Abstract
Sex determining region Y-box 2 (SOX2) is a transcription factor involved in self-renewal and pluripotency. Dysregulation of SOX2 expression has been found in squamous cell carcinoma (SCC), including esophageal SCC. Recently, high SOX2 expression was found to be a negative predictor of occult lymph node metastasis in early oral SCC, but the clinical significance of SOX2 expression in esophageal SCC remains controversial. Here we investigated SOX2 expression by immunohistochemistry in 75 cases of surgically resected esophageal SCC. Similar to oral SCC, we found for the first time that high SOX2 expression correlates with absence of clinical nodal metastasis (P = 0.011). Podoplanin is a glycoprotein which is variably expressed by esophageal SCC. Since we previously found that podoplanin expression correlates with nodal metastasis in esophageal SCC, we also assessed podoplanin expression in these cases. Interestingly, SOX2 expression correlates negatively with podoplanin expression (P = 0.018). It is in contrast with a recent finding that SOX2 can up-regulate podoplanin expression in SCC of the skin. Our result suggests that SOX2 might suppress nodal metastasis through down-regulation of podoplanin in esophageal SCC. Further studies are needed to clarify the exact mechanism of regulation.
Keywords: Sex determining region Y-box 2 (SOX2), esophagus, squamous cell carcinoma, podoplanin, nodal metastasis
Introduction
Sex determining region Y (SRY)-box 2 (SOX2) is a member of the SRY-related high-mobility group (HMG)-box transcription factors [1]. It is an important key transcription factor involved in self-renewal and pluripotency of embryonic stem cells [2]. Dysregulation of SOX2 expression has been found in different human malignancies. Of note, expression of SOX2 was recently found to be associated with progression and invasiveness of esophageal squamous cell carcinoma (SCC) [3]. In accord with this, SOX2 has been found to be a commonly amplified lineage-specific oncogene in esophageal SCC [4]. Recently, high SOX2 expression was found to be a negative predictor of occult lymph node metastasis in early oral SCC [5]. Oral SCC and esophageal SCC are known to share common risk factors such as consumption of alcohol, tobacco and betel quid [6]. Synchronous or metachronous SCC involving both oral cavity and esophagus is also frequently seen [7]. These findings suggest that the pathogenesis and tumor biology in SCCs of these two sites may be similar. Since the clinical significance of SOX2 expression in esophageal SCC remains controversial [8-10], it would be interesting to check whether high SOX2 expression also prevents nodal metastasis in our esophageal SCC patients.
Podoplanin is a type 1 transmembrane mucin-like glycoprotein which was originally named due to its expression in renal glomerular podocytes of rats [11]. It is expressed in various normal human tissues and is involved in different physiologic functions, such as lymphangiogenesis [12], platelet aggregation [13], and regulation of glomerular filtration [14]. It is also variably expressed by SCC in different organs, and there is growing evidence that podoplanin is involved in lymph node metastasis, carcinogenesis, cell motility, tumor invasiveness, and hematogenous metastasis [15]. Previously, we found that tumor cell expression of podoplanin correlates with lymph node metastasis in esophageal SCC [16]. A recent study showed that SOX2 expression can up-regulate podoplanin expression in SCC of the skin [17]. To date, only a small cohort (n = 20) of esophageal SCC has been studied for both SOX2 and podoplanin expression, and the correlation between SOX2 and podoplanin has not been assessed [10]. It would also be interesting to know if the transcription factor SOX2 could regulate podoplanin expression in esophageal SCC.
In this study, we investigated tumor cell expression of SOX2 and podoplanin by immunohistochemistry in 75 cases of surgically resected esophageal SCC. The result was correlated with clinicopathologic features and patient survival.
Materials and methods
Patients
A total of 75 cases of surgically resected esophageal SCC were recruited for this study. Some of these cases had been previously studied for tumor cell expression of podoplanin [16]. Fifty-one of the patients received pre-operative concurrent chemoradiotherapy (CCRT). Pathologic and pre-operative clinical staging was performed according to the 7th edition of AJCC Cancer Staging Manual [18].
