Abstract
Objective: This study aimed to investigate the role of glucose regulated protein 78 (GRP-78) in the apoptosis of neutrophils in rats with severe acute pancreatitis. Methods: A total of 54 SD male rats were randomly assigned into 2 groups: sham group (n=24) and pancreatitis group (n=30). Severe acute pancreatitis was induced by retrograde cholangiopancreatography injection of sodium taurocholate. Rats were sacrified at 3 h, 6 h and 12 h after injection. In control group, rats received laparotomy, but the pancreates remained intact. The serum amylase was detected at different time points, and flow cytometry was done to detect the apoptosis of neutrophils. Proteins were extracted from neutrophils and subjected to detection of GRP78 and Mcl-1 expression by Western blot assay. HE staining was performed for pathological scoring of the pancreas. Results: The serum amylase in pancreatitis group increased markedly when compared with control group (P<0.01). In SAP group, the serum amylase increased gradually over time (P<0.01). HE staining showed a lot of inflammatory cells and infiltration of red blood cells and the apoptosis rate of neutrophils reduced gradually (P<0.01). Western blot assay showed the protein expression of GRP-78 and Mcl-1 increased in neutrophils over time. Conclusion: In rats with SAP, the apoptosis rate of neutrophils reduced over time, which may be associated to the stress induced expression of GRP78 and subsequent activation of Mcl-1 resulting in suppression of neutrphil apoptosis over time.
Keywords: 78 kDa glucose-regulated protein, myeloid cell leukemia 1, neutrophils, phosphatidylinositol kinase-3/serine-threonine protein kinase pathway, severe acute pancreatitis, cell apoptosis
Introduction
Severe acute pancreatitis is a disease with high mortality [1]. To clear the neutrophils in the inflammatory foci via the apoptosis pathway is a mechanism of restricting tissue damage [2]. Neutrophils are a group of important cells in the defense system and also a group of major inflammatory cells involved in the inflammatory injury. The 78 kDa glucose regulated protein is a stress protein and its expression increases significantly following stress to maintain the stability of endoplasmic reticulum and protect cells [3]. In the present study, serum biochemistry, flow cytometry, Western blot assay and pathological examination were performed to investigate the association between GRP78 expression and apoptosis of neutrophils in rats with SAP. Our findings may provide evidence for the investigation of pathogenesis, prevention and therapy of SAP.
Materials and methods
Materials
A total of 54 male SD rats (specific pathogen free) weighing 260-310 g were purchased from the Experimental Center of the 6th People Hospital in Shanghai. Sodium taurocholate (Sigma, USA), neutrophil separation medium LZS1091 (Tianjin Haoyang Biological Products Co., Ltd, China), BCA protein quantification kit, Annexin V-FITC cell apoptosis detection kit, SDS-PAGE kit, erythrocyte lysate (Beyotime Biotech Co., Ltd), ECL chemiluminescence kit, protein marker (Thermo Scientific, USA), PVDF membrane (Biosharp, China), rabbit anti-rat GRP-78 and Mcl-1, β-actin (epitomics, CA, USA), and horseradish peroxidase conjugated goat anti-rabbit IgG (Hangzhou Hua’an Biotech Co., Ltd, China) were used in the present study.
Establishment of animal model
SD rats were housed for 1 week and fasted for 12 h before surgery. Animals were anesthetized with 1% ketamine hydrochloride at 1 ml/100 g intraperitoneally. Rats were fixed in a surgery table, and abdominal skin was sterilized. A midline incision was made at the abdomen (2 cm in length) and the abdominal cavity was exposed. A transparent pancreaticobiliary tract was exposed along the stomach and duodenum connection. A swab was used to gently separate the pancreaticobiliary tract from the tissues surrounding the stomach and duodenum connection and the lower end of the pancreaticobiliary tract was exposed. The upper end of the pancreaticobiliary tract was clamped with an arterial clamp. A 24-gauge needle was retrogradely inserted 1 cm along the pancreaticobiliary tract through an avascular zone of the duodenum and then fixed. The core was extracted and the needle was connected to a syringe, followed by slow injection of 5% sodium taurocholate at 0.1 ml/100 g. Two minutes later, the clamp was released, and the wound was closed. After surgery, rats received food deprivation (but no water deprivation). At 3 h, 6 h and 12 h after surgery, rats were intraperitoneally anesthetized and 6-8 ml of blood was collected from the femoral vein. The pancreatic tissues at the same site were harvested for following experiments. In control group, the pancrease was exposed, but not injected with sodium taurocholate.
