common respiratory tumors, the incidence and fatality rate of lung cancer are increasing, which greatly threatens human health [1,2]. About 85% of the lung cancer cases are non-small-cell lung cancer (NSCLC), and when the patients visit doctors, most of them are with terminal lung cancer and metathesis in adjacent or distant organs. Even for the patients with early NSCLC, quite a few have relapse after radical operation [3,4]. Therefore, it is crucial to in-depth study the molecular biological mechanism of lung cancer for improving the treatment of lung cancer. rapamycin (RAPA) is a new macrolides immunosuppressive agent. It stops T lymphocyte and other cells from progressing from stage G1 to S by breaking signal transmission via different cytokines, so as to obtain immunologic suppression [5-7]. RAPA has been proved to be able to inhibit the growth of many tumor cells, including rhabdomyosarcoma, neuroblastoma, small lung cancer, osteosarcoma, pancreatic cancer, breast cancer, leukemia cells and B cellular lymphoma [8-10]. As the research into the effects of RAPA on human NSCLC model mice is rare, we chose different concentrations of RAPA to treat mice with A549 lung cancer to observe the changes of tumor growth, survival rates and weight, and thus to study how RAPA inhibit transplanted tumor in nude mice and related mechanisms.
Materials and methods
Model construction and grouping
Balb/c nude mice at SPF grade were purchased from Henan Experimental Animal Center, Zhengzhou, China. They were 8-weeks old, weighing 20±1.5 g and kept in animal house. The human lung adenocarcinoma cells A549 (ATCC, Manassas, VA, USA) were cultured in DMEM medium (Hyclone, Logan, Utah, USA) containing 10% FBS (Hyclone, Logan, Utah, USA). When the cells grew to be in exponential growth phase, it was digested with 0.25% pancreatic enzyme and then centrifugated at 1500 g for 5 min. The cell precipitation was washed with PBS twice, and the serum was removed, and then the cells were suspended in PBS; the cell concentration was adjusted to be 1×107/ml, and 100 μl was inoculated into each mouse’ s fat pad near armpit in the left rib. After 9 days, the tumor grew to be 100-130 mm3, the mice with tumors were randomly divided into model group, low RAPA dose group and high RAPA dose group, 15 mice for each group. The low dose group underwent intraperitoneal injection of 1.5 mg/kg RAPA (Sigma, St. Louis, MO, USA), while the high dose group underwent intraperitoneal injection of 4.5 mg/kg RAPA, and the control group was given the same volume of PBS. Starting from the day of administration, the death of mice of each group was observed and recorded. 30 days after administration, the mice were dissected and the tumor was stripped off (the animals of the normal group were excluded) and weighed with analytical balance. The tumor growth inhibition rate was calculated, the average tumor inhibition rate (%) = (model group-administration group)/model group × 100%. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA). Eighth Edition, 2010. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Second Affiliated Hospital of Zhengzhou University.
RT-PCR
21 d after administration, the mice were killed respectively. 0.1 g of tumor tissue was taken to be put into liquid nitrogen. The tumor tissue was grinded, and 1 ml Trizol (TaKaRa, Dalian, China) was added, and then placed in 1.5 ml tube. 200 μl chloroform was added into the centrifuge tube, and the solution was oscillated greatly to be mixed up, and then placed on ice for 15 min for stratification, and centrifugated at 15,000 g for 15 min. After stratification, the supernatant (about 500 μl) was moved to 500 μl isopropanol to be mixed up, and placed on ice for separating out RNA precipitation. 15 min later, it was centrifugated at 15,000 g for 10 min, and the sediment was washed twice with pre-cooled 75% anhydrous ethanol, and finally dissolved in double distilled water without RNA enzyme. The sample concentration was detected, and RNA was transcripted to be cDNA with reverse transcription kit (TaKaRa, Dalian, China) as the template of PCR. The mice Caspase-3 primer was designed as follows: Caspase-3-F: 5’-GGTATTGAGACAGACAGTGG-3’, Caspase-3: 5’-CATGGGATCTGTTTCTTTGC-3’; β-actin-F: 5’-GCGGGAAATCGTGCGTGAC-3’, β-actin-R: CGTCATACTCCTGCTTGCTG-3’. The following reaction system was conducted to prepare reaction mixture: 2×SYBR Green common qPCR Master Mix (Roche, Basel, Switzerland) 10 µl, upstream/downstream primers (10 µmmol•L-1) 1 µl, respectively, cDNA 1 µl, adding double distilled water to get the final volume of 20 µl. PCR was carried out based on the following reaction conditions: pre-denaturation: 95°C, 30 s; denaturation: 95°C, 3 s; annealing and extension: 60°C, 30 s; constructing dissolution curve. Finally, read the data from RT-PCR instrument (Bio-Rad, Hercules, CA, USA).