Immunohistochemistry
Resected esophageal SCC and adjacent normal tissue were fixed in 10% buffered neutral formalin, dehydrated and embedded in paraffin. Sections were stained with hematoxylin and eosin for morphologic evaluation. Additional 4-µm-thick sections were taken, deparaffinized and rehydrated for immunohistochemical study. We used a mouse anti-human SOX2 monoclonal antibody (Clone GT1352, Abcam, 1:250) as the primary antibody. Immunohistochemistry for SOX2 was performed using a fully automated machine Bond-Max (Leica). Heat-induced epitope retrieval was achieved using a pH 9.0 EDTA-based solution (ER2, Leica) at 121°C for 20 minutes. The staining pattern for SOX2 was nuclear in all cases. Positive staining for basal cells of normal esophageal mucosa served as internal control. For podoplanin, we used a mouse anti-human podoplanin monoclonal antibody (Clone D2-40, Dako, 1:100) as the primary antibody and followed a previously published protocol for immunohistochemical study [16]. The staining pattern for podoplanin was cytoplasmic in all cases. Positive staining for lymphatic endothelial cells served as internal control.
The immunostained slides were evaluated by two pathologists (W.-Y. C. and C.-J. Y.) under a dual-head microscope without knowing the clinicopathologic information. For SOX2 expression, a Histo-score (H-score; range = 0~300) was calculated by multiplying the intensity score (0 = negative; 1 = weak; 2 = intermediate; 3 = strong; Figure 1) and the fraction score (percentage of positive tumor cells; range = 0~100). The tumor cell expression of podoplanin was evaluated as previously described [16].
Figure 1.
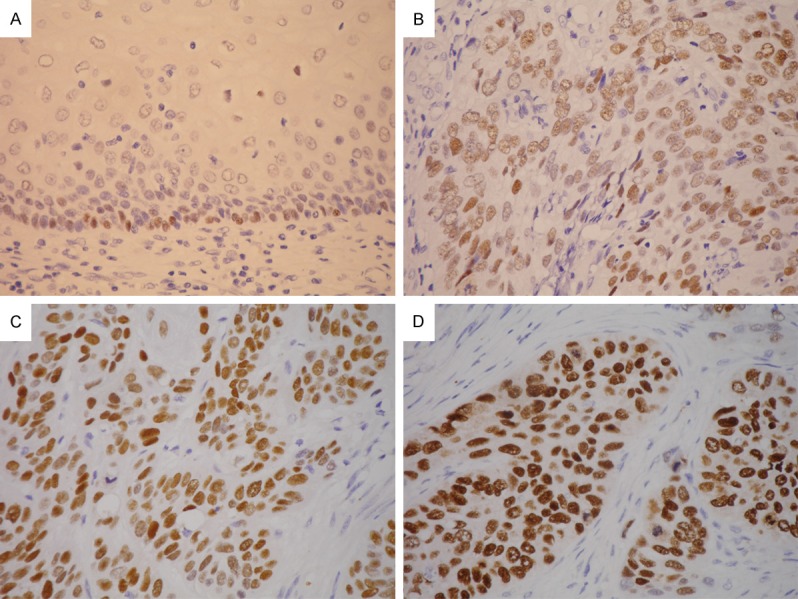
Immunohistochemistry for SOX2 showed positive nuclear staining in basal cells of normal esophageal mucosa (A. × 400). The intensity scores of tumor cells were graded as 1 (weak, B. × 400), 2 (intermediate, C. × 400), or 3 (strong, D. × 400).
Statistical analysis
Differences in categorical data were assessed by a chi-square test, and Yates’ correction was performed if expected frequencies less than 5 were encountered. Difference in age between groups was assessed by the Mann-Whitney U-test. Overall survival was analyzed by the Kaplan-Meier method and compared by log-rank tests. The influence of parameters on survival was analyzed using Cox regression. P value < 0.05 was considered statistically significant. All statistical analyses were performed using the WinSTAT® for Excel (R. Fitch Software, Bad Krozingen, Germany).