Detection of serum amylase
In brief, 1 ml of blood was collected and centrifuged, and the concentration of amylase was detected by using an automatic biochemical analyzer.
Separation of neutrophils
Neutrophils were separated by density gradient centrifugation. In brief, 4 ml of heparin treated blood was mixed with whole blood and solution for tissue homogenation at a ratio of 1:1. The mixture was allowed to stay at room temperature for 30 min and the supernatant was harvested onto solution A (1:1), followed by centrifugation at 1500 r/min for 20 min. Four layers were observed in the centrifuge tube, and the third layer contained a large amount of neutrophils. The third layer was harvested and transferred into a tube containing 4-5 ml of washing buffer, followed by centrifugation at 1500 r/min for 15 min. This was repeated once. When there were red blood cells, 5 volumes of erythrocyte lysate were added, followed by incubation for 2 min. After centrifugation at 400 g for 5 min, the supernatant was removed, and the remaining cells were neutrophils. The concentration of neutrophils was adjusted to 1×106/L. Detection showed more than 95% of PMN was intact and trypan blue staining revealed that more than 99% of cells were viable.
Detection of apoptotic neutrophils
In brief, 5-10×104 cells were collected and centrifuged at 1000 g for 5 min. The supernatant was removed. Then, cells were re-suspended in annexin V-FITC binding buffer (195 µl), followed by addition of Annexin V-FITC (5 µl). Incubation was done in dark at room temperature for 10 min, followed by centrifugation at 1000 g for 5 min. The supernatant was removed, and annexin V-FITC binding buffer (190 µl) was used to re-suspend these cells. After addition of 10 µl of propidium iodide staining solution, incubation was done in dark on ice, and this cell suspension was used for flow cytometry.
HE staining for pathological examination
The pancreatic tissues were fixed in formalin, followed by routine embedding with paraffin and sectioning. The sections were then subjected to deparaffinization and HE staining. Experienced pathologists who were blind to this study were asked for pathological examination according to the Schmids method [4]. The pancrease was observed under a light microscope.
Detection of GRP78 and Mcl-1 expression by western blot assay
In brief, 50-100×104 neutrophils were mixed with 150 µl of RIPA lysis buffer (900 µl RIPA + 100 µl PMSF), followed by incubation. Then, cell lysate was centrifuged at 12000 g for 5 min, and the supernatant was collected for protein quantification with BCA method. Then, 40 µg of proteins per sample were loaded onto gel for SDS-PAGE at 80 V for 30 min and then 120 V for 90 min. The proteins were transferred onto PVDF membrane at 250 mA for 60 min which was then blocked in 5% non-fat milk for 1 h. This membrane was incubated with rabbit anti-rat GRP78 antibody, β-actin antibody (1:3000) or Mcl-1 antibody (1:1000) at 4°C over night. After washing in T-BST, the membrane was incubated with HRP conjugated anti-rabbit IgG (1:3000) for 1 h. After washing, chemiluminescence method was used for visualization, and Image Quant LAS4000 min was used for detect the protein bands.
Statistical analysis
Data were expressed as mean ± standard deviation (x ± s) and analyzed with analysis of variance. Statistical analysis was done with SPSS version 17.0. Intragroup comparisons of data at different time points were done with t test, and independent sample t test was employed for intergroup comparisons of data at the same time point. A value of P<0.05 was considered statistically significant.
Results
Serum concentration of amylase (U/L)
In SAP group, the serum concentration of amylase increased gradually and reached a peak at 12 h (6246.00±602.931). The amylase concentration in SAP group at different time points after surgery was significantly higher than that in SO group (P<0.01). In SO group, the serum concentration of amylase remained at a low level (Table 1).
Table 1.
Serum concentration of amylase at different time points (U/L)
| Group | 3 h | 6 h | 12 h |
|---|---|---|---|
| SO | 1532.00±214.812 | 1606.00±205.557 | 1608.38±231.369 |
| SAP | 3573.00±424.317a,b | 5183.70±761.834a,b | 6246.00±602.931a,b |
P<0.01 vs. SO group at different time points in;
P<0.01 at different time points in SAP group.
Macroscopic findings of the pancrease
In SO group, there was a small amount of white fluid and the pancrease was intact. At 3 h after SAP, there was a small amount of light red ascites, the pancrease was edema. At 6 h after SAP, the ascites increased. At 12 h after SAP, a large amount of red ascites was observed, and the pancrease became hemorrhagic and necrotic.
