Abstract
Background: Esophageal squamous cell carcinoma (ESCC) is a malignant tumor with a strong tendency toward familial aggregation and a higher incidence as well as mortality in Kazakh population. Tumor necrosis factor-alpha (TNF-α) is an important inflammatory cytokine that plays a role in controlling the progression of lung cancer, hepatocellular cancer, breast cancer and gastric cancer. But the association between TNF-α-308G/A and ESCC still remains unclarified. Materials and Methods: Here, we investigated the potential associations between the TNF-α-308G/A and susceptibility to ESCC in 212 cases and 200 controls from a pure ethnic population of Kazakh. DNA extraction and Real-time PCR were performed to detect the TNF-α-308G/A expression levels and odd ratios (ORs) with the corresponding 95% confidence interval (CI) were to evaluate their association with TNF-α-308G/A polymorphism. Results: We found that the frequencies of TNF-α-308G/A in the cases were similar to that of the controls with no differences being statistically significant (χ2=1.23, P>0.05). Using the G allele as the reference genotype, individuals who carried A allele had a significantly increased risk of developing ESCC (OR=2.64, 95% CI=1.31~5.35). Especially, the G/A+A/A genotype are associated with increased risk of metastatic as compared with GG genotype individuals (OR=2.08, 95% CI=1.14-3.80, P=0.02). Conclusions: Our findings suggest that though the TNF-α-308G/A polymorphism may not be correlated with the susceptibility to Kazakh’s ESCC in Xinjiang, patients who carry A allele tend to poorly differentiated and lymph node metastasis.
Keywords: Tumor necrosis factor, gene polymorphism, esophageal cancer, Kazakh
Introduction
Esophageal squamous cell carcinoma (ESCC), a primary cause of death worldwide, approximately 300,000 cases die of ESCC each year, and >50% of these cases occur in China. It is indicated that ESCC has been one of the most common causes of cancer-related deaths in China [1], which ranks the sixth in mortality and the fourth in cancer-related deaths in China [2]. Kazakh is one of the major minorities in China and most of them reside in the Ili Kazakh Autonomous Prefecture within the Xinjiang Uyghur Autonomous Region [3]. ESCC as a major cause of cancer- related deaths in the Kazak ethnic group, which is characterized by a strong tendency toward familial aggregation and a higher incidence and mortality (age standardized rates of 90-150/100,000) as compared with other ethnic populations residing within Xinjiang, China [4-6]. Since very few Kazakh marries people from other ethnicities as Kazakh’s unique cultural background, they are a relatively isolated population with a pure genetic background. It makes the Kazakh population as ideal population for the study of the genetic mechanisms of ESCC. However, the etiology of ESCC is not well clarified [4]. Thus some genetic factors as well as polymorphism, as significant factors of molecular level, may contribute to the carcinogenic mechanism [6].
Tumor necrosis factor α (TNF-α), as a multifunctional cytokine, is one of the earliest cytokines produced in inflammatory processes, plays a significant role in the autoimmunity inflammation, proliferation and apoptosis [7]. As a potent cytokine mediating inflammatory reaction and immune responses, TNF-α has various biological activities, especially in the expression of adhesion molecules, facilitating the invasion of metastatic tumor cells [8,9]. Expression of TNF-α is in part regulated at the transcriptional level. Several single nucleotide polymorphisms (SNPs) in the TNF-α, which may affect the transcription and expression, have been identified. The presence of the functional polymorphisms in cytokine genes affects cytokine expression, and thus may have an important role in the genetic regulation of the inflammatory response and resistance or susceptibility to infections. Single nucleotide conversion from guanine (G) to adenine (A) at position -308 is the most common polymorphism in general populations and this transition has been shown to influence the expression of TNF-α [10]. Of these reasons, the TNF-α-308G > A polymorphism is the best studied. An A to G transition directly affecting TNF-α expression is located at the 308 nucleotide position upstream of the transcription initiation site (TNF-α-308G/A). Dysregulation of TNF-α production has been involved in a variety of human diseases including hepatocellular cancer, breast cancer, gastric cancer and breast cancer [11]. Furthermore, if ESCC associated SNPs could be identified in coding regions, we would hypothesize that these genetic SNPs could have functional manifestations, a genotype-phenotype correlation could exist. So, we further detected the association between polymorphism of tumor necrosis factor-α-308G/A (TNF-α-308G/A) and Kazakh’s esophageal squamous cell carcinoma (ESCC) in Xinjiang.
