Abstract
Prostate cancer is a common malignant tumor in urinary system. Curcumin has curative effect on many kinds of cancers and can inhibit prostate cancer (PC)-3 cells proliferation. This study aimed to explore the curcumin induced prostate cancer cell apoptosis and apoptosis related proteins Bcl-2 and Bax expression. PC-3 cells were injected subcutaneously to the nude mice to establish the tumor model. The nude mice were randomly divided into group C (normal saline), group B (6% polyethylene glycol and 6% anhydrous ethanol), group H, M, L (100 mg/kg, 50 mg/kg, and 25 mg/kg curcumin). The tumor volume was measured every 6 days to draw the tumor growth curve. The mice were killed at the 30th day after injection to weight the tumor. TUNEL assay was applied to determine cell apoptosis. Immunohistochemistry was used to detect Bcl-2 and Bax expression. The tumor volume and weight in group H, M, L were significantly lower than the control group (C, B) (P<0.05), and the inhibitory rate increased following the curcumin dose increase. Compared with the control group, Bcl-2 expression in group H, M, L gradually decreased, while Bax protein expression increased (P<0.05). The cell apoptosis rate showed no statistical difference between group B and C, while it increased in curcumin group H, M, and L (P<0.05). Curcumin could inhibit PC-3 growth, decrease tumor volume, reduce tumor weight, and induce cell apoptosis under the skin of nude mice by up-regulating Bax and down-regulating Bcl-2.
Keywords: Curcumin, prostate cancer, Bcl-2, Bax
Introduction
Prostate cancer is a common malignant tumor in male reproductive system with high mortality rate. Endocrine therapy is the main treatment for advanced prostate cancer [1,2]. However, most patients progress to hormone independent prostate cancer in the treatment course. Radiotherapy, chemotherapy, and biological treatment showed no ideal effect on hormone independent prostate cancer. The main obstacle is hormone independent transformation [3,4]. Chemotherapy is limited by its side effects and multi-resistance. It is important to reduce side effect and slow down tumor multi-drug resistance formation in treating prostate cancer. Curcumin is a type of natural phenolic pigment extracted from rhizoma zedoariae and turmeric rhizome zingiberaceae plants with diversiform pharmacological activities including anti-inflammation, anti-tumor, and antioxidant action [5,6]. Studies have shown that curcumin has significant clinical efficacy on many kinds of cancers, such as pancreatic cancer and colon cancer [7]. Its antitumor mechanism might be related to the induction of cell apoptosis through regulating cell cycle, oncogenes, tumor suppressor genes, and their protein expression. Curcumin can also inhibit tumor angiogenesis through down-regulating VEGF and tumor metastasis related factors expression [8-10]. Bax is a type of apoptosis promoting factor, while the Bcl-2 is a kind of apoptosis inhibiting factor. Their expression level is related to cell apoptosis. This research used curcumin in vivo to detect its role on tumor proliferation establishing androgen independence prostate cancer cell PC-3 subcutaneous transplantation tumor model in nude mice.
Materials and methods
Cell line and cell culture
Human prostate cancer cell PC-3 was bought from the Chinese academy of sciences, Shanghai institute of biochemistry and cell biology. The cells were cultivated in IMDM medium containing 10% fetal bovine serum, 100 u/ml penicillin-streptomycin at 37°C and 5% CO2. The medium was changed every two days and the cells were passaged every 5 days.
Animals
40 male SPF BALB/c nude mice weight 18-20 g at 4-6 weeks old were provided by the institute of Chinese academy of medical sciences tumor animal center and raised in SPF animal laboratory. The mice eating and drinking accord with experimental animal standard.
Reagents
Curcumin was provided by the Sigma Company. Ethanol and polyethylene glycol were purchased from Xi’an Tianzheng medicinal materials co., LTD. In situ terminal-deoxynucleoitidyl transferase mediated nick end labeling apoptosis detection kit, Bcl-2 and Bax monoclonal antibody, and the Bcl-2, Bax immunohistochemical kit were supplied by the Wuhan Boster biotechnology company.
