Abstract
Since effective therapies for melanoma with BRAFV600E mutation are being developed, interest has been shown in the development of therapies for melanoma without BRAFV600E mutation. Recently, interest has also been shown in medical application of non-nequilibrium atmospheric pressure plasmas (NEAPPs). We previously suggested that repeated NEAPP irradiation to spontaneously developed benign melanocytic tumors in RFP-RET-transgenic mice (RET-mice) not only suppresses tumor growth but also prevents malignant transformation. In this study, we first confirmed that transcript expression levels of tumor growth regulators (CyclinD1, D2, E1, E2, G2 and PCNA but not CyclinG1) and tumor invasion regulators [Matrix metalloproteinase (MMP)-2, -9 and -14 and melanoma cell adhesion molecule (MCAM)] in melanomas were significantly higher than those in benign melanocytic tumors in RET-mice. We then showed that transcript expression levels of CyclinE1, G1 and G2 and MMP-2 and -9 in melanomas from RET-mice were significantly decreased by single NEAPP irradiation, whereas transcript expression levels of CyclinD1, D2, E2, PCNA, MCAM and MMP-14 were comparable in untreated and NEAPP-treated melanomas. Since no BrafV600E mutation melanomas have been found in RET-mice, our results suggest that single NEAPP irradiation is a potential therapeutic tool for melanoma without BRAFV600E mutation through modulation of the expression levels of tumor growth and invasion regulators.
Keywords: Melanoma, non-equilibrium atmospheric pressure plasmas, tumor growth, invasion
Introduction
Melanoma, the most aggressive skin cancer, is rapidly increasing worldwide because of increased ultraviolet irradiation caused by depletion of the ozone layer. Generally, melanoma has been reported to be resistant to various therapies including X-ray irradiation. Recently, interest has been shown in a molecular target therapy for BRAFV600E mutation as a new therapy for melanoma [1]. However, therapy for melanoma without BRAFV600E mutation remains difficult. Since the results of a previous study showed that about half of melanoma cases have no mutation of BRAFV600E [1,2], development of a novel therapy for melanoma without BRAFV600E mutation is one of the key elements in melanoma research.
Interest has recently been shown in application of non-nequilibrium atmospheric pressure plasmas (NEAPPs) for the medical field including cancer therapy. In fact, there are some reports showing anti-cancer effects of NEAPPs for various cancers including melanoma in vitro [3-5]. However, studies showing an in vivo effect of NEAPPs on transplanted cancer in animals are limited [6], and there are even fewer studies an in vivo effect of NEAPPs on spontaneously developed cancer in animals and humans.
Multistep melanomagenesis, melanoma that has developed from a melanocytic nevus, has been reported in patients with large congenital melanocytic nevi [7]. We previously developed RFP-RET-transgenic mice (RET-mice) under the control of the metallothionein-I promoter/enhancer, in which benign melanocytic tumors and melanoma spontaneously develop with an intact immune system [8]. The entire process of melanomagenesis in RET-mice resembles that of a human melanocytic nevus [9,10]. Since macroscopic and microscopic appearances of melanoma used in RET-mice are comparable with those of melanoma in humans, RET-mice are used worldwide as a standard animal model for melanoma [8-11].
Previous studies showed that cell cycle promoters such as CyclinD1, E1 and E2 and PCNA are potentially involved in the development of melanoma from benign melanocytic tumors in RET-mice [12]. Matrix metalloproteinase (MMP)-2 and -9 were suggested to be associated with tumor growth [9,13] and malignant transformation [9,14,15] in addition to tumor invasion and metastasis [9,15] in tumors in RET-mice. MMP-14 and melanoma cell adhesion molecule (MCAM) have been reported to be regulators of the activity and expression of MMP-2, respectively [16,17] and have been suggested to be associated with invasion of melanoma from RET-mice [9,18]. Our previous study also suggested that MCAM and MMPs are involved in melanoma development from benign melanocytic tumors in RET-mice [15,18]. Since repeated NEAPP irradiation for benign melanocytic tumors in RET-mice for 11 weeks decreased transcript expression levels of Cyclins, MCAM and MMPs in our previous study, we proposed that NEAPP irradiation might suppress malignant transformation of benign melanocytic tumors in RET-mice [12,15,18]. Based on our previous results, we examined the effect of NEAPP irradiation on cutaneous tumors after transformation (melanoma) in RET-mice in this study.