Given TNF-α-308G/A, was identified in a racially mixed Uygur population which differs genetically from pure Kazakh population, we accordingly hypothesized that, in addition to TNF-α-308G/A, there would be more SNPs in the TNF-α-308G/A gene potentially associated with ESCC in pure Kazakh population [3]. Therefore, in the present study, using DNA sequencing analysis and assessment, we performed genotype analyses potentially functional SNPs in TNF-α-308 and assessed their associations, alone and in accumulation, with risk of ESCC in ethnic Kazakh subjects of 212 ESCC cases and 200 controls. To the best of our knowledge, this is the first paper to evaluate the association between the TNF-α-308G/A polymorphism and risk of Kazakh’s ESCC.
Materials and methods
Subjects
All subjects were ethnically homogeneous Kazakh nationality. In a case-control study, 212 patients with esophageal cancer and 200 healthy individuals were recruited. The inclusion criteria for the patients were the primary diagnosis of esophageal cancer based on clinical work-up and definitive pathological diagnosis. The control group consisted of normal healthy individuals with no history of malignancies or autoimmune diseases in their immediate relatives. They were matched with the case group according to age and gender. This study was approved by the Medical Ethics Committee of Shihezi University of Medical Sciences. Participants were informed that blood samples would be used for a research project and informed consent was obtained.
DNA extraction
A 5 mL sample of venous blood from each subject was drawn in test tubes containing EDTA as anticoagulant and stored at 4°C. After sampling by using proteinase K (Merck, Darmstadt, Germany) digestion followed by a salting out procedure according to the method published by Miller et al, genomic deoxyribonucleic acid (DNA) was extracted within one week.
Polymorphism genotype
TNF-α-308 gene polymorphism was typed by PCR, using forward primer 5’-AGGCAATAGGTTTTGAGG GCCAT-3’ and reverse primer 5’-TCCTCCCTGCTCTGATTCCG-3’. The 20 μl final PCR volume was as follows: 200 ng DNA, 10 μl 2×PCR mix, 5 pmol each primer. The following cycling conditions were used: 95°C for 5 min, followed by 35 cycles of 94°C for 60 s, 58°C for 30 s and 72°C for 60 s, with a final extension at 72°C for 10 min. PCR product was digested by restriction enzyme and visualized in 3% agarose gel stained with ethidium bromide. Different genotypes were then identified in blood sample and ESCC issue after gel electrophoresis and staining as illustrated in Figures 1, 2. The data of TNF-α-308G/A genotype was verified by automated DNA sequencing as mentioned above (Figure 3).
Figure 1.
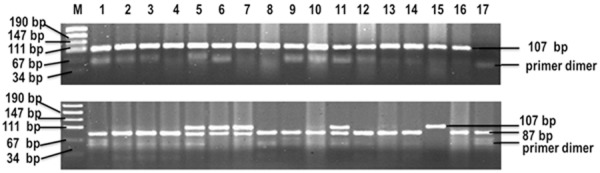
Genotyping of TNF-α-308G/A in blood sample. PCR product was digested by restriction enzyme and visualized in 3% agarose gel stained with ethidium bromide. Lane 1, 3, 5 show positive control; Lane 2, 4, 8, 9, 10, 12, 13, 14, 16 show G/G genotype; Lane 6, 7, 11 show G/A genotype; Lane 15 shows A/A; Lane 17 shows blank control; M shows 100 bp DNA standard marker.
Figure 2.
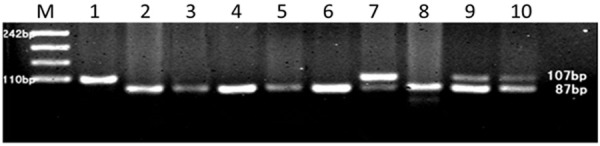
PCR-RFLP assay for analyzing the TNF-α-308G/A polymorphism in ESCC tissue. PCR products were separated by 3% agarose. Lane 1, A/A; Lane 2, 3, 4, 5, 6, 8, G/G; Lane 7, 9, 10, G/A; M, molecular weight marker.