Animal model
PC-3 cells in logarithmic phase were digested into single cell suspension at 1×107/L. 0.1 ml cells were injected to the left subcutaneous anterior axillary of the nude mice under aseptic condition. The tumor diameter a and short diameter b were measured every day to draw the tumor growth curve.
Grouping and administration
When the tumor volume reached 300 mm3, the nude mice were randomly divided into group C (normal saline), group B (6% polyethylene glycol and 6% anhydrous ethanol), group H, M, L (100 mg/kg, 50 mg/kg, and 25 mg/kg curcumin) with eight mice in each group. The mice received abdominal cavity injection every two days at 2 ml in each time. The tumor volume was measured every 6 days to draw the tumor growth curve. Tumor volume V=π/6 (a×b2). The mice were killed at the 30th day after injection to weight the tumor. Tumor inhibitory rate (%)=(control mean tumor weight-test mean tumor weight)/control mean tumor weight ×100%. TUNEL method was applied to detect tumor cell apoptosis. Apoptosis index AI= apoptosis cell number/total cell number ×100%.
Immunohistochemical detection
Bcl-2 and Bax expression in tumor tissue were detected by immunohistochemical SP method. Bcl-2 and Bax positive expression in tumor tissue were observed under the microscope. The result was judged as the yellow or tan particle appeared on cell membranes or in the cytoplasm. The staining intensity was analyzed quantitatively to determine the positive area average integral absorbance.
Statistical analysis
All statistical analyses were performed using SPSS19.0 software (Chicago, IL). Numerical data were presented as means and standard deviation (±S). Differences between multiple groups were analyzed using one-ANOVA or LSD test. P<0.05 was considered as significant difference.
Results
Tumor growth and nude mice survival
After 1 week, small bump could be touched in the inoculation place but with no significant difference among the groups. All of the nude mice appeared tumor after two weeks with diameter at 0.5-0.6 cm. The mice activity, hair, eating and drinking showed no significant abnormity. After 7 days, tumor volume gradually increased in group C and B, while it grew slowly in group H, M, and L. After 14 days the tumor volume decreased in group H, M, and L. The tumor volume exhibited obvious difference among different groups at the 30th day after inoculation (P<0.05). Mice in group C and B presented less hair, no activity, and fewer eating and drinking. Mice in group H had rapid response, normal eating and drinking, and smooth hair. Mice in group M showed no abnormal action and eating, and smooth hair. Mice in group L exhibited slow action, dry hair, and poor eating (Figure 1).
Figure 1.
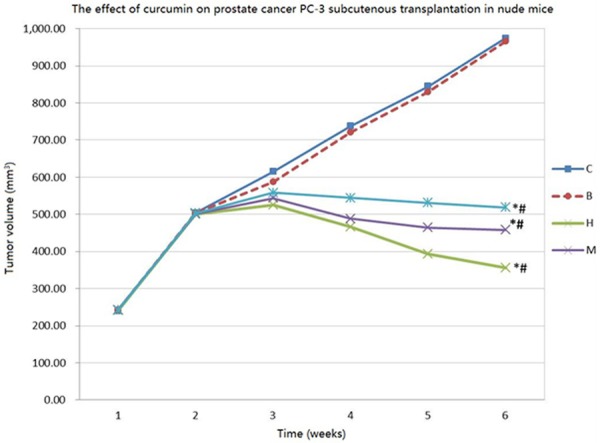
Tumor growth curve in nude mice. *P<0.05, compared with group B and C; #P<0.05, compared within group H, M, and L.
Effect of curcumin on prostate cancer tumor weight and tumor inhibitory rate in nude mice
After 30th days, the tumor volume and weight in curcumin groups were significantly smaller than that in group C and B (P<0.05), while the inhibitory rate was markedly higher than group C and B (P<0.05). Furthermore, their also showed statistical differences among curcumin groups (P<0.05). Group H showed the most obvious tumor inhibitory rate, and certain dose dependence could be found among each dose group (Table 1).
