Abstract
Objective: This study aimed to investigate the effect of curcumin on the retinal structure and the expressions of interleukin-23 (IL-23) and IL-17 in the rat retina after retinal ischemia-reperfusion injury (RIRI). Methods: 150 Sprague-Dawley rats were randomly divided into RIRI group (MG), low-dose curcumin group (LDCG) and high-dose curcumin group (HDCG), (n = 50 per group). RIRI was generated by anterior chamber perfusion of normal saline to the right eye. The left eye served as a normal control group (NCG). Rats in LDCG and HDCG received an intraperitoneal injection of 20 mg/kg/d and 100 mg/kg/d curcumin respectively, at 30 min before RIRI and once daily after RIRI. Results: The morphological changes in HDCG group were improved as compared to MG and LDCG groups. Immunohistochemistry showed that IL-23 and IL-17 were mainly expressed in the ganglion cell layer and the inner nuclear layer of the retina. Low IL-23 and IL-17 expressions were observed in NCG, but increased significantly in MG and LDCG groups. Western blot assay and ELISA also showed that IL-23 and IL-17 expressions increased significantly after RIRI (vs. NCG, P<0.01). Moreover, the IL-23 expression reached a peak at 24 h, whereas IL-17 expression peaked at 72 h after RIRI. Curcumin reduced IL-23 and IL-17 expressions significantly in a dose-dependent manner (vs. MG, P<0.01). Conclusion: The IL-23 and IL-17 expressions increase after RIRI and curcumin significantly reduces retinal IL-23 and IL-17 expressions in a dose-dependent manner and is able to prevent the RIRI induced damage to the retina.
Keywords: Retinal ischemia-reperfusion injury, curcumin, interleukin-23, interleukin-17
Introduction
Retinal ischemia-reperfusion injury (RIRI) is a common pathological process and can result in vision loss. It primarily occurs due to reduction in the intraocular pressure as a consequence of ocular hypertension, thrombolytic treatment of retinal vascular occlusion or other ophthalmic operations able to affect the retinal blood flow. Although its mechanism has not yet been fully elucidated, inflammatory factor-mediated immune inflammation is particularly involved, while classic mechanisms such as oxygen free radical generation, calcium ion overload and change in nitric oxide concentration also have roles [1-3]. In the subacute phase of RIRI (several hours to several days after RIRI), the expression of inflammation related genes is activated, thereby resulting in the increased production of interleukin-8 (IL-8), IL-6, tumor necrosis factor-α (TNF-α), interferon regulatory factor (IRF), nuclear factor-kappa B (NF-κB) and other inflammatory mediators [3,4]. These inflammatory mediators, through a series of cascade reactions, may induce leukocyte infiltration to the lesions. The infiltrating leukocytes further release numerous cytokines and chemokines, causing blood-retinal barrier destruction and ultimately resulting in retinal neuronal cell death [3,5,6]. It has been reported that, in an acute high-intraocular-pressure model, T- and B-lymphocyte-combined immunodeficient mice had a higher retinal ganglion cell survival and improved retinal damage when compared with wild-type mice [7], indicating that the inflammatory response plays a very important role in the subacute phase of RIRI.
T helper cells (Th) are traditionally divided into two cell subgroups: Th1 (mainly producing interferon γ and IL-2) and Th2 (mainly producing IL-4, -5, and -13). Recently, a new type of CD4+ effector cell, T helper cell 17 (Th17), is identified. Th17 cells mainly produce IL-17, which plays a key role in the inflammation, infection and defense response [8,9]. Subsequent studies reveal that IL-17 is not only produced by Th17 cells but secreted by epithelial cells, neuroglial cells, endothelial cells, NK cells, γδT cells, eosinophils, neutrophils and other cells [10]. Moreover, the production and activity of IL-17 can be regulated by other cytokines. For example, IL-23, a cytokine produced by dendritic cells, phagocytes, microgliocytes and other antigen-presenting cells can not only promote the differentiation of Th cells into Th17 cells but stimulate T cells to secrete IL-17. It has been reported that IL-23, as an upstream positive regulatory factor of IL-17, can participate in the IL-17-mediated pathophysiological process [11]. In rat cerebral ischemia-reperfusion model, the IL-23 and IL-17 expressions significantly increased in the brain [12,13]. Moreover, brain injury in this model could be attenuated by IL-23 antibody or IL-17 antibody or in gene knockout IL-17- or IL-23p19- animals [14,15]. Similarly, IL-23/IL-17 related ischemia-reperfusion injury is also confirmed in the heart, liver, and lung [16-18]. Therefore, IL-17 can aggravate tissue damage, and has now become a new focus in studies on ischemia-reperfusion injury.