Materials and methods
Tumors
Benign melanocytic tumors and melanomas in our original RET-mice [8] were used after confirming no BrafV600E mutation in melanomas by DNA sequence analysis according to the method described previously [19].
NEAPP irradiation
Transcript expression levels of cell cycle promoters, MCAM and MMPs at 6 hours after single NEAPP irradiation for 30 seconds were examined in pathologically diagnosed benign melanocytic tumors and melanomas from RET-mice. The method used for NEAPP irradiation (Figure 1A and 1B) in this study was the same as our previous method [12] except for the difference of single or repeated irradiation.
Figure 1.
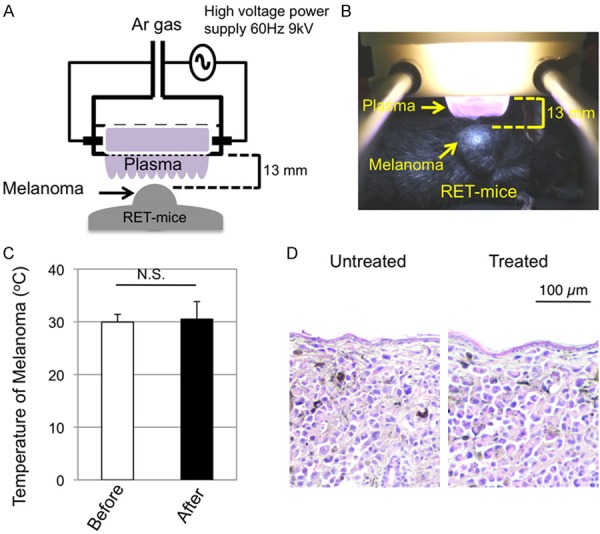
Single non-nequilibrium atmospheric pressure plasma (NEAPP) irradiation to melanomas. (A, B) A schema (A) and a photograph with a dark field (B) of NEAPP irradiation to melanomas in RET-mice are presented. (C) Temperatures (means ± SD) measured by an infrared thermometer (CA380; CASON, Inc) on the melanoma surface before (n=6) and just after (n=6) single NEAPP irradiation are shown. Statistical significance was evaluated by the paired Student’s t-test. (D) HE-stained microscopic appearances of melanomas in RET-mice before (Untreated) and 6 hours after (Treated) single NEAPP irradiation.
Quantitative PCR (Q-PCR)
Q-PCR was performed according to the method previously described [12,15,18].
Approval
The experiments using recombinant DNA were approved by the Recombination DNA Advisory Committee of Nagoya University (approval No. 12-59, 13-59, 13-76) and Chubu University (approval No. 12-04). The animal experiments were approved by the Animal Care and Use Committee of Nagoya University (approval No. 27241) and Chubu University (approval No. 2710048).
Results
Effects of NEAPP irradiation on temperature and morphology of melanoma
The temperatures before and just after single NEAPP irradiation on the melanoma surface were comparable (Figure 1C). Histopathological analysis showed comparable microscopic appearances of untreated and NEAPP-treated melanoma at 6 hours after irradiation (Figure 1D).
Effect of NEAPP irradiation on expression of regulators of the cell cycle
Constitutive expression levels of cell cycle regulatory genes, CyclinD1, D2, E1, E2, G1, G2 and PCNA, in benign tumors and melanomas from RET-mice are shown in Figure 2A-G. Transcript expression levels of CyclinD1, E1, E2 and PCNA, but not that of CyclinG1, in melanomas were significantly higher than those in benign melanocytic tumors, as was observed in our previous study [12]. Increased transcript expression levels of CyclinD2 and G2 in melanomas from RET-mice compared to those in benign melanocytic tumors were newly shown in this study.