Pathological findings of the pancrease
HE staining showed no hemorrhage and normal structure of the pancrease under a light microscope in control group. The pancreatic injury in SO group deteriorated over time. The mild pancreatic injury was characterized by alveolar interstitial edema, infiltration of inflammatory cells including neutrophils and lymphocytes, and scattered hemorrhagic and necrotic foci, and the change in blood vessels was not obvious. The severe pancreatic injury was characterized by evident hemorrhage in the pancrease, necrotic alveolar cells, and lobular destruction and disordered lobular structure; there was a large amount of red blood cells and inflammatory cells in the lobular septum (Figure 1; Table 2).
Figure 1.
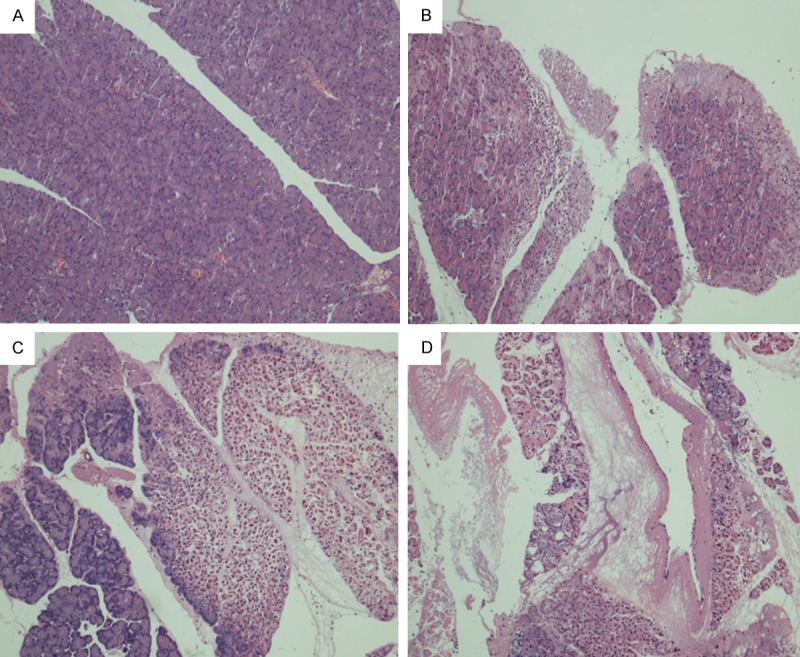
HE staining of the pancreatic in different groups at different time points (×100; A: 3 h SO; B: 3 h SAP; C: 6 h SAP; D: 12 h SAP).
Table 2.
Pathological score of the pancreas in different groups
P<0.01 vs. SO group at different time points;
P<0.01 at different time points in SAP group.
Apoptosis rate of neutrophils (%)
After establishment of animal model, the apoptosis rate of neutrophils in SAP increased gradually over time and reached a peak at 12 h (1.300±0.5812). At the corresponding time points, the apoptosis rate in SAP group was significantly higher than that in SO group (P<0.01; Table 3; Figure 2).
Table 3.
Apoptosis rate of peripheral neutrophils in rats of different groups at different time points
| Group | 3 h | 6 h | 12 h |
|---|---|---|---|
| SO | 7.452±0.4621 | 7.125±0.5148 | 6.900±0.5682 |
| SAP | 4.800±0.8340a,b | 3.030±0.4923a,b | 1.300±0.5812a,b |
P<0.01 vs. SO group at different time points;
P<0.01 at different time points in SAP group.
Figure 2.