Curcumin is a natural product extracted from turmeric (Curcumalonga) and has been used as an herb for centuries. Because of its anti-inflammatory, anti-oxidant, anti-cell proliferation and anti-apoptotic properties (among its other biological activities), curcumin has been widely used in studies on systemic diseases (e.g., diabetes, cardiovascular disease, multiple sclerosis, Alzheimer’s disease, and cancers) as well as ocular diseases, including diabetic retinopathy, retinitis pigmentosa, and glaucoma [19-21]. Numerous studies have shown that curcumin may exert a neuroprotective effect on the retina, acting mainly as an inhibitor of retinal ganglion cell apoptosis, retinal pigment cell proliferation and angiogenesis [22-25], and it may also protect the retinal neurons and microvessels against RIRI through inhibiting the injury-induced activation of NF-κB and STAT3, and the over-expression of monocyte chemotactic protein 1 (MCP-1) [26].
This study was undertaken to explore whether the IL-23/IL-17 pathway participated in the pathological process of RIRI and to investigate the effect of curcumin on the IL-23 and IL-17 expressions in the retina of Sprague-Dawley (SD) rats following RIRI, thus providing a new theoretical basis for the treatment of RIRI with curcumin.
Materials and methods
Grouping and preparation of animal model
A total of 150 healthy male SD rats weighing 200-300 g (8-week-old) were purchased from the Experimental Animal Center of Three Gorges University in China. All the experimental and animal-handling procedures were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Committee on the Use of Living Animals in Teaching and Research at Wuhan University. SD rats were randomly divided into RIRI group (MG), low-dose curcumin group (LDCG) and high-dose curcumin group (HDCG) (n = 50 per group). RIRI was induced to the right eye of each rat by elevating the intraocular pressure. Briefly, the anterior chamber was cannulated with a 27-gauge needle attached to an infusion line of sterile saline. Intraocular pressure was increased to 110 mmHg by raising the infusion saline bottle to 150 cm over the eye ball. The left eye of each rat was not treated and served as a normal control group (NCG). Whitening of the iris and loss of the red reflex of the retina were used to confirm the retinal ischemia. One hour later, the needle was withdrawn, and the reflow of the retinal circulation was documented visually. Rats in LDCG and HDCG were injected intraperitoneally with 20 mg/kg and 100 mg/kg curcumin (Sigma, USA) in DMSO, respectively, at 30 min before RIRI and once daily after RIRI. Rats in MG were intraperitoneally treated with DMSO in sterile saline.
Hematoxylin and eosin staining and morphological examination
Five rats were randomly selected from each group and euthanized by intrapertioneal injection of an overdose of pentobarbital (100 mg/kg) at 72 h after RIRI. The eyes were immediately enucleated, fixed with 4% paraformaldehyde overnight at room temperature, embedded in paraffin, and cut into 5-µm sagittal sections (the retina near the optic disc), followed by hematoxylin and eosin (H&E) staining [27]. Briefly, the sections were treated with toluene for 2 h to remove paraffin and hydrated in a series of alcohol solutions, followed by H&E staining. After dehydration, sections were mounted for microscopic examination. Photographs were captured under a microscope (Nikon Eclipse Ti-SR, Japan) with a digital camera (Nikon DS-U3, Japan) and the retinal structure was evaluated as in our previous study [27]. The inflitrated inflammatory cells in the ganglion cell layer (GCL) were counted over 200 micrometer in 4 retinal sections of each group.
Immunohistochemistry for IL-23 and IL-17 in the retina
Five rats were randomly selected from each group and euthanized at 72 h after RIRI. To detect the IL-23 and IL-17 protein expressions in the retina, immunohistochemistry was performed as previously reported on paraffin sections. Three sections of each paraffin-embedded eye sample from each group were processed for immunohistochemistry [27]. In brief, eye sections were deparaffinized in xylene and treated in a series of ethanol solutions. Then, antigen retrieval was performed in EDTA retrieval buffer. After washing with phosphate buffer saline (PBS), endogenous peroxidase was blocked by incubation in methanol containing 0.3% hydrogen peroxide for 25 min. Subsequently, sections were incubated with primary antibody against IL-23 (1:100, ab115759, Abcam, England) or IL-17 (1:100, ab79056, Abcam, England) overnight at 4°C in a humidified environment. After washing, sections were incubated with biotinylated goat anti-rabbit IgG (1:1, K5007, Dako, Denmark) at 37°C for 50 min. Finally, sections were counterstained, dehydrated and mounted after DAB (K5007, Dako, Denmark) staining. Photographs were captured under a microscope with a digital camera, and the protein expressions of IL-23 and IL-17 were evaluated with the Image-Pro Plus 6.0 Software (Media Cybernetics, USA) [27].