Figure 3.
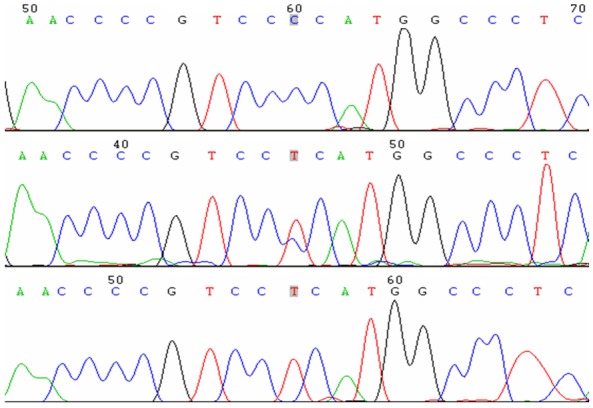
Sequencing map of the genotype for the TNF-α-308G/A polymorphisms.
Statistical analysis
All statistical analyses were performed using Statistical Products and Services Solutions software (SPSS version 13.0, U.S.). The TNF haplotype frequencies were estimated using EH Linkage Software (1.2 version, Rockefeller University, New York). The odds ratio (OR) and 95% confidence interval (CI) were calculated using an unconditional Logistic regression model and adjusted by age and sex accordingly. Hardy-Weinberg equilibrium was evaluated using a goodness-of-fit χ2 test for comparison of the observed genotype frequencies with those expected among the controls. Comparison of the TNF-α-308G/A patients and healthy controls were performed by two-sided contingency tables using Chi-square test. Chi-square values for comparing the distribution of the TNF haplotypes in cancer patients and healthy controls were calculated according to the instruction of the EH program. A probability level of 5% was considered as significant for all statistical tests.
Results
Studies and populations characteristics
In the present case-control study, a total of 412 Kazakh subjects were enrolled, which consisting of 212 ESCC patients and 200 cancer-free controls. Table 1 showed the general population characteristics. There were 127 males and 85 females in the patients group, and 118 males and 82 females in the control group. The mean age of ESCC patients (52.5±8.8) years (ranging from 24 to 79 years) was similar to that of the healthy controls [(54.4±7.9) years, ranging from 22 to 77 years]. There were no significant differences between ESCC cases and cancer-free controls with regard to gender and age distribution. (P>0.05, χ2=2.61; P>0.05, χ2=2.28) (Table 1).
Table 1.
Basic characteristic of the study population
| Group | Patients | Controls | χ2 | P value | |
|---|---|---|---|---|---|
| Sex | M* | 127 (59.7%) | 102 (51.8%) | ||
| F* | 85 (40.3%) | 95 (48.2%) | 2.61 | 0.11 | |
| Age | ≤70 | 4 (1.8%) | 9 (4.2%) | ||
| >70 | 216 (98.2%) | 201 (95.8%) | 2.28 | 0.13 | |
| Genotype | G/G | 150 (70.8%) | 140 (70.0%) | ||
| G/A | 57 (26.9%) | 58 (29.0%) | |||
| A/A | 5 (2.4%) | 2 (1.0%) | 1.23 | 0.58 |
M: Male; F: female.
The observed genotype distribution in the controls did not differ from those expected from Hardy-Weinberg equilibrium (P>0.05). The genotype and allele frequencies of TNF-α-308G/A gene polymorphisms for patients with ESCC and healthy controls are shown in Table 1. The GG, GA and AA genotype frequencies were respectively similar in the case as 70.8, 26.9 and 2.4%, and control groups as 70.0, 29.0 and 1.0%. Thus, genotypic frequencies in the cases were similar to those in the controls with no differences being statistically significant (both P=0.58, χ2=1.23, shown in Table 1).
Relation of the TNF-α-308G/A polymorphism and ESCC differentiation
Frequency distribution and association of the selected polymorphisms with differentiation of ESCC was summarized in Table 2. The GG genotype of TNF-α-308 was found to be dominantly associated with well-differentiated ESCC whereas the GA/AA genotype of TNF-α-308 was found to be significantly associated with middle/poorly-differentiated ESCC (OR=2.64, 95% CI=1.31~5.35) (Table 2). Using the G allele as the reference genotype, individuals who carried A allele had a significantly increased risk of cancer developing in patients with ESCC.