Western blot
After the three groups’ mice were sentenced to death, their tumor tissues were taken to be rinsed in cold saline; when the blood was removed, the tissues were dried with filter paper and weighed; 1 g of tissues were placed in the mortar with liquid nitrogen to be grinded, and 300 µl pre-cooled lysates were added for suspension; 3 µl protease inhibitor was added, and then it was placed on ice for 30 min, centrifugated at 15,000 g for 15 min; the supernatant was taken, and the total protein content was measured according to the instruction of BCA protein concentration determination kit (Beyotime Biotechnology, Shanghai, China); 4× buffer solution was added, and boiled for 30 min; after centrifugation, the sample was added, while SDS-PAGE was conducted, and the protein was moved onto PVEF membrane, sealed with 5% skimmed milk powder, enveloped for overnight with Caspase-3 primary antibody (Abcam, Cambridge, UK), washed for three times with PBST, enveloped for 1 h at room temperature with HRP-coupling goat anti-rat secondary antibody (Boster, Wuhan, China), washed for three times with PBST; luminous liquid was added for color development and photo-taking. Meanwhile, with GAPDH as internal reference, the target protein band gradation was analyzed with gradation calculation software so as to calculate the relative expression of target protein.
TUNEL staining
After 20 d of treatment, the three groups’ mice were killed to get the tumor tissues, which were embedded with OCT, cut into 7 µm slices with freezing microtome, and fixed for 30 min with 4% paraformaldehyde solution. The solution was abandoned, and the slides were washed for three times with PBS, and each washing lasted 5 min; Proteinase K solution was diluted to an appropriate proportion to get a final concentration of 20 μg/ml. On each slide, Proteinase K solution was drop wise added, incubated at room temperature for 10min, and then rinsed for three times, each rinse lasting 5 min. 5× Equilibration Buffer was diluted by double distilled water, dropped to 1× Equilibration Buffer and samples to be tested, and incubated at room temperature for 30 min. TdT incubation buffer was dropped onto the samples, which were incubated at 37°C for 60 min away from light. After the incubation, the samples were washed with PBS for three times, each washing lasting 5 min, and then observed under inverted fluorescence microscope (DM IRBE; Leica, Bensheim, Germany). The apoptotic cells in which brown yellow granules exist in the nucleus are defined to be positive cells. The observation was carried out with 40× object lens and 10× eye lens. 10 visual fields in the infarction zone and surrounding areas were randomly selected to calculate the proportion of apoptotic cells to the total cells, which is taken as the apoptotic rate of brain cells.
Statistical analysis
All data were analyzed with SPSS 17.0 software (SPSS Inc, Chicago, IL, USA); measurement data were expressed by X ± S; the group comparison of measurement data was conducted with analysis of variance; the survival rates were compared with Kaplan-Meier method and Log-Rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibition of RAPA to the growth of mice’s transplanted tumors and the effect on survival rate
Low dose and high dose of RAPA can inhibit the growth of tumors in nude mice. The tumor weight of low and high dose groups is significantly lower than that of the model group, and the difference is statistical significant (P<0.05). The tumor inhibition rates of the low dose and high dose group are 48.85% and 53.44% respectively, and the difference between the low and high dose groups is of no statistical significance (P>0.01) (Figure 1). The survival rates of the low dose and high dose groups are 60% and 55% respectively, which are remarkably higher than 35%, the survival rate of the model group, and the difference between the low dose and high dose groups is not statistically significant (P>0.05) (Figure 2).
Figure 1.
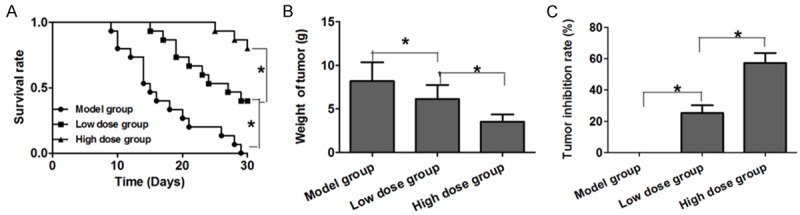
The inhibition of RAPA to the growth of mice’s transplanted tumors. A. Survival rate in three groups; B. Average tumor weight of mice in three groups; C. Tumor inhibition rate in three groups.