Table 1.
The effect of curcumin on prostate cancer tumor weight and tumor inhibitory rate in nude mice (x̅±S, N=8)
| Group | Dose, mg/(kg•d) | Tumor volume (mm3) | Tumor weight (g) | Inhibitory rate (%) |
|---|---|---|---|---|
| C | - | 974.51±8.79 | 0.86±0.04 | - |
| B | - | 965.98±6.88 | 0.81±0.03 | - |
| H | 100 | 356.32±8.09**,##,&,Δ | 0.34±0.02**,##,&,Δ | 58.02**,##,&,Δ |
| M | 50 | 458.33±7.68**,##,& | 0.44±0.03**,##,& | 45.68**,##,& |
| L | 25 | 518.34±7.31**,## | 0.51±0.03**,## | 37.04**,## |
*P<0.05,
P<0.01, compared with group C;
#P<0.05,
P<0.01, compared with group B;
P<0.05, compared with group L;
P<0.05, compared with group M.
Effect of curcumin on Bcl-2 and Bax expression in nude mice
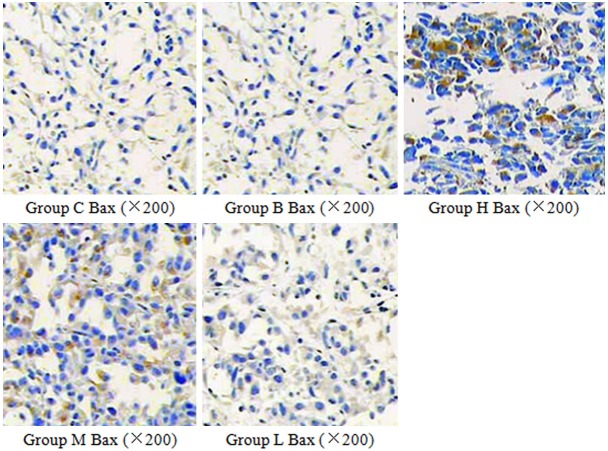
Bax expressed weak in group C and B, and it increased in group H, M, and L (P<0.05). Its expression showed statistical difference among group H, M, and L. Compared with group C and B, Bcl-2 expression obviously reduced in group H, M, and L, and it exhibited significant differences among the curcumin groups (P<0.05), (Table 2; Figures 2 and 3). The effect of curcumin on cancer cell apoptosis in nude mice. The cell apoptosis rate showed no statistical difference between group B and C, while it increased in curcumin group H, M, and L (P<0.05) (Table 3).
Table 2.
The effect of curcumin on Bcl-2 and Bax expression in nude mice (±S, N=8)
| Group | Dose, mg/(kg•d) | Bcl-2 | Bax |
|---|---|---|---|
| C | - | 0.163±0.031 | 0.001±0.000 |
| B | - | 0.171±0.025 | 0.001±0.000 |
| H | 100 | 0.017±0.014**,##,&,△ | 0.141±0.037**,##,&,△ |
| M | 50 | 0.024±0.017**,##,& | 0.107±0.019**,##,& |
| L | 25 | 0.055±0.012**,## | 0.082±0.028**,## |
*P<0.05,
P<0.01, compared with group C;
#P<0.05,
P<0.01, compared with group B;
P<0.05, compared with group L;
P<0.05, compared with group M.
Figure 2.
The effect of curcumin on Bax expression in nude mice. Bax protein positively expressed in cytoplasm. Its expression increased in curcumin group with dose dependence.
Figure 3.