Table 2.
Frequency distribution and association of the selected polymorphisms with differentiated of esophageal squamous cell carcinoma (ESCC)
| Genotype | n | Differentiated n (%) | χ2 | P value | OR | (95% CI) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| High-differentiated | Middle/poor-differentiated | ||||||
| G/G | 134 | 58 (43.3) | 76 (56.7) | ||||
| G/A | 53 | 13 (24.5) | 40 (75.5) | 5.67 | 0.02 | 2.35 | (1.15-4.79) |
| A/A | 5 | 0 (0) | 5 (100) | 5.53 | 0.02 | 0.57 | (0.49-0.66) |
| G/A+A/A | 58 | 13 (22.4) | 45 (77.6) | 7.57 | 0.01 | 2.64 | (1.31-5.35) |
Linkage of TNF-α-308G/A polymorphisms and lymph node metastases
Nevertheless, when analyses between TNF-α-308G/A polymorphisms and lymph node metastatic were conducted, significant differences were found in the G/A+A/A genotype as it was associated with increased risk of metastases as compared with GG genotype individuals. Using the GG genotype as the reference genotype, AA showed 7.32-fold increased ESCC lymph node metastasis in a dominant model. Similarly, and AG + AA genotype was significantly associated with increased risk of metastasis (adjusted OR = 2.08, 95% CI = 1.14-3.80) (Table 3).
Table 3.
Frequency distribution and association of the selected polymorphisms with metastasis of esophageal squamous cell carcinoma (ESCC)
| Genotype | n | Metastasis n (%) | χ2 | P value | OR | (95% CI) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Non-metastasis | Metastasis | ||||||
| G/G | 150 | 97 (64.7) | 53 (35.3) | ||||
| G/A | 57 | 28 (49.1) | 29 (50.9) | 4.17 | 0.04 | 1.9 | (1.02-3.52) |
| A/A | 5 | 1 (20.0) | 4 (80.0) | 4.05 | 0.04 | 7.32 | (1.31-5.35) |
| G/A+A/A | 62 | 29 (46.8) | 33 (53.2) | 5.83 | 0.02 | 2.08 | (1.14-3.80) |
Discussion
ESCC is a malignant tumor which precise mechanism is largely unknown. Inter-individual variation in ESCC may be partly attributed to genetic variants in inflammatory and immune-responsive genes [1]. As ESCC in the Kazakh population in the Xinjiang area is characterized by a strong tendency toward familial aggregation and racial differences, an individual’s genetic makeup may play an important role in the carcinogenesis of ESCC [12]. The present study investigated for the first time the role of the multiple TNF-α-308G/A gene polymorphisms that have never been reported to be associated with ESCC in pure Kazakh population. In this case-control study, we investigated the association between the polymorphism of TNF-α-308G/A and susceptibility to Kazakh’s ESCC in Xinjiang.
Recently, a number of studies indicate that TNF-αG-308A polymorphism is associated with higher susceptibility for a variety of inflammatory and autoimmune diseases, and the TNF-α-308 polymorphism has been described as the most important TNF polymorphism in human disease susceptibility [13-17]. We have noticed that some studies have been conducted to explore TNF-αG-308A polymorphism on inflammatory and autoimmune diseases. For example, Hua’s study has found that TNF-α-308 G > A polymorphism alters the risk of hepatocellular carcinoma in a Han Chinese population. In contrast to their findings, we found that the frequencies of TNF-α-308G/A in the cases were similar to the controls. Our findings are consistent with two previous studies that did not find independent association of TNF-α-308G>A polymorphism with susceptibility to ESCC [18].