Figure 2.
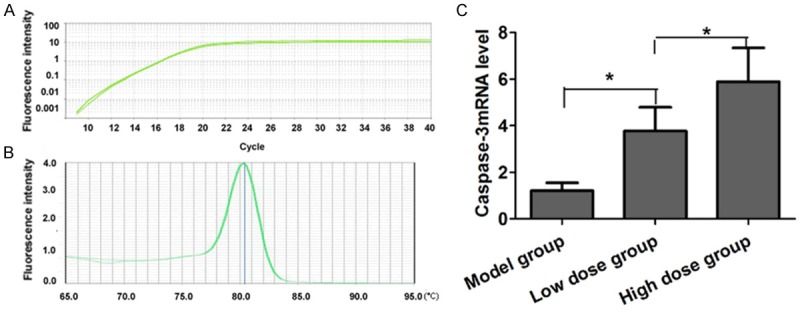
Comparison of the expression of Caspase-3 in mice’s tumor tissues of three groups. A. Amplification curve of Caspase-3 primer by real time PCR. B. Melt curve of Caspase-3 primer by real time PCR. C. Caspase-3 mRNA level in three groups.
Effect of RAPA on the expression of Caspase-3 in mice’s tumor tissues
RT-PCR results indicate that the Caspase-3 primer designed in this research has good specificity, and the dissolution curve has no impure peak (Figure 2A, 2B); compared with the model group, the low dose group and high dose group have significantly increased Caspase-3 mRNA level in the mice’s tumor tissues (P<0.05), and the high dose group has more significant higher Caspase-3 mRNA level, and the difference is statistically significant (P>0.05) (Figure 2C).
Western blot analysis results are shown by Figure 3A and 3B. Compared with the model group, the low dose group and the high dose group have significantly increased Caspase-3 protein level in the tumor tissues of the mice (P<0.05), and the comparison between the two groups is of no statistical significance (P>0.05).
Figure 3.
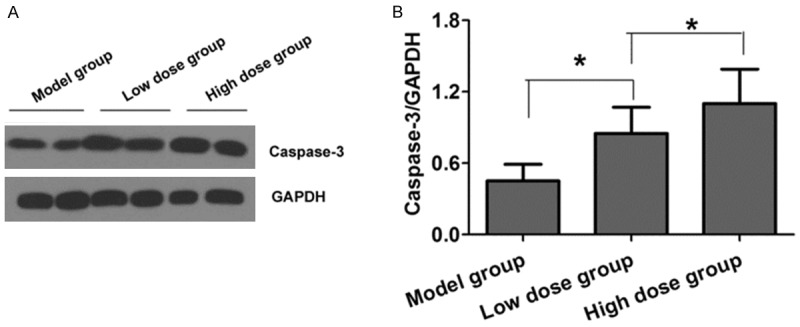
Comparison of Caspase-3 protein in mice’s tumor tissues of three groups. A. Caspase-3 protein level in three groups by Western blot; B. Quantitative analysis of Caspase-3 protein level in three groups.
Effect of RAPA on the apoptosis of tumor cells in mice
Compared with the model group, the low dose group and the high dose group have markedly increased cellular apoptotic levels in the mice’s tumor tissues (P<0.05), while the high dose group has obviously higher apoptotic level than the low dose group, and the difference is of statistical significance (P<0.05) (Figure 4).
Figure 4.
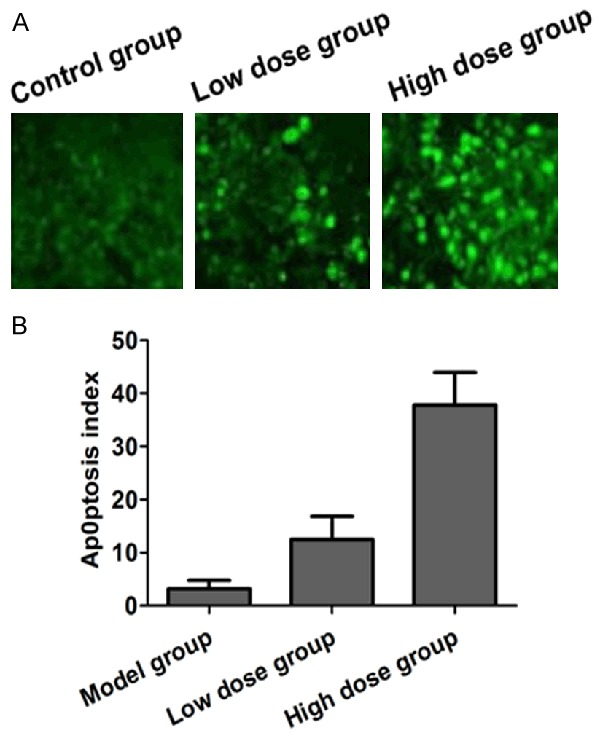
Comparison of the apoptosis levels in three groups. A. TUNEL staining showed apoptosis of tumor tissue in three groups; B. Quantitative analysis of apoptosis of tumor tissue in three groups by TUNEL staining.