Results
SOX2 expression and clinicopathologic characteristics
For SOX2, the H-scores of the tumors ranged from 0 to 195, with a median of 15. Therefore, an H-score of 15 or higher was considered high SOX2 expression (n = 40), whereas an H-score of 14 or lower was considered low expression (n = 35). Tumor cell expression of SOX2 was not correlated with patient survival (P = 0.92; Figure 2). The clinicopathologic characteristics of patients grouped by SOX2 expression were listed in Table 1. High SOX2 expression was significantly associated with absence of clinical nodal metastasis (cN0; P = 0.011). The result is similar to the previous study on oral SCC [5]. We found no correlation of SOX2 expression with age at diagnosis, gender, preoperative CCRT, tumor grade, pT classification, pN, pM, pathologic stage, cT, cM or clinical stage.
Figure 2.
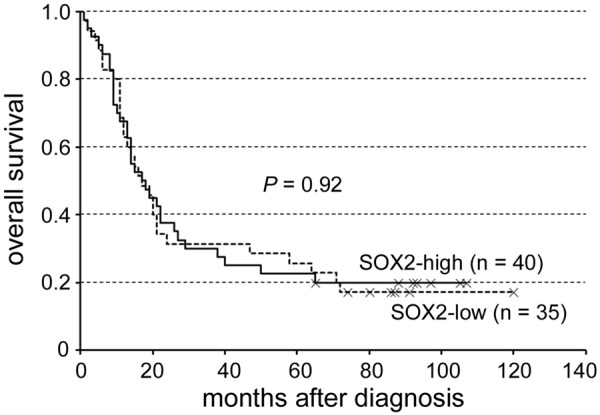
SOX2 expression was not correlated with patient survival (P = 0.92).
Table 1.
Clinicopathologic characteristics of cases grouped by SOX2 expression
| Characteristic | SOX2 expression | Total (n = 75) | P value | |
|---|---|---|---|---|
|
| ||||
| Low (n = 35) | High (n = 40) | |||
| Age at diagnosis | ||||
| Mean ± SD | 57 ± 12 | 58 ± 12 | 57 ± 12 | 0.33 |
| Median (min; max) | 59 (37; 100) | 60 (32; 85) | 58 (32; 100) | |
| Gender (%) | ||||
| Female | 2 (6) | 1 (3) | 3 (4) | 0.91 |
| Male | 33 (94) | 39 (97) | 72 (96) | |
| Pre-operative CCRT (%) | ||||
| Yes | 27 (77) | 24 (60) | 51 (68) | 0.11 |
| No | 8 (23) | 16 (40) | 24 (32) | |
| Tumor grade (%) | ||||
| Grade 1 | 4 (11) | 1 (3) | 5 (7) | 0.51 |
| Grade 2 | 25 (72) | 29 (72) | 54 (72) | |
| Grade 3 | 6 (17) | 10 (25) | 16 (21) | |
| pT (%) | ||||
| pT1-2 | 12 (34) | 13 (33) | 25 (33) | 0.87 |
| pT3-4 | 23 (66) | 27 (67) | 50 (67) | |
| pN (%) | ||||
| pN0 | 20 (57) | 25 (63) | 45 (60) | 0.64 |
| pN1-3 | 15 (43) | 15 (37) | 30 (40) | |
| pM (%) | ||||
| pM0 | 32 (91) | 38 (95) | 70 (93) | 0.88 |
| pM1 | 3 (9) | 2 (5) | 5 (7) | |
| Pathologic stage (%) | ||||
| I/II | 20 (57) | 24 (60) | 44 (59) | 0.80 |
| III/IV | 15 (43) | 16 (40) | 31 (41) | |
| cT (%)a | ||||
| cT1-2 | 10 (32) | 15 (43) | 25 (38) | 0.38 |
| cT3-4 | 21 (68) | 20 (57) | 41 (62) | |
| cN (%)a | ||||
| cN0 | 6 (19) | 16 (50) | 22 (35) | 0.011 |
| cN1-3 | 25 (81) | 16 (50) | 41 (65) | |
| cM (%)a | ||||
| cM0 | 29 (97) | 26 (96) | 55 (96) | 1.0 |
| cM1 | 1 (3) | 1 (4) | 2 (4) | |
| Clinical stage (%)a | ||||
| I/II | 8 (35) | 13 (52) | 21 (44) | 0.23 |
| III/IV | 15 (65) | 12 (48) | 27 (56) | |
| Podoplanin expression (%) | ||||
| High | 20 (57) | 12 (30) | 32 (43) | 0.018 |
| Low | 15 (43) | 28 (70) | 43 (57) | |
SD: standard deviation; CCRT: concurrent chemoradiotherapy.