TNF-α plays an important role in the regulation of cell differentiation, proliferation, death as well as in inflammation and the innate and adaptive immune response [13]. The substitution of guanine (G) with adenine (A) at the -308 site of the TNF-α gene generates two alleles, TNF-α-308G and TNF-α-308A. The less common TNF-α-308A allele is considered to be associated with higher TNF-α gene transcription and TNF-α overproduction [5]. It is reported that individuals carrying the TNF-α-308A allele has an increased risk for several cancers, such as hepatocellular cancer, breast cancer, gastric cancer and breast cancer. There are some evidences that the polymorphism of TNF-α-308G/A is linked to carcinogenic processes. For example, Hua’s study showed that TNF-α-308G/A polymorphism was associated with increased hepatocellular carcinoma risk in a Han Chinese population [13]. Our findings indicated that though the TNF-α-308G/A polymorphism might not be correlated with the susceptibility to Kazakh’s ESCC in Xinjiang, patients who carried A allele were tend to poorly differentiated and lymph node metastasis. Our observations are consistent with a study by Guo et al. in 2005 that focused on a population of high incidence region of north China with ESCC, and a study by Meenakshi Umar et al.’s conclusion in 2013 that TNF-α-308G/A polymorphism was associated with enhanced risk of ESCC especially in females and in patients with regional lymph node involvements [18,19].
Literature suggests strong association of TNFA-308A allele with HCC that AA was significantly associated with increased risk of HCC, and AG + AA genotype showed 5.59-fold increased HCC risk [13]. Their results suggest that TNF-α-308G/A polymorphism might significantly contribute to cancer susceptibility. However, there is no research to show the role of TNF-α-308G/A polymorphism in Kazakh population. The present case-control study was performed to assess the association of ESCC risk and TNF-α-308G/A polymorphism in a Kazakh Chinese population. There is no significant difference in age and gender as well as genotypic frequencies in cases and controls. We observed the GG genotype of TNF-α-308 was found to be dominant associated with well-differentiated ESCC whereas the GA/AA genotype of TNF-α-308 was found to be significantly associated with poorly-differentiated ESCC. Similarly, using the GG genotype as the reference genotype, AA genotype showed 7.32-fold increased ESCC lymph node metastatic in a dominant model. Furthermore, we found individuals who carried A allele had a significantly increased risk of developing in patients with ESCC. Our results suggest TNF-α-308G/A polymorphism might not be correlated with the susceptibility to Kazakh’s ESCC in Xinjiang. However, TNF-α-308G/A polymorphism is associated with enhanced risk of ESCC developing especially in patients with regional lymph node involvements.
Conclusions
In summary, the present study showed that though the TNF-α-308G/A polymorphism might not be correlated with the susceptibility to Kazakh’s ESCC in Xinjiang, patients who carried A allele were tend to poorly differentiated and lymph node metastasis. This conclusion may be helpful to evaluate the prognosis of ESCC patients. Further prospective studies on large and different ethnic populations will be necessary to confirm our findings and elucidate the underlying molecular mechanism for the development of ESCC.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (2012 AA02A503) and the National Natural Science Foundation of China (No. 81160301, 81360358, 81460362, 81560399, 81260301), the Major science and technology projects of Shihezi University (No. gxjs2014-zdgg06), the jointly foundation for nurturing the outstanding young scientists of Shihezi University (No. 2013 ZRKXYQ-YD19). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Tytgat GN, Bartelink H, Bernards R, Giaccone G, van Lanschot JJ, Offerhaus GJ, Peters GJ. Cancer of the esophagus and gastric cardia: recent advances. Dis Esophagus. 2004;17:10–26. doi: 10.1111/j.1442-2050.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- 2.Cui XB, Pang XL, Li S, Jin J, Hu JM, Yang L, Liu CX, Li L, Wen SJ, Liang WH, Chen YZ, Li F. Elevated expression patterns and tight correlation of the PLCE1 and NF-kappaB signaling in Kazakh patients with esophageal carcinoma. Med Oncol. 2014;31:791. doi: 10.1007/s12032-013-0791-5. [DOI] [PubMed] [Google Scholar]