Discussion
Lung cancer is a heterogeneous disease, being the first cancer-related death factor in the world. It consists of two major histological subtypes: NSCLC and small cell lung cancer [11]. NSCLC accounts for 85% of the cases. Although the diagnosis and treatment methods are increasingly developing, no breakthrough has been made in the treatment of NSCLC during the past decades. Even for the NSCLC patients at stage I, their five-year survival rate is only 50%, only 30% for the patients at stage II, and only less than 15% for those at advanced stage. The reason for this is that the occurrence and development of lung cancer is a complex progress involving many factors, multiple gene changes and several stages [12-14]. As a result, an in-depth study of lung cancer’s molecular biological features is of great clinical significance for the early diagnosis, treatment and prognosis improvement of lung cancer. Besides, a high efficient treatment method with low toxicity or without toxicity is always a difficulty in treating tumor.
As an immunosuppressant, RAPA has been widely applied in clinics. As is shown by researches, the occurrence of many tumors is related to the abnormal activation of RAPA protein signal pathway of mammals. It combines with the target protein m-TOR of the immune affinity protein FKBP12, and thus affects the proliferation in vitro and the growth cycle of many tumor cells. In the cells of NSCLC, the abnormal phosphorylation of this protein also exists [15-17]. Li et al. [18] chose human NSCLC strain A549 as object to observe and analyze the effects of RAPA on the growth and proliferation of human NSCLC strain A549. The results indicate that RAPA significantly inhibits cellular morphology, cycle and proliferation of human lung cancer strain A549, which is time- and concentration-dependent. Liu et al. [19] studied the influence of RAPA on different tumor cells’ Bax/Bcl-2 and active caspase-3 expression, finding that after RAPA exerts its effect on adenocarcinoma A549, the cells’ relative proliferation is decreased, and the Bax/Bcl-2 and active caspase-3 expression is up-regulated, which suggests that RAPA may inhibit the proliferation of cell A549 and promote the apoptosis. However, most local and foreign researches on RAPA are on the molecular scale, and the researches on animal scale are few. In this research, the effects of RAPA on the tumor growth in the mice with human adenocarcinoma cell A549 was observed. The results indicate that both low dose and high dose of RAPA can significantly inhibit the tumor growth in the mice. In addition, compared with the model group, the two groups have higher survival rates. Meanwhile, the research further analyzes the expression of caspase-3 and cellular apoptosis in the tumor tissues of the three groups. The results manifest that RAPA can markedly increase the expression of Caspase-3 in the tumor tissues and promote the tumor cells’ apoptosis, resulting in excellent anti-tumor effects, so it can guide the clinical treatment of lung cancer to some degree.
Acknowledgements
We are grateful to all the participants during performing this study.
Disclosure of conflict of interest
None.