Western blot assay of IL-23 and IL-17 expression in the retina
Five rats from each group were sacrificed and the retinal tissues were collected at 12, 24, 72 and 144 h after RIRI. The retinal tissues were homogenized in ice-cold lysis buffer (20 mM HEPES, pH 7.5, 1% Triton X-100, 1 mM EDTA, and 0.1 mol/L NaCl) and centrifuged for 15 min at 4°C. The supernatant was collected, and the protein concentration was measured by a bicinchoninic acid protein assay (Beyotime, Jiangsu, China). An equal amount of protein from each sample (60 μg/lane) was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and then transferred onto a polyvinylidene fluoride membrane. The membranes were blocked in 5% skim milk for 2 h at room temperature before incubation with a primary antibody against IL-23 or IL-17 (Abcam, England) overnight at 4°C. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h, and visualization was done using an enhanced chemiluminescence kit (Pierce Biosciences, Rockford, IL). β-actin (Santa Cruz, USA) served as a loading control. The experiments were repeated 3 times. The optical intensity of each band was semiquantitatively determined with the Image-Pro Plus 6.0 software (Media Cybernetics, USA).
ELISA of IL-23 and IL-17 expression in the retina
Five rats from each group were sacrificed and the retinal tissues were collected at 12, 24, 72 and 144 h after RIPI. The retinal tissues were homogenized and centrifuged, and the protein concentration of the supernatant was quantified. ELISA was employed to detect the IL-23 and IL-17 expression according to the manufacturer’s instructions (R&D Company, USA). Biotin-labeled antibody (100 μl) was added to each well followed by incubation for 2 h at 37°C. The supernatant was removed, and the plate was washed 5 times and then incubated with 100 μl of horseradish peroxidase conjugated avidin working solution for 1 h at 37°C. Subsequently, 90 μl of substrate solution was added to each well, and visualization was performed for 15-30 min at 37°C in dark. Finally, the stop solution was added, and the absorbance (A) was measured at 450 nm with a microplate reader. Standard curve was delineated, and the expressions of IL-23 and IL-17 were calculated according to the standard curve.
Statistical analysis
Data are expressed as the mean ± standard deviation. Two-way ANOVA with post-hoc correction was employed for the comparisons of means among groups, and statistical analysis was done with SPSS version 19.0 (New York, NY). A value of P<0.05 was considered statistically significant.
Results
Protective effects of curcumin on the retina
Figure 1 shows the morphology of rat retinas following different treatments. In NCG, the retinal layers and structure were clear, and the arrangements of GCL, inner nuclear layer (INL) and outer nuclear layer (ONL) were regular. GCL was a single cell layer composed of cells with large cell bodies and clear nuclei. In MG, obvious cell infiltration was observed in GCL and other inner layers. An increase in the infiltrated cells was observed in the retina of MG (vs. NCG, P<0.01), LDCG and HDCG. Retinal edema, evidenced by numerous empty spaces and loosely packed cells, was observed in INL and GCL. The retinal structure of LDCG was similar to that in MG group, but the infiltrated cells reduced and retinal edema was improved to a certain extent. In HDCG, the retina mostly retained its normal morphology, with clear retinal layers and less infiltrated cells.
Figure 1.
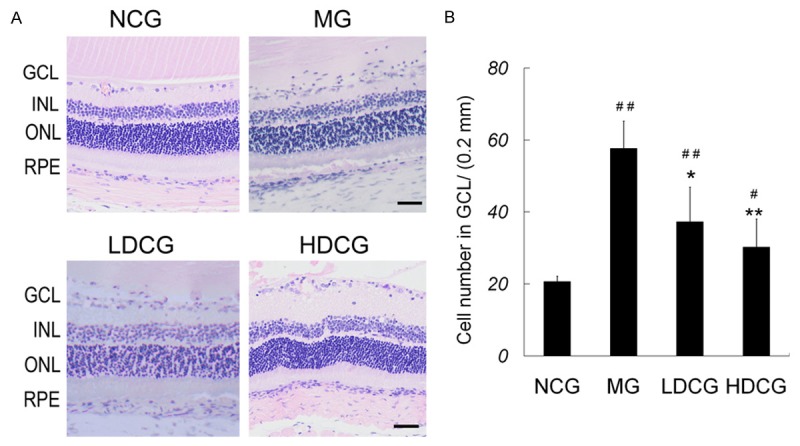
Protective effect of curcumin on the retinal injury after RIRI. A: At 72 h after RIRI, retinal sections were subjected to H&E staining and photographed. Scale bar = 40 mm. B: Number of cells per 200 mm in GCL. # P<0.05 & ## P<0.01, vs. NCG; *P<0.05 & **P<0.01 vs. MG.