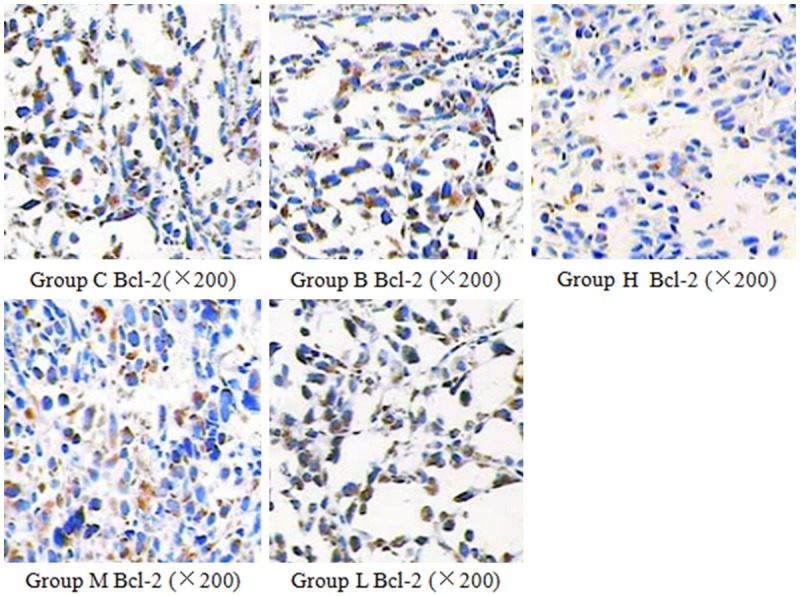
The effect of curcumin on Bcl-2 expression in nude mice. Bcl-2 protein positively expressed in cytoplasm and cell membrane. Its expression decreased in curcumin group with dose dependence.
Table 3.
The effect of curcumin on cancer cell apoptosis in nude mice (±S, N=8)
| Group | Dose, mg/(kg•d) | Apoptosis index |
|---|---|---|
| C | - | 2.33±0.11 |
| B | - | 2.67±0.25 |
| H | 100 | 49.35±2.87**,##,&,△ |
| M | 50 | 39.85±1.62**,##,& |
| L | 25 | 27.65±1.57**,## |
*P<0.05,
P<0.01, compared with group C;
#P<0.05,
P<0.01, compared with group B;
P<0.05, compared with group L;
P<0.05, compared with group M.
Discussion
There is still lack of effective drugs for clinical treatment of prostate cancer. The side effect and tumor cells resistance limited chemotherapy drugs’ application. As a type of phenolic pigment extracted from turmeric zingiberaceae plants, curcumin exhibited many pharmacological activities. It showed a variety of curative effect on tumor, such as colon cancer, breast cancer, and lymphoma. Moreover, it has few side effects. Previous studies confirmed that curcumin could regulate tumor factor expression, inhibit prostate cancer cell proliferation and induce apoptosis in vivo through multiple pathways [11,12]. Tumor progression is associated with cell apoptosis, and curcumin can down-regulate MDM2 protein or affect its combination with p53 through calyculin A or PP2A dephosphorylation mediated Akt/mTOR signaling pathway. Also, it can induce apoptosis by inhibiting the effect of AP-1 and NF-κB signaling pathway. Furthermore, it might promote the Bak and Bax gene effects on cancer cell apoptosis by inhibiting mitochondrial Akt. Several studies considered that curcumin induced PC-3 cell apoptosis is associated with mitochondria destruction and cell ceramide accumulation [13]. Androgen receptor signal activation plays an important role in the process of prostate cancer hormone independent transformation. Curcumin can down-regulate androgen receptor transcription and expression. In vitro test showed that curcumin could be used for hormone refractory prostate cancer treatment, strengthen the cytotoxic effect of taxol, and delay the paclitaxel resistance [14,15]. It could inhibit LNCaP transplant tumor growth, control tumor angiogenesis, and suppress tumor metastasis in Balb/c nude mice [15]; it also could make the PC-3 stay in G2/M phase and assist radiation [15]. Researchers have shown that curcumin might inhibit HepG 2 cell growth by up-regulating Bax gene and down-regulating Bcl-2 gene [16]. Currently, it is still not clarified the mechanism of curcumin inducing prostate cancer cell apoptosis. Bax is an apoptosis promoting factor, while Bcl-2 is an apoptosis inhibitory factor. Bcl-2 and Bax expression level is related to cell apoptosis. This study investigate the curcumin effect on androgen independent prostate cancer cells proliferation by establishing PC-3 nude mice model, aiming to provide basis for application of traditional Chinese medicine in prostate cancer treatment.