References
- 1.Cufer T, Knez L. Update on systemic therapy of advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2014;14:1189–1203. doi: 10.1586/14737140.2014.940327. [DOI] [PubMed] [Google Scholar]
- 2.Di Maio M, De Marinis F, Hirsch FR, Hirsch FR, Gridelli C. Diagnostic and therapeutic issues for patients with advanced non-small cell lung cancer harboring anaplastic lymphoma kinase rearrangement: European vs. US perspective (review) Int J Oncol. 2014;45:509–515. doi: 10.3892/ijo.2014.2453. [DOI] [PubMed] [Google Scholar]
- 3.Varela G, Thomas PA. Surgical management of advanced non-small cell lung cancer. J Thorac Dis. 2014;6:S217–223. doi: 10.3978/j.issn.2072-1439.2014.04.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parums DV. Current status of targeted therapy in non-small cell lung cancer. Drugs Today (Barc) 2014;50:503–525. doi: 10.1358/dot.2014.50.7.2185913. [DOI] [PubMed] [Google Scholar]
- 5.Heuer M, Benko T, Cicinnati VR, Kaiser GM, Sotiropoulos GC, Baba HA, Treckmann JW, Broelsch CE, Paul A. Effect of low-dose rapamycin on tumor growth in two human hepatocellular cancer cell lines. Transplant Proc. 2009;41:359–365. doi: 10.1016/j.transproceed.2008.10.090. [DOI] [PubMed] [Google Scholar]
- 6.Fasolo A, Sessa C. mTOR inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2008;17:1717–1734. doi: 10.1517/13543784.17.11.1717. [DOI] [PubMed] [Google Scholar]
- 7.Jerjees DA, Negm OH, Alabdullah ML, Mirza S, Alkaabi M, Hameed MR, Abduljabbar R, Muftah A, Nolan CC, Green AR, Tighe PJ, Band V, Ellis IO, Rakha EA. The mammalian target of rapamycin complex 1 (mTORC1) in breast cancer: the impact of oestrogen receptor and HER2 pathways. Breast Cancer Res Treat. 2015;150:91–103. doi: 10.1007/s10549-015-3308-4. [DOI] [PubMed] [Google Scholar]
- 8.Beck JT, Ismail A, Tolomeo C. Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: an emerging treatment strategy for squamous cell lung carcinoma. Cancer Treat Rev. 2014;40:980–989. doi: 10.1016/j.ctrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Krześniak M, Zajkowicz A, Matuszczyk I, Rusin M. Rapamycin prevents strong phosphorylation of p53 on serine 46 and attenuates activation of the p53 pathway in A549 lung cancer cells exposed to actinomycin D. Mech Ageing Dev. 2014;139:11–21. doi: 10.1016/j.mad.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Jiang RY, Pei HL, Gu WD, Huang J, Wang ZG. Autophagic inhibitor attenuates rapamycin-induced inhibition of proliferation in cultured A549 lung cancer cells. Eur Rev Med Pharmacol Sci. 2014;18:806–810. [PubMed] [Google Scholar]
- 11.Stojiljkovic D, Santrac N, Stojiljkovic T, Miletic N, Gavrilovic D. Correlation of tumor size as independent factor and disease stage with local recurrence of non-small cell lung carcinoma and its operability. J BUON. 2015;20:166–172. [PubMed] [Google Scholar]
- 12.Chen YJ. Individualized altered fractionation as a more effective radiotherapy for non-small cell lung cancer. J Thorac Dis. 2014;6:E161–162. doi: 10.3978/j.issn.2072-1439.2014.06.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lievens Y, Obyn C, Mertens AS, Van Halewyck D, Hulstaert F. Stereotactic Body Radiotherapy for Lung Cancer: How Much Does it Really Cost? J Thorac Oncol. 2015;10:454–461. doi: 10.1097/JTO.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 14.Lee CJ, Yue CH, Lin YJ, Lin YY, Kao SH, Liu JY, Chen YH. Antitumor activity of acriflavine in lung adenocarcinoma cell line A549. Anticancer Res. 2014;34:6467–6472. [PubMed] [Google Scholar]
- 15.Luo Y, Liu L, Wu Y, Singh K, Su B, Zhang N, Liu X, Shen Y, Huang S. Rapamycin inhibits mSin1 phosphorylation independently of mTORC1 and mTORC2. Oncotarget. 2015;6:4286–4298. doi: 10.18632/oncotarget.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawabata S, Mercado-Matos JR, Hollander MC, Donahue D, Wilson W 3rd, Regales L, Butaney M, Pao W, Wong KK, Jänne PA, Dennis PA. Rapamycin prevents the development and progression of mutant epidermal growth factor receptor lung tumors with the acquired resistance mutation T790M. Cell Rep. 2014;7:1824–1832. doi: 10.1016/j.celrep.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawabata S, Chiang CT, Tsurutani J, Shiga H, Arwood ML, Komiya T, Gills JJ, Memmott RM, Dennis PA. Rapamycin downregulates thymidylate synthase and potentiates the activity of pemetrexed in non-small cell lung cancer. Oncotarget. 2014;5:1062–1070. doi: 10.18632/oncotarget.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li WY, Zhu J. Effects of rapamycin on growth and proliferation of human non-small cell lung cancer cell line A549. Journal of Shanghai Jiaotong University (Medical Science) 2011;31:275–278. [Google Scholar]
- 19.Liu YD, Zheng QX, Wu HB, Guo XD, Li JF, Hao SF. The effects of rapamycin on expression ratio of Bax/Bcl-2 and the expression of activated caspase-3 in different types of tumor cells. Tumor. 2013;33:138–144. [Google Scholar]