Effects of curcumin on IL-23 expression in the retina
Immunohistochemistry showed that IL-23 was mainly expressed in the cytoplasm of GCL and INL cells, and a few cells in ONL were also positive for IL-23 (Figure 2A). Positive cells were rarely found in the retina of NCG, whereas many brown cells were observed in rats of MG. In LDCG, many IL-23 positive cells were found in GCL and INL, but fewer positive cells were noted than in MG. In HDCG, the IL-23 positive cells further reduced.
Figure 2.
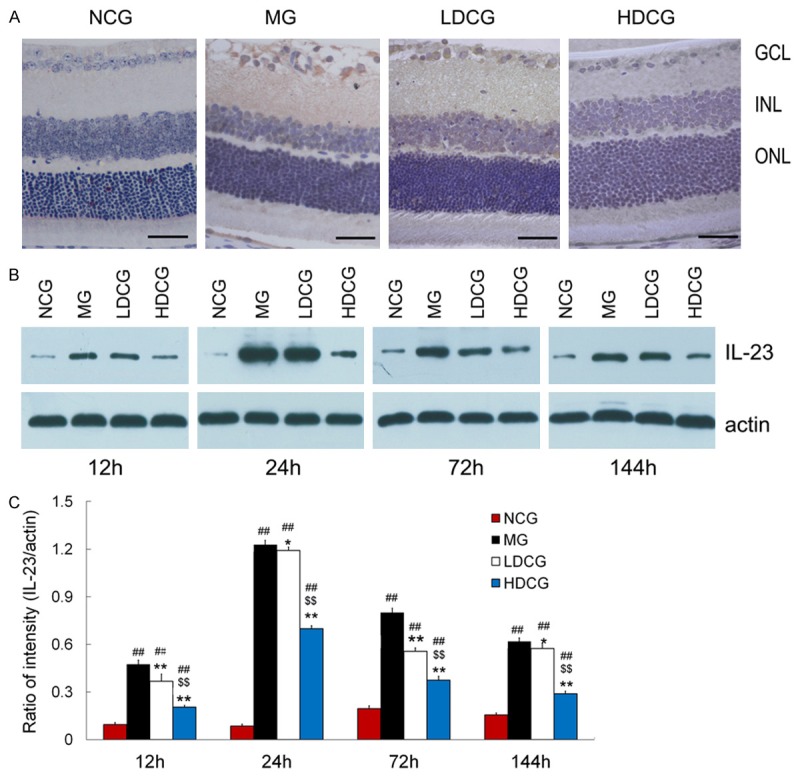
Effect of curcumin on IL-23 expression in the retina after RIRI. A: Retinal sections were processed for immunohistochemistry for IL-23 (scale bar = 40 mm, n = 5 per group). IL-23 expression was expressed in the cytoplasm of cells mainly in the GCL and INL. B: Western blot assay of IL-23 protein expression in the retina. C: Quantification of IL-23 protein expression following Western blot assay (B). Data are from an average of 3 individual experiments and expressed as mean ± standard deviation. # P<0.05 & ## P<0.01, vs. NCG; *P<0.05 & **P<0.01 vs. MG; $ P<0.05 & $$ P<0.01 vs. LDCG.
Western blot assay showed a very low IL-23 protein expression in the retina of NCG (Figure 2B, 2C). However, IL-23 expression increased significantly in MG. IL-23 expression in MG were 0.46 ± 0.03, 1.25 ± 0.02, 0.80 ± 0.04 and 0.63 ± 0.03 at 12, 24, 72 and 144 h, respectively. A similar trend in IL-23 expression was observed in LDCG and HDCG, but the overall IL-23 expression in LDCG and HDCG reduced by 21.74% and 56.52%, respectively, at 12 h, 4.00% and 44.00%, respectively, at 24 h, 28.75% and 53.75%, respectively, at 72 h, and 9.52% and 53.57%, respectively, at 144 h as compared to MG group. The IL-23 expression in MG group was significantly higher than that in NCG and HDCG at the corresponding time points (P<0.01).