Some cases were excluded due to incomplete pre-treatment clinical staging.
Correlation between SOX2 expression and podoplanin expression
Podoplanin expression was evaluated as previously described [16]. Thirty-two cases had high podoplanin expression, whereas the other 43 cases had low podoplanin expression. In contrast to the previously found up-regulation of podoplanin by SOX2 in SCC of the skin [17], we found that high SOX2 expression was significantly associated with low podoplanin expression (P = 0.018; Table 1; Figure 3).
Figure 3.
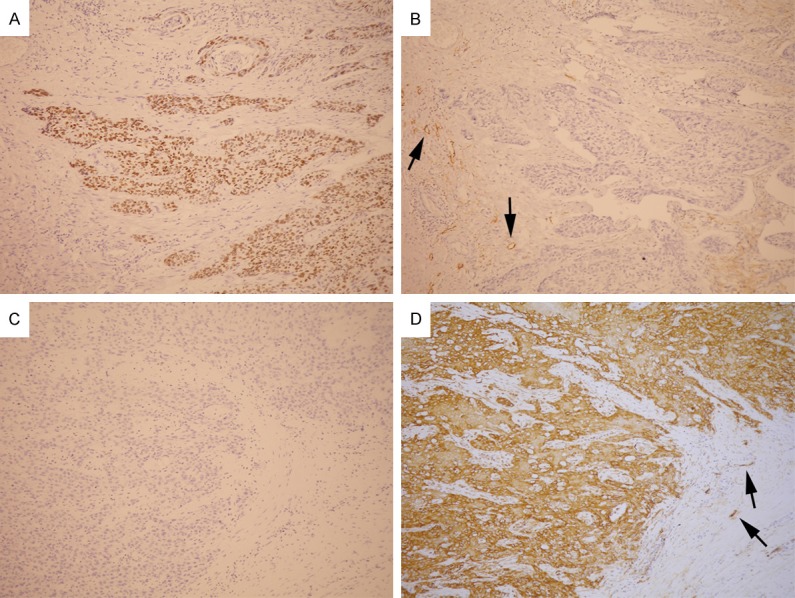
A tumor with high SOX2 expression (A. × 100) showed low podoplanin expression (B. × 100). Another tumor with low SOX2 expression (C. × 100) showed high podoplanin expression (D. × 100). Lymphatic endothelial cells (arrows) served as internal positive control for podoplanin immunostaining.
Discussion
Our study showed that SOX2 expression correlates negatively with clinical lymph node metastasis and podoplanin expression in esophageal SCC.
SOX2 is an SRY-related HMG-box transcription factor which has a critical role in self-renewal and pluripotency of embryonic stem cells [2]. It was found to be critical in the development of multiple organs including esophagus in mice. Developmental fate mapping showed that SOX2 (+) adult stem cells at the basal layer of esophageal mucosa originate from fetal SOX2 (+) tissue progenitors [19]. SOX2 gene is commonly amplified and functions as an oncogene in lung and esophageal SCC [4]. Conditional overexpression of SOX2 in basal cells expands the progenitor population in esophagus of mice, and co-overexpression of SOX2 and activated STAT3 drives malignant transformation of esophageal basal cells [20].