- 3.Cui XB, Chen YZ, Pang XL, Liu W, Hu JM, Li SG, Yang L, Zhang WJ, Liu CX, Cao YW, Jiang JF, Gu WY, Pang J, Yang L, Yuan XL, Yu SY, Li F. Multiple polymorphisms within the PLCE1 are associated with esophageal cancer via promoting the gene expression in a Chinese Kazakh population. Gene. 2013;530:315–322. doi: 10.1016/j.gene.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 4.Chen YZ, Cui XB, Hu JM, Zhang WJ, Li SG, Yang L, Shen XH, Liu CX, Pan QF, Yu SY, Yuan XL, Yang L, Gu WY, Chen JZ, Wang LD, Li F. Overexpression of PLCE1 in Kazakh esophageal squamous cell carcinoma: implications in cancer metastasis and aggressiveness. APMIS. 2013;121:908–918. doi: 10.1111/apm.12095. [DOI] [PubMed] [Google Scholar]
- 5.Abraham LJ, Kroeger KM. Impact of the -308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: relevance to disease. J Leukoc Biol. 1999;66:562–566. doi: 10.1002/jlb.66.4.562. [DOI] [PubMed] [Google Scholar]
- 6.Lu JB, Yang WX, Liu JM, Li YS, Qin YM. Trends in morbidity and mortality for oesophageal cancer in Linxian County, 1959-1983. Int J Cancer. 1985;36:643–645. doi: 10.1002/ijc.2910360603. [DOI] [PubMed] [Google Scholar]
- 7.Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16:3152–3167. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick A, Bidwell J, van den Brule AJ, Meijer CJ, Pawade J, Glew S. TNFalpha polymorphism frequencies in HPV-associated cervical dysplasia. Gynecol Oncol. 2004;92:675–679. doi: 10.1016/j.ygyno.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21:S447–463. doi: 10.1097/00003246-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang L, Ma W, Cai D, Zhong H, Sun Q. Associations between tumor necrosis factor-alpha polymorphisms and risk of psoriasis: a meta-analysis. PLoS One. 2013;8:e68827. doi: 10.1371/journal.pone.0068827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Hu Z, He Z, Jia W, Wang F, Zhou Y, Liu Z, Zhan Q, Liu Y, Yu D, Zhai K, Chang J, Qiao Y, Jin G, Liu Z, Shen Y, Guo C, Fu J, Miao X, Tan W, Shen H, Ke Y, Zeng Y, Wu T, Lin D. Genome-wide association study identifies three new susceptibility loci for esophageal squamous-cell carcinoma in Chinese populations. Nat Genet. 2011;43:679–684. doi: 10.1038/ng.849. [DOI] [PubMed] [Google Scholar]
- 13.Feng H, Kuai JH, Zhang MY, Wang GC, Shi YJ, Zhang JY. Tumor necrosis factor-alpha gene -308G > A polymorphism alters the risk of hepatocellular carcinoma in a Han Chinese population. Diagn Pathol. 2014;9:199. doi: 10.1186/s13000-014-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kou S, Wu Y. Meta-analysis of tumor necrosis factor alpha -308 polymorphism and knee osteoarthritis risk. BMC Musculoskelet Disord. 2014;15:373. doi: 10.1186/1471-2474-15-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jevtovic-Stoimenov T, Despotovic M, Pesic Z, Cosic A. Lack of Association of Tumor Necrosis Factor-alpha G-308A and Transforming Growth Factor-beta1 C-509T Polymorphisms in Patients with Deep Neck Space Infections. Balkan J Med Genet. 2013;16:59–66. doi: 10.2478/bjmg-2013-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos TM, Passos ST, Novais FO, Beiting DP, Costa RS, Queiroz A, Mosser D, Scott P, Carvalho EM, Carvalho LP. Matrix Metalloproteinase 9 Production by Monocytes is Enhanced by TNF and Participates in the Pathology of Human Cutaneous Leishmaniasis. PLoS Negl Trop Dis. 2014;8:e3282. doi: 10.1371/journal.pntd.0003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Wang Q, Wu Z, Wu Q, Li P, Li Y, Li J, Deng C, Wu C, Gao L, Zhang F, Li Y. Associations between TNF-alpha-308A/G polymorphism and susceptibility with dermatomyositis: a meta-analysis. PLoS One. 2014;9:e102841. doi: 10.1371/journal.pone.0102841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Wang N, Li Y, Zhang JH. Polymorphisms in tumor necrosis factor genes and susceptibility to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma in a population of high incidence region of North China. Chin Med J (Engl) 2005;118:1870–1878. [PubMed] [Google Scholar]
- 19.Umar M, Upadhyay R, Kumar S, Ghoshal UC, Mittal B. Association of common polymorphisms in TNFA, NFkB1 and NFKBIA with risk and prognosis of esophageal squamous cell carcinoma. PLoS One. 2013;8:e81999. doi: 10.1371/journal.pone.0081999. [DOI] [PMC free article] [PubMed] [Google Scholar]