ELISA indicated that IL-23 expression increased significantly in MG and LDCG after RIRI (vs. NCG, P<0.01) (Table 1). Moreover, IL-23 expression began to increase at 12 h and reached a peak at 24 h in MG after RIRI. In HDCG, IL-23 expression also increased, and was still significantly higher than in NCG group (P<0.05) except at 12 h. When compared with MG, IL-23 expression significantly decreased in LDCG and HDCG at different time points (P<0.01).
Table 1.
IL-23 expression in the retina (ELISA; pg/ml)
| Group | 12 h | 24 h | 72 h | 144 h |
|---|---|---|---|---|
| NCG | 9.70 ± 0.28 | 9.8 ± 0.24 | 9.84 ± 0.26 | 9.77 ± 0.22 |
| MG | 24.93 ± 1.81## | 48.67 ± 0.51## | 32.44 ± 1.99## | 18.45 ± 1.1## |
| LDCG | 17.18 ± 1.05##,** | 41.27 ± 1.51##,** | 25.35 ± 1.25##,** | 13.73 ± 0.45##,** |
| HDCG | 10.11 ± 0.13**,$$ | 29.21 ± 0.78##,**,$$ | 18.01 ± 0.32##,**,$$ | 10.84 ± 0.45##,**,$$ |
Footnotes: Data are expressed as mean ± standard deviation. # P<0.05 & ## P<0.01 vs. NCG; * P<0.05 & ** P<0.01 vs. MG; $ P<0.05 & $$ P<0.01 vs. LDCG; n = 3 per group.
Effects of curcumin on IL-17 expression in the retina
Immunohistochemistry showed no positive cells in NCG, whereas a variety of positive cells were found in MG (Figure 3A). IL-17 positive cells were mostly found in GCL and INL, and few in ONL. In LDCG, fewer positive cells were observed than in MG. In HDCG, few positive cells were noted.
Figure 3.
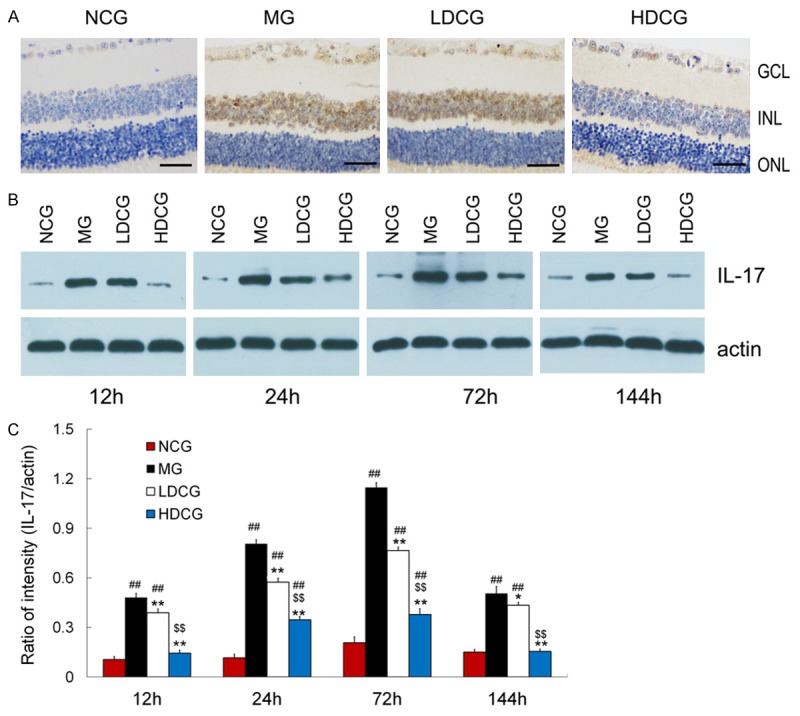
Effect of curcumin on IL-17 expression in the retina. (A) Immunohistochemistry showed IL-17 expression in the retina of different groups (scale bar = 40 mm, n = 5 per group). IL-17 expression was expressed in the cytoplasm of cells mainly in the GCL and INL. (B) Western blot assay indicated IL-17 expression in 4 groups. (C) Quantification of IL-17 protein expression after Western blot assay (B). Data are from an average of 3 individual experiments and expressed as mean ± standard deviation. # P<0.05 & ## P<0.01, vs. NCG; *P<0.05 & **P<0.01 vs. MG; $ P<0.05 & $$ P<0.01 vs. LDCG.