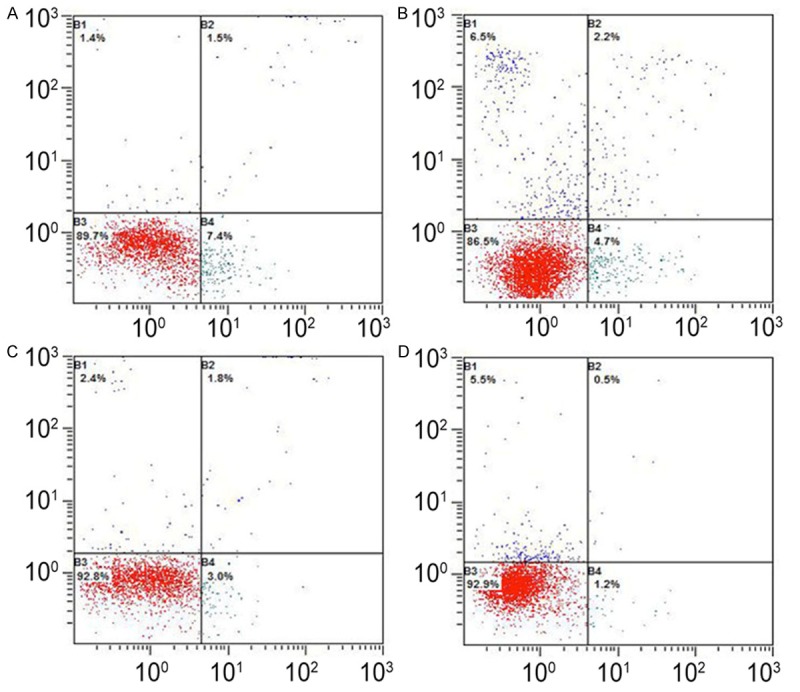
Apoptosis rate of peripheral neutrophils in rats of different groups at different time points. A: 3 h SO; B: 3 h SAP; C: 6 h SAP; D: 12 h SAP.
Protein expression of GRP78 and Mcl-1 in neutrophils
Western blot assay showed the protein expression of GRP78 and Mcl-1 in SAP was significantly higher than that in SO at corresponding time point. In addition, the protein expression of GRP78 and Mcl-1 in SAP increased gradually over time and that in SO group remained at a low level (Figure 3).
Figure 3.
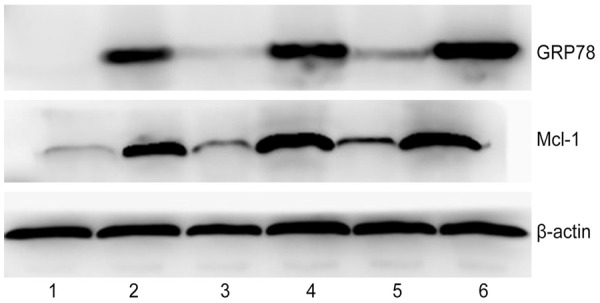
Western blot assay of protein expression of GRP78 and Mcl-1 in neutrophils (1, 3, 5: 3 h, 6 h and 12 h in SO; 2, 4, 6: 3 h, 6 h and 12 h in SAP group).
Discussion
Acute pancreatitis is an acute abdominal disease, has an acute onset, progresses rapidly and has a high mortality. Especially, severe acute pancreatitis (SAP) progresses more rapidly, has a complex pathogenic mechanism, is difficult to treat and has a mortality of higher than 30%. It has been confirmed that multiple factors are involved in the pathogenesis of SAP [5]. The increase in neutrophils reflects the presence of inflammatory response and neutrophils play an important role in the occurrence, development and outcome of inflammation. Apoptosis refers to programmed cell death and the change in the survival environment may affect the process of apoptosis [6]. The normal aging PMN dies in the form of apoptosis and the dead PMN is then recognized, phagocytized and cleared. In the apoptosis, there is no rupture of cell membrane and no release of cytotoxic contents after phagocytosis, which are important to promote the attenuation and restriction of inflammation. In SAP, the function cycle of PMN prolongs when the apoptosis of PMN is inhibited, and a large amount of harmful substances such as free radicals, proteolytic enzymes and inflammatory mediators are released, resulting in sustained tissue injury [7]. In the present study, results showed, at 3 h, 6 h and 12 h after SAP, the apoptosis rate of peripheral neutrophils reduces gradually and reached a minimal level at 12 h. In SO group, the apoptosis rate of neutrophils remained at a high level. Statistical analysis showed the apoptosis rate of neutrophils in SAP was significantly lower than that in SO group at corresponding time points (P<0.01). In addition, marked difference was also observed at any two time points in SAP group (P<0.01).