The clinical significance of SOX2 expression in esophageal SCC is controversial. A previous study on Chinese esophageal SCC patients showed that high SOX2 expression correlates with poor prognosis and high tumor grade [9], but a later study on another large cohort of Chinese esophageal SCC patients showed no influence of SOX2 expression on survival [8]. Another study on a small group (n = 20) of Japanese esophageal SCC patients after chemoradiotherapy showed correlation of SOX2 expression with high tumor grade, lymphatic invasion, and vascular invasion [10]. Regarding SCC of other organs, SOX2 gene amplification and protein overexpression were associated with better prognosis in SCC of the lung [21,22]. A study on hypopharyngeal, laryngeal and sinonasal SCC found no correlation between SOX2 expression and survival [23].
The controversial results regarding clinical significance of SOX2 expression in SCCs of different organs and different cohorts of patients might reflect different tumor biology. In addition, in SCCs of both lung and skin, SOX2 was found to regulate dozens of different downstream genes, reflecting its pleiotropic oncogenic role [17,24]. Moreover, different co-factors could act as either a co-activator or a co-repressor depending on different microenvironment and complex interactions among signaling networks regulated by SOX2 [24]. The different results of clinical significance most likely reflect the complexity of gene regulation by SOX2 in SCC.
Of note, a previous study showed that high SOX2 expression is a negative predictor of occult lymph node metastasis in early SCC of the oral cavity [5]. Oral SCC and esophageal SCC are known to share common risk factors [6], and synchronous or metachronous SCC involving both oral cavity and esophagus is also frequently seen [7]. Since the pathogenesis and tumor biology in SCCs of these two sites is likely similar, it is not surprising that we also found that high SOX2 expression correlated with absence of nodal metastasis in esophageal SCC.
Esophageal cancer is a highly lethal disease, and most patients who present with symptoms have either locally advanced or metastatic disease [25]. Radiotherapy and chemotherapy could improve disease control by downstaging cancer to increase respectability [26]. Therefore, preoperative CCRT is widely used in potentially resectable esophageal cancer [25]. Since a large proportion (68%) of our patients received CCRT before surgery, the pN classification might have been underestimated due to eradication of metastatic tumor cells in lymph nodes. Thus the cN classification could reflect better the pre-treatment nodal status. This might explain our result that SOX2 expression negatively correlates with clinical but not pathologic nodal metastasis.
Podoplanin is a 38 kDa type 1 transmembrane mucin-like glycoprotein [27]. In normal human tissue, it is expressed in lymphatic endothelial cells, renal podocytes, skeletal muscle, lung, heart, placenta, myoepithelial cells of glands, osteoblasts, mesothelial cells, follicular dendritic cells, Schwann cells, and occasionally in basal layer of epidermis and esophageal mucosa [15]. Variable podoplanin expression is also found in different tumors, including esophageal and oral SCC. Podoplanin has been found to play important roles in lymph node metastasis, carcinogenesis, cell motility, tumor invasiveness, platelet aggregation and hematogenous metastasis of these tumors [15]. We also found that high tumor cell expression of podoplanin correlates with clinical nodal metastasis in esophageal SCC [16]. We later found that concordant podoplanin expression in tumor cells and cancer-associated fibroblasts is an adverse prognostic factor in esophageal SCC [28].