Western blot assay showed a very low IL-17 protein expression in the retina of NCG (Figure 3B, 3C). However, its expression increased after RIRI. In MG, IL-17 expression was 0.48 ± 0.03, 0.81 ± 0.03, 1.16 ± 0.02 and 0.51 ± 0.06 at 12, 24, 72 and 144 h, respectively. Although the IL-17 protein expression increased and reached a peak at 72 h in both LDCG and HDCG, it reduced after curcumin treatment and a significant decrease was observed in HDCG (P<0.01). Curcumin decreased IL-17 expression in LDCG and HDCG by 20.83% and 33.62%, respectively, at 12 h, 70.83% and 67.24%, respectively, at 24 h, 29.63% and 13.73%, respectively, at 72 h, and 56.80% and 68.63%, respectively, at 144 h, when compared with MG.
Similarly, ELISA indicated that IL-17 expression began to increase at 12 h and reached a peak at 72 h after RIRI. In MG and LDCG, IL-17 expression significantly increased when compared with NCG at corresponding time points (P<0.01) (Table 2). In HDCG, IL-17 expression tended to be higher than in NCG at 12, 24 and 144 h, although significant differences were not observed (P>0.05). When compared with MG, IL-17 expression in LDCG and HDCG significantly reduced (P<0.01).
Table 2.
IL-17 expression in the retina (ELISA; pg/ml)
| Group | 12 h | 24 h | 72 h | 144 h |
|---|---|---|---|---|
| NCG | 5.45 ± 0.41 | 5.24 ± 0.56 | 5.26 ± 0.73 | 5.23 ± 0.55 |
| MG | 24.17 ± 0.87## | 32.84 ± 3.2## | 51.16 ± 1.73## | 32.28 ± 0.96## |
| LDCG | 16.23 ± 0.88##,** | 16.14 ± 1.99##,** | 30.83 ± 0.58##,** | 20.65 ± 1.80##,** |
| HDCG | 5.71 ± 0.63**,$$ | 5.87 ± 0.46**,$$ | 13.06 ± 2.39##,**,$$ | 6.63 ± 1.16**,$$ |
Footnotes: Data are expressed as mean ± standard deviation. # P<0.05 & ## P<0.01 vs. NCG; * P<0.05 & ** P<0.01 vs. MG; $ P<0.05 & $$ P<0.01 vs. LDCG; n = 3 per group.
Discussion
RIRI is a common pathophysiological process and characterized by the thinning of retinal nerve fibers and GCL and INL cellular apoptosis. RIRI usually results in decreased visual acuity or even leads to vision loss [3]. Our results showed that the retina in MG displayed edema, infiltration of leukocytes and structural disorder. Immunohistochemistry showed the IL-17- and IL-23-positive cells increased in the GCL and INL of MG. Western blot assay also revealed very low IL-23 and IL-17 protein expression in the retina of NCG, but they increased significantly after RIRI. Moreover, ELISA indicated that IL-23 and IL-17 expressions began to increase at 12 h, IL-23 expression peaked at 24 h and IL-17 expression peaked at 72 h. These findings suggest that IL-23 and IL-17 may participate in the pathology of RIRI, possibly aggravating the retinal injury. After curcumin treatment, retinal IL-23 and IL-17 expressions were significantly inhibited. Furthermore, curcumin-mediated inhibition of IL-23 and IL-17 expression was associated with the dose of curcumin. These results indicate that curcumin is able to reduce the expressions of IL-23 and IL-17 following RIRI, and then attenuated the inflammatory response of the retina.
Studies have shown that the inflammatory cascade produced after retinal ischemia is the main cause of delayed neuronal damage and usually caused by the up-regulated expression of inflammatory factors [2,4]. IL-17 acts as a powerful pro-inflammatory cytokine and can promote the activation of T cells and stimulate fibroblasts, endothelial cells, macrophages, and epithelial cells to secrete various pro-inflammatory factors, such as IL-6, TNF-α, iNOS, metalloproteinases, and prostaglandin E2, and chemokines (such as MCP-1 and granulocyte colony-stimulating factor), amplifying the inflammatory response [28,29]. However, IL-17 may also attract and activate neutrophils, mediating the inflammatory response and leading to tissue injury. Our results indicated that IL-23 and IL-17 expression increased following RIRI, which attributed to the inflammatory response in the retina. Studies have revealed that IL-17 may activate mitogen-activated protein kinase (MAPK) and NF-κB pathways [30,31]. After IL-17 binds to its receptor, the MAPK pathway induces the up-regulation of iNOS and cyclooxygenase-2 expressions, while the NF-κB pathway may stimulate the secretion of IL-6, TNF-α, MCP-1, intercellular adhesion molecule-1 and other inflammatory factors [32].