GRP was first identified in 1977. Shiu et al [8] found that the chicken embryo fibroblasts in the glucose free medium could produce 2 proteins with the relative molecular weight of 78 kDa and 94 kDa, respectively. Thus, they named these two proteins as GRP78 and GRP94. Pathological states such as hypoxia, low glucose, low calcium or viral infection may induce the production of a large amount of unfolded proteins in the endoplasmic reticulum, and then induce a series of adaptive responses which are known as a unfolded protein response (UPR) [9]. UPR has three branches to control the transcription and expression of molecular chaperone proteins including PRK like protein kinase (PERK), inositol requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6) [10-14]. GRP78 is also known as immunoglobulin heavy chain binding protein and is a calcium binding protein. Under normal conditions, GRP78 binds to the sensors on the endoplasmic reticulum including PERK, IRE1 and AFT6, to inhibit the signaling pathways related to UPR [15]. In the presence of endoplasmic reticulum stress, the unfolded proteins or misfolded proteins release GRP78 from the sensors and the GRP78 expression increases significantly. Then, GRP78 binds to misfolded or unfolded proteins to restore the normal conformation. Thus, proteins can be normally synthesized under stress state, which maintain the calcium stability in the endoplasmic reticulum and the homeostasis [16]. Thus, the rapid increase in GRP78 expression has been regarded a sensitive marker of endoplasmic reticulum stress (ERS) [17]. In SAP, the low calcium and toxic substances may increase the GRP78 expression and initiate ERS. In the present study, at 3 h, 6 h and 12 h after SAP, the GRP78 expression increased gradually in the peripheral neutrophils of SAP rats and reached a peak at 12 h. In addition, the GRP78 expression in SAP group was significantly higher than that in SO group at corresponding time point.
GRP78 signaling pathways include Ras/MAPK, PI3-kinase/Akt, and PAK2 dependent signaling pathways. The increase in GRP78 expression may activate above signaling pathways [18-21]. Which pathway is involved in the regulation of Mcl-1 expression in neutrophils is still unclear. There is evidence showing that PI3k/Akt signaling pathway may phosphorylate Akt in the help of 3-phosphate inositol dependent protein kinase 1 (PDK-1) and then increase the activity of Akt, which may influences the expression of apoptosis related proteins (such as BAD, Mcl-1), exerting anti-apoptotic effect [22]. Currently, studies focus on the key molecules in the PI3k/Akt signaling pathway which may become new targets for the anti-tumor therapy. However, no studies have been conducted on the apoptosis of neutrophils in the SAP [23]. Mcl-1 is a anti-apoptotic protein of Bcl-2 family and can bind to the pro-apoptotic proteins of Bcl-2 family to block the change in mitochondrial permeability and the release of pro-apoptotic factors from the mitochondria, exerting anti-apoptotic effect [24,25]. In the present study, at 3 h, 6 h and 12 h after SAP, the Mcl-1 expression increased gradually in the peripheral neutrophils of SAP rats and reached a peak at 12 h. In addition, the Mcl-1 expression in SAP group was significantly higher than that in SO group at corresponding time points. Semi-quantification showed Mcl-1 expression was negatively associated with the apoptosis rate of neutrophils. However, the specific mechanism is required to be further studied.
Our results showed the GRP78 expression increased over time, but the apoptosis rate of neutrophils reduced gradually in rats with sodium taurocholate induced SAP. These indicate that GRP78 can activate PI3K/Akt signaling pathway to induce the expression of Mcl-1 (a pro-apoptotic protein), resulting in delayed apoptosis of peripheral PMN and deterioration of inflammation in SAP rats. Thus, GRP78 and Mcl-1 may become new targets for the therapy of injury due to neutrophil apoptosis, and blocker of PI3K/Akt signaling pathway may promote the apoptosis of neutrophils and then attenuate the tissue injury and inflammation. However, our results are required to be further confirmed in future studies.
Disclosure of conflict of interest
None.