Our result showed that high SOX2 expression of esophageal SCC correlated with low podoplanin expression and less clinical nodal metastasis. It suggests that the transcription factor SOX2 could down-regulate podoplanin and thus inhibit nodal metastasis. This is in contrast to the recent finding that SOX2 can up-regulate podoplanin expression in SCC of the skin [17]. Previously, there was a study which worked on the expression of SOX2 and podoplanin in a small cohort (n = 20) of esophageal SCC patients after neoadjuvant chemoradiotherapy [10]. However, the correlation between SOX2 and podoplanin expression was not analyzed, and the number of patients was considerably small. It is noteworthy that a Swiss group found podoplanin expression correlated with sentinel lymph node metastasis in early SCC of the oral cavity and oropharynx [29]. Later on, the same group also found that high SOX2 expression is a negative predictor of occult lymph node metastasis in early SCC of the oral cavity [5]. But the correlation between SOX2 and podoplanin expression was again not performed. It would be of interest to see whether similar negative correlation between SOX2 and podoplanin expression is also present in oral SCC.
In conclusion, we demonstrated for the first time that high SOX2 expression in esophageal SCC correlates with low podoplanin expression and absence of clinical lymph node metastasis. It suggests that SOX2 might suppress nodal metastasis through down-regulation of podoplanin in tumor cells. Further studies are warranted to elucidate their exact regulatory mechanism in esophageal SCC.
Acknowledgements
This work was supported by grants from the Department of Education, Taiwan, ROC (EMRPD1E1391) and the Chang Gung Medical Research Program (CMRPG3E0341 and CMRPD1C0041).
Disclosure of conflict of interest
None.
References
- 1.Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 2.Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–1938. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- 3.Forghanifard MM, Ardalan Khales S, Javdani-Mallak A, Rad A, Farshchian M, Abbaszadegan MR. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med Oncol. 2014;31:922. doi: 10.1007/s12032-014-0922-7. [DOI] [PubMed] [Google Scholar]
- 4.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O’Kelly M, Dutt A, Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS, Cecconello I, Jr UR, Marie SK, Dahl O, Shivdasani RA, Tsao MS, Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK, Meyerson M. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Züllig L, Roessle M, Weber C, Graf N, Haerle SK, Jochum W, Stoeckli SJ, Moch H, Huber GF. High sex determining region Y-box 2 expression is a negative predictor of occult lymph node metastasis in early squamous cell carcinomas of the oral cavity. Eur J Cancer. 2013;49:1915–1922. doi: 10.1016/j.ejca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Lee CH, Lee KW, Fang FM, Wu DC, Shieh TY, Huang HL, Chen CH, Chen PH, Chen MK, Kuo SJ, Chang CH, Tsai YS, Chiang SL, Tu HP, Chen BH, Ko YC. The use of tobacco-free betel-quid in conjunction with alcohol/tobacco impacts early-onset age and carcinoma distribution for upper aerodigestive tract cancer. J Oral Pathol Med. 2011;40:684–692. doi: 10.1111/j.1600-0714.2011.01022.x. [DOI] [PubMed] [Google Scholar]
- 7.Wind P, Roullet MH, Douard R, Laccoureye O, Brasnu D, Cugnenc PH. Experience in the treatment of synchronous and metachronous carcinoma of the oesophagus and the head and neck. J Surg Oncol. 2000;73:138–142. doi: 10.1002/(sici)1096-9098(200003)73:3<138::aid-jso5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Sun LL, Wu JY, Wu ZY, Shen JH, Xu XE, Chen B, Wang SH, Li EM, Xu LY. A three-gene signature and clinical outcome in esophageal squamous cell carcinoma. Int J Cancer. 2015;136:E569–77. doi: 10.1002/ijc.29211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, He W, Lu C, Wang Z, Wang J, Giercksky KE, Nesland JM, Suo Z. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res. 2009;29:1233–1241. [PubMed] [Google Scholar]