Our results showed that the tumor volume and weight in curcumin group H, M, L were significantly lower than that in the control group (C, B), while the inhibitory rate increased following the dose augment. It indicated that curcumin could obviously suppress androgen independent prostate cancer PC-3 cell growth in nude mice in the early phase. Apoptosis index in curcumin group H, M, L increased compared with group C and B, suggesting that certain doses of curcumin could induce PC-3 cell apoptosis. The cancer lesions process is associated with cell apoptosis, whereas cell apoptosis is associated with multiple genes and pathways. Compared with the control group, Bcl-2 expression in curcumin group H, M, L gradually declined and Bax protein expression increased. Curcumin regulating on apoptosis related proteins Bcl-2 and Bax expression plays an important role in prostate cancer cells apoptosis. Bcl-2 and Bax both belong to the Bcl-2 family but have the opposite function. Their ratio between decides cell apoptosis or anti-apoptosis. Bax can antagonist the biology activity of Bcl-2 and promoting cell apoptosis. Bcl-2 can promote cell apoptosis. Bcl-2 can bind with Bcl-2 family such as Bax, Bcl-X1, Bad, and Mc1-1 to form homologous dimers. Bax can form dimer with Bcl-2 protein. Higher relative amount of Bcl-2 may inhibit cell apoptosis, whereas higher relative amount of Bax result in promoting cell apoptosis. Some researches thought that after the cell receives apoptosis signal, Bak and Bax occur oligomerization. Then, some proteins shift to the outer membrane of the mitochondria. Oligomerization product may combine with the voltage dependent anion channel on the mitochondrial membrane to make it open, resulting in cytochrome c release and apoptosis [17,18]. Our immunohistochemistry results showed that Bax expression up-regulated and Bcl-2 down-regulated in PC-3 transplanted tumor tissue after curcumin intervention, resulting in the increase of Bcl-2 heterologous dimer and decrease of homologous dimer to promote cell apoptosis. Curcumin can mediate mitochondrial outer membrane permeability by up-regulating Bax protein expression and down-regulating Bcl-2 expression. Bax can let the mitochondria open the mitochondria permeability transition channels, leading to cytochrome c release and Caspase family activation, to activate the downstream triggering apoptosis. Bcl-2 inhibits Ca2+ release, while Bax promotes Ca2+ release Curcumin may control Bax and Bcl-2 expression to induce Ca2+ overload in the mitochondria, resulting mitochondrial permeability transition channels open, mitochondria swelling broken, and tumor cell apoptosis [19,20]. In this study, group H exhibited great effect on prostate cancer PC-3 transplanted tumor inhibitory rate, tumor cell apoptosis index, and the Bcl-2 and Bax protein expression with dose dependence.
Apoptosis is believed to be achieved through mainly two pathways: caspase dependent and independent pathways. Bcl-2 and Bax are the upstream signals, and their specific mechanism in curcumin antitumor still needs further research. Above all, curcumin can effectively regulate apoptosis related proteins expression to inhibit prostate cancer cell proliferation and induce apoptosis in vitro. Also, it can inhibit tumor growth, reduce tumor weight, decline tumor volume, and promote tumor cell apoptosis in PC-3 transplanted nude mice in vivo.
Disclosure of conflict of interest
None.