Curcumin is a phenolic pigment extracted from Curcuma longa L and Curcuma aromatica Salisb and possesses a powerful anti-inflammatory activity [24]. Curcumin is a promising candidate in the clinical treatment of rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease and other inflammatory diseases [33-35]. Studies on the anti-inflammatory activity of curcumin in eye diseases mainly focus on diabetic retinopathy, retinal degeneration, and glaucoma [19]. In a diabetic retinopathy model, curcumin was found to significantly reduce the hyperglycemia-induced oxidation and inhibit the IL-1, VEGF and NF-κB [36]. In a retinal light injury model, curcumin was found to not only inhibit NF-κB expression but suppress NF-κB activation by blocking IkB phosphorylation, resulting in the down-regulated expressions of downstream inflammatory factors [37]. In the present study, RIRI was induced by anterior chamber perfusion of normal saline, whether IL-23 and IL-17 participated in RIRI was investigated, and the effects of curcumin at different concentrations on the retinal IL-23 and IL-17 expressions and the retinal structure were explored at different times after RIRI. Our results provide a new theoretical basis for the anti-inflammatory activity of curcumin in RIRI.
Our results not only showed that the IL-23/IL-17 pathway participated in the pathology of RIRI but revealed that IL-23 reached its peak at 24 h, whereas IL-17 peaked at 72 h after RIRI. In a study on the cerebral ischemia-reperfusion, results also showed the induction of IL-17 expression was dependent on IL-23, suggesting that IL-23 acts immediately after injury, whereas IL-17 plays an important role in the delayed phase of the injury [14]. Therefore, to inhibit IL-17 production in the late phase is promising for the treatment of RIRI. However, RIRI is a complex pathological process involving numerous factors and a variety of pathways. Which type of retinal cells secrete IL-23 and IL-17 and the exact mechanism underlying the inhibitory effect of curcumin on the IL-23 and IL-17 expressions remain to be further studied.
In conclusion, our findings reveal that IL-23 and IL-17 expressions increase in the retinal after RIRI and curcumin may attenuate the retinal inflammation and reduce the IL-23 and IL-17 expressions in a dose-dependent manner following RIRI. These results provide evidence for the treatment of RIRI with curcumin.
Acknowledgements
This study was supported in part by the Doctoral Program of Higher Education, Ministry of Education and the Ophthalmologic State Key Laboratory, Sun-Yat Sun University, P. R. China.
Disclosure of conflict of interest
None.
References
- 1.Kramer M, Dadon S, Hasanreisoglu M, Monselise Y, Avraham BR, Feldman A, Eldar I, Weinberger D, Goldenberg-Cohen N. Proinflammatory cytokines in a mouse model of central retinal artery occlusion. Mol Vis. 2009;15:885–894. [PMC free article] [PubMed] [Google Scholar]
- 2.Andreeva K, Zhang M, Fan W, Li X, Chen Y, Rebolledo-Mendez JD, Cooper NG. Time-dependent Gene Profiling Indicates the Presence of Different Phases for Ischemia/Reperfusion Injury in Retina. Ophthalmol Eye Dis. 2014;6:43–54. doi: 10.4137/OED.S17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Dvoriantchikova G, Barakat DJ, Hernandez E, Shestopalov VI, Ivanov D. Liposome-delivered ATP effectively protects the retina against ischemia-reperfusion injury. Mol Vis. 2010;16:2882–2890. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Luo C, Penalva R, Xu H. Paraquat-induced retinal degeneration is exaggerated in CX3CR1-deficient mice and is associated with increased retinal inflammation. Invest Ophthalmol Vis Sci. 2013;54:682–690. doi: 10.1167/iovs.12-10888. [DOI] [PubMed] [Google Scholar]
- 6.Jo N, Wu GS, Rao NA. Upregulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2003;44:4054–4060. doi: 10.1167/iovs.02-1308. [DOI] [PubMed] [Google Scholar]
- 7.Huang P, Huo Y, Lou LX, Li H, Barnstable CJ, Zhang C, Zhang SS. CD4 positive T helper cells contribute to retinal ganglion cell death in mouse model of ischemia reperfusion injury. Exp Eye Res. 2013;115:131–139. doi: 10.1016/j.exer.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Kramer JM, Gaffen SL. Interleukin-17: a new paradigm in inflammation, autoimmunity, and therapy. J Periodontol. 2007;78:1083–1093. doi: 10.1902/jop.2007.060392. [DOI] [PubMed] [Google Scholar]