- 10.Saigusa S, Mohri Y, Ohi M, Toiyama Y, Ishino Y, Okugawa Y, Tanaka K, Inoue Y, Kusunoki M. Podoplanin and SOX2 expression in esophageal squamous cell carcinoma after neoadjuvant chemo-radiotherapy. Oncol Rep. 2011;26:1069–1074. doi: 10.3892/or.2011.1408. [DOI] [PubMed] [Google Scholar]
- 11.Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 12.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, Yamazaki Y, Narimatsu H, Ozaki Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 14.Koop K, Eikmans M, Wehland M, Baelde H, Ijpelaar D, Kreutz R, Kawachi H, Kerjaschki D, de Heer E, Bruijn JA. Selective loss of podoplanin protein expression accompanies proteinuria and precedes alterations in podocyte morphology in a spontaneous proteinuric rat model. Am J Pathol. 2008;173:315–326. doi: 10.2353/ajpath.2008.080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang WY, Chang YS, Yeh CJ, Wu YC, Hsueh C. Role of podoplanin expression in squamous cell carcinoma of upper aerodigestive tract. Histol Histopathol. 2013;28:293–299. doi: 10.14670/HH-28.293. [DOI] [PubMed] [Google Scholar]
- 16.Chuang WY, Yeh CJ, Wu YC, Chao YK, Liu YH, Tseng CK, Chang HK, Liu HP, Hsueh C. Tumor cell expression of podoplanin correlates with nodal metastasis in esophageal squamous cell carcinoma. Histol Histopathol. 2009;24:1021–1027. doi: 10.14670/HH-24.1021. [DOI] [PubMed] [Google Scholar]
- 17.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohée S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, Blanpain C. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 18.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Esophagus and Esophagogastric Junction. In: Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th edition. New York: Springer; 2010. pp. 103–115. [Google Scholar]
- 19.Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu K, Jiang M, Lu Y, Chen H, Sun J, Wu S, Ku WY, Nakagawa H, Kita Y, Natsugoe S, Peters JH, Rustgi A, Onaitis MW, Kiernan A, Chen X, Que J. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, Maier S, Reischl M, Mikut R, Altorki NK, Moch H, Fend F, Staebler A, Bass AJ, Meyerson M, Rubin MA, Soltermann A, Lengerke C, Perner S. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol. 2011;24:944–953. doi: 10.1038/modpathol.2011.49. [DOI] [PubMed] [Google Scholar]
- 22.Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, Dhodapkar K, Deng Y, Gettinger S, Rimm DL. High SOX2 levels predict better outcome in non-small cell lung carcinomas. PLoS One. 2013;8:e61427. doi: 10.1371/journal.pone.0061427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Márquez R, Llorente JL, Rodrigo JP, García-Pedrero JM, Álvarez-Marcos C, Suárez C, Hermsen MA. SOX2 expression in hypopharyngeal, laryngeal, and sinonasal squamous cell carcinoma. Hum Pathol. 2014;45:851–857. doi: 10.1016/j.humpath.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Hussenet T, du Manoir S. SOX2 in squamous cell carcinoma: amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle. 2010;9:1480–1486. doi: 10.4161/cc.9.8.11203. [DOI] [PubMed] [Google Scholar]
- 25.van Meerten E, van der Gaast A. Systemic treatment for oesophageal cancer. Eur J Cancer. 2005;41:664–672. doi: 10.1016/j.ejca.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545–553. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- 27.Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br J Cancer. 2007;96:1–5. doi: 10.1038/sj.bjc.6603518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang WY, Yeh CJ, Chao YK, Liu YH, Chang YS, Tseng CK, Chang HK, Wan YL, Hsueh C. Concordant podoplanin expression in cancer-associated fibroblasts and tumor cells is an adverse prognostic factor in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:4847–4856. [PMC free article] [PubMed] [Google Scholar]
- 29.Huber GF, Fritzsche FR, Züllig L, Storz M, Graf N, Haerle SK, Jochum W, Stoeckli SJ, Moch H. Podoplanin expression correlates with sentinel lymph node metastasis in early squamous cell carcinomas of the oral cavity and oropharynx. Int J Cancer. 2011;129:1404–1409. doi: 10.1002/ijc.25795. [DOI] [PubMed] [Google Scholar]