References
- 1.Wardle J, Robb K, Vernon S, Waller J. Screening for prevention and early diagnosis of cancer. Am Psychol. 2015;70:119–133. doi: 10.1037/a0037357. [DOI] [PubMed] [Google Scholar]
- 2.Nordstrom T, Clements M, Karlsson R, Adolfsson J, Gronberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer. 2015;51:725–33. doi: 10.1016/j.ejca.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Liu M, Yao XD, Li W, Geng J, Yan Y, Che JP, Xu YF, Zheng JH. Nrf2 sensitizes prostate cancer cells to radiation via decreasing basal ROS levels. Biofactors. 2015;41:52–57. doi: 10.1002/biof.1200. [DOI] [PubMed] [Google Scholar]
- 4.Graff RE, Pettersson A, Lis RT, DuPre N, Jordahl KM, Nuttall E, Rider JR, Fiorentino M, Sesso HD, Kenfield SA, Loda M, Giovannucci EL, Rosner B, Nguyen PL, Sweeney CJ, Mucci LA Transdisciplinary Prostate Cancer Partnership ToPCaP. The TMPRSS2:ERG fusion and response to androgen deprivation therapy for prostate cancer. Prostate. 2015;75:897–906. doi: 10.1002/pros.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 6.Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32:1119–1123. [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Dabrosin C, Yin X, Fuster MM, Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B, Ribatti D, Chen YC, Honoki K, Fujii H, Georgakilas AG, Nowsheen S, Amedei A, Niccolai E, Amin A, Ashraf SS, Helferich B, Yang X, Guha G, Bhakta D, Ciriolo MR, Aquilano K, Chen S, Halicka D, Mohammed SI, Azmi AS, Bilsland A, Keith WN, Jensen LD. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015 doi: 10.1016/j.semcancer.2015.01.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Wang Z, Hu Z, Zeng X, Li Y, Su Y, Zhang C, Ye Z. Anti-tumor activity of curcumin against androgen-independent prostate cancer cells via inhibition of NF-kappaB and AP-1 pathway in vitro. J Huazhong Univ Sci Technolog Med Sci. 2011;31:530–534. doi: 10.1007/s11596-011-0485-1. [DOI] [PubMed] [Google Scholar]
- 9.Rana C, Piplani H, Vaish V, Nehru B, Sanyal SN. Downregulation of PI3-K/Akt/PTEN pathway and activation of mitochondrial intrinsic apoptosis by Diclofenac and Curcumin in colon cancer. Mol Cell Biochem. 2015;402:225–241. doi: 10.1007/s11010-015-2330-5. [DOI] [PubMed] [Google Scholar]
- 10.Shankar S, Ganapathy S, Chen Q, Srivastava RK. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer. 2008;7:16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar S, Srivastava RK. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis. 2007;28:1277–1286. doi: 10.1093/carcin/bgm024. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Wang B, Chung S, Wu Y, Henning SM, Vadgama JV. Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells. RSC Adv. 2014;4:35242–35250. doi: 10.1039/C4RA06616B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentini A, Conforti F, Crispini A, De Martino A, Condello R, Stellitano C, Rotilio G, Ghedini M, Federici G, Bernardini S, Pucci D. Synthesis, oxidant properties, and antitumoral effects of a heteroleptic palladium(II) complex of curcumin on human prostate cancer cells. J Med Chem. 2009;52:484–491. doi: 10.1021/jm801276a. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell Cycle. 2007;6:2953–2961. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 15.Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3’-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- 17.Hilchie AL, Furlong SJ, Sutton K, Richardson A, Robichaud MR, Giacomantonio CA, Ridgway ND, Hoskin DW. Curcumin-induced apoptosis in PC3 prostate carcinoma cells is caspase-independent and involves cellular ceramide accumulation and damage to mitochondria. Nutr Cancer. 2010;62:379–389. doi: 10.1080/01635580903441238. [DOI] [PubMed] [Google Scholar]
- 18.Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/beta-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010;13:343–349. doi: 10.1038/pcan.2010.26. [DOI] [PubMed] [Google Scholar]
- 19.Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL, Lubahn DB. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 20.Cabrespine-Faugeras A, Bayet-Robert M, Bay JO, Chollet P, Barthomeuf C. Possible benefits of curcumin regimen in combination with taxane chemotherapy for hormone-refractory prostate cancer treatment. Nutr Cancer. 2010;62:148–153. doi: 10.1080/01635580903305383. [DOI] [PubMed] [Google Scholar]