- 9.Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11:625–630. doi: 10.1016/j.micinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 11.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Shichita T, Sakaguchi R, Suzuki M, Yoshimura A. Post-ischemic inflammation in the brain. Front Immunol. 2012;3:132. doi: 10.3389/fimmu.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li HL, Kostulas N, Huang YM, Xiao BG, van der Meide P, Kostulas V, Giedraitas V, Link H. IL-17 and IFN-gamma mRNA expression is increased in the brain and systemically after permanent middle cerebral artery occlusion in the rat. J Neuroimmunol. 2001;116:5–14. doi: 10.1016/s0165-5728(01)00264-8. [DOI] [PubMed] [Google Scholar]
- 14.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 15.Konoeda F, Shichita T, Yoshida H, Sugiyama Y, Muto G, Hasegawa E, Morita R, Suzuki N, Yoshimura A. Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochem Biophys Res Commun. 2010;402:500–506. doi: 10.1016/j.bbrc.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 16.Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, Yuan J, Jevallee H, Wei F, Shi GP, Cheng X. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012;59:420–429. doi: 10.1016/j.jacc.2011.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi P, Yuan Q, Torres D, Delbauve S, Laute MA, Lalmand MC, Petein M, Goriely S, Goldman M, Flamand V. Interferon regulatory factor 3 deficiency leads to interleukin-17-mediated liver ischemia-reperfusion injury. Hepatology. 2013;57:351–361. doi: 10.1002/hep.26022. [DOI] [PubMed] [Google Scholar]
- 18.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Med. 2014;80:249–254. doi: 10.1055/s-0033-1351074. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burugula B, Ganesh BS, Chintala SK. Curcumin attenuates staurosporine-mediated death of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011;52:4263–4273. doi: 10.1167/iovs.10-7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie P, Zhang W, Yuan S, Chen Z, Yang Q, Yuan D, Wang F, Liu Q. Suppression of experimental choroidal neovascularization by curcumin in mice. PLoS One. 2012;7:e53329. doi: 10.1371/journal.pone.0053329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldebasi YH, Aly SM, Rahmani AH. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int J Physiol Pathophysiol Pharmacol. 2013;5:194–202. [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, You ZP. Curcumin inhibits human retinal pigment epithelial cell proliferation. Int J Mol Med. 2014;34:1013–1019. doi: 10.3892/ijmm.2014.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Li C, Guo H, Kern TS, Huang K, Zheng L. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS One. 2011;6:e23194. doi: 10.1371/journal.pone.0023194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Yang F, Cheng H, Liu C, Sun M, Wu K, Ai M. Protective effects of total flavonoids from Flos Puerariae on retinal neuronal damage in diabetic mice. Mol Vis. 2013;19:1999–2010. [PMC free article] [PubMed] [Google Scholar]
- 28.Kawanokuchi J, Shimizu K, Nitta A, Yamada K, Mizuno T, Takeuchi H, Suzumura A. Production and functions of IL-17 in microglia. J Neuroimmunol. 2008;194:54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 30.Song X, Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 32.Chen Y, Kijlstra A, Chen Y, Yang P. IL-17A stimulates the production of inflammatory mediators via Erk1/2, p38 MAPK, PI3K/Akt, and NF-kappaB pathways in ARPE-19 cells. Mol Vis. 2011;17:3072–3077. [PMC free article] [PubMed] [Google Scholar]
- 33.Park C, Moon DO, Choi IW, Choi BT, Nam TJ, Rhu CH, Kwon TK, Lee WH, Kim GY, Choi YH. Curcumin induces apoptosis and inhibits prostaglandin E(2) production in synovial fibroblasts of patients with rheumatoid arthritis. Int J Mol Med. 2007;20:365–372. [PubMed] [Google Scholar]
- 34.Xie L, Li XK, Takahara S. Curcumin has bright prospects for the treatment of multiple sclerosis. Int Immunopharmacol. 2011;11:323–330. doi: 10.1016/j.intimp.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Salh B, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- 36.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