References
- 1.Nesvaderani M, Eslick GD, Cox MR. Acute pancreatitis: update on management. Med J Aust. 2015;202:420–423. doi: 10.5694/mja14.01333. [DOI] [PubMed] [Google Scholar]
- 2.Yu C, Merza M, Luo L, Thorlacius H. Inhibition of Ras signalling reduces neutrophil infiltration and tissue damage in severe acute pancreatitis. Eur J Pharmacol. 2015;746:245–251. doi: 10.1016/j.ejphar.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Bi K, Nishihara K, Machleidt T, Hermanson S, Wang J, Sakamuru S, Huang R, Xia M. Identification of known drugs targeting the endoplasmic reticulum stress response. Anal Bioanal Chem. 2015;407:5343–51. doi: 10.1007/s00216-015-8694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li JY, Wang WX. Severe Acute Pancreatitis and Microcirculatory Disturbance. Occup Heal. 2010;26:2519–2521. [Google Scholar]
- 6.Zhao ZY, He RH, Gao YQ, Yan ZJ, Feng GX, Yu XJ. Effect of Extracorporeal Circulation On Neutrophil Apoptosis during and after Coronary Artery Bypass Grafting. Chin Gene Prac. 2008;11:654–657. [Google Scholar]
- 7.Lichtenberger GS, Flavell RA, Alexopoulou L. Innate immunity and apoptosis in IBD. Inflamm Bowel Dis. 2004;10(Suppl 1):S58–62. doi: 10.1097/00054725-200402001-00012. [DOI] [PubMed] [Google Scholar]
- 8.Shiu RP, Pastan IH. Properties and purification of a glucose-regulated protein from chick embryo fibroblasts. Biochim Biophys Acta. 1979;576:141–150. doi: 10.1016/0005-2795(79)90493-8. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 10.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 11.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 12.Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–328. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumashiro N, Erion DM, Zhang D, Kahn M, Beddow SA, Chu X, Still CD, Gerhard GS, Han X, Dziura J, Petersen KF, Samuel VT, Shulman GI. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K, Zhang L, Xiang X, Gong S, Ma L, Xu L, Wang G, Liu Y, Ji X, Liu S, Chen P, Zeng H, Li J. Arsenic trioxide alleviates airway hyperresponsiveness and promotes apoptosis of CD4+ T lymphocytes: evidence for involvement of the ER stress-CHOP pathway. Ir J Med Sci. 2013;182:573–583. doi: 10.1007/s11845-013-0928-8. [DOI] [PubMed] [Google Scholar]
- 16.Yang GH, Li S, Pestka JJ. Down-regulation of the endoplasmic reticulum chaperone GRP78/BiP by vomitoxin (Deoxynivalenol) Toxicol Appl Pharmacol. 2000;162:207–217. doi: 10.1006/taap.1999.8842. [DOI] [PubMed] [Google Scholar]
- 17.Zhang HY, Liu BQ, Deng WW, Du ZX, Wang HQ. Role of GRP78 in apoptosis of human thyroid cancer cells induced by proteasome inhibitors. Chin J Cancer Prev Treat. 2009;16:1379–1382. [Google Scholar]
- 18.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 19.Misra UK, Deedwania R, Pizzo SV. Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J Biol Chem. 2005;280:26278–26286. doi: 10.1074/jbc.M414467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra UK, Pizzo SV. Potentiation of signal transduction mitogenesis and cellular proliferation upon binding of receptor-recognized forms of alpha2-macroglobulin to 1-LN prostate cancer cells. Cell Signal. 2004;16:487–496. doi: 10.1016/j.cellsig.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Misra UK, Gonzalez-Gronow M, Gawdi G, Hart JP, Johnson CE, Pizzo SV. The role of Grp 78 in alpha 2-macroglobulin-induced signal transduction. Evidence from RNA interference that the low density lipoprotein receptor-related protein is associated with, but not necessary for, GRP 78-mediated signal transduction. J Biol Chem. 2002;277:42082–42087. doi: 10.1074/jbc.M206174200. [DOI] [PubMed] [Google Scholar]
- 22.Bassili M, Birman E, Schor NF, Saragovi HU. Differential roles of Trk and p75 neurotrophin receptors in tumorigenesis and chemoresistance ex vivo and in vivo. Cancer Chemother Pharmacol. 2010;65:1047–1056. doi: 10.1007/s00280-009-1110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, Zhou Q, Zhu LX, Zhao YF. Curcumin dually inhibits both mammalian target of rapamycin and nuclear factor-kappaB pathways through a crossed phosphatidylinositol 3-kinase/Akt/IkappaB kinase complex signaling axis in adenoid cystic carcinoma. Mol Pharmacol. 2011;79:106–118. doi: 10.1124/mol.110.066910. [DOI] [PubMed] [Google Scholar]
- 24.London N, Gulla S, Keating AE, Schueler-Furman O. In silico and in vitro elucidation of BH3 binding specificity toward Bcl-2. Biochemistry. 2012;51:5841–5850. doi: 10.1021/bi3003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodust PM, Fecker LF, Stockfleth E, Eberle J. Activation of mitochondrial apoptosis pathways in cutaneous squamous cell carcinoma cells by diclofenac/hyaluronic acid is related to upregulation of Bad as well as downregulation of Mcl-1 and Bcl-w. Exp Dermatol. 2012;21:520–525. doi: 10.1111/j.1600-0625.2012.01516.x. [DOI] [PubMed] [Google Scholar]