Figure 2.
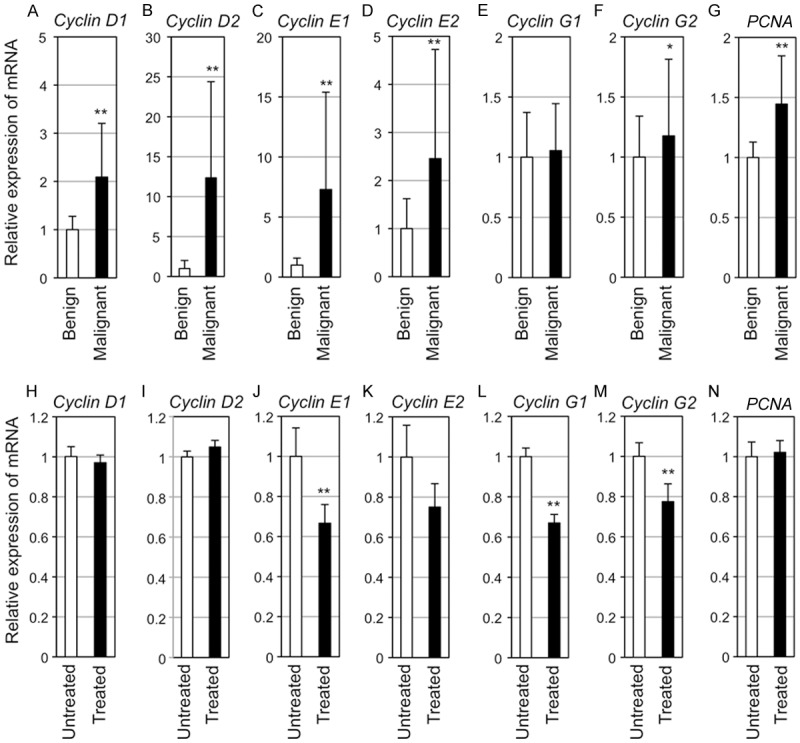
Transcript expression levels of CyclinD1, CyclinD2, CyclinE1, CyclinE2, CyclinG1, CyclinG2, and PCNA in tumors from RET-mice. (A-G) Relative transcript expression levels (means ± SD) of CyclinD1 (A), CyclinD2 (B), CyclinE1 (C), CyclinE2 (D), CyclinG1 (E), CyclinG2 (F), and PCNA (G) in benign melanocytic tumors (Benign, n=6) and melanoma (Malignant, n=6) of RET-mice are presented. (H-N) Relative transcript expression levels (means ± SD) of CyclinD1 (H), CyclinD2 (I), CyclinE1 (J), CyclinE2 (K), CyclinG1 (L), CyclinG2 (M), and PCNA (N) in NEAPP-irradiated (n=6) and unirradiated (n=5) melanomas from RET-mice are presented. The transcript expression levels were determined by quantitative PCR and normalized with the transcript expression level of hypoxanthine ribosyltransferase (Hprt) (A-N). Statistical significance was evaluated by the Mann-Whitney U test. *p<0.05, **p<0.01.
Transcript expression levels of CyclinE1, G1 and G2 in melanoma from RET-mice were significantly decreased by single NEAPP irradiation (Figure 2J, 2L, 2M). There was no statistical difference in CyclinD1, D2, E2 and PCNA transcript expression levels between untreated and NEAPP-treated melanomas (Figure 2H, 2I, 2K, 2N).
Effect of NEAPP irradiation on expression of regulators of invasion
Constitutive transcript expression levels of invasion regulatory genes, MCAM and MMP-2, -9 and -14, in benign melanocytic tumors and melanomas from RET-mice are shown in Figure 3A-D. Transcript expression levels of all of the molecules in melanomas were significantly higher than those in benign melanocytic tumors, as was observed in our previous studies [15,18].
Figure 3.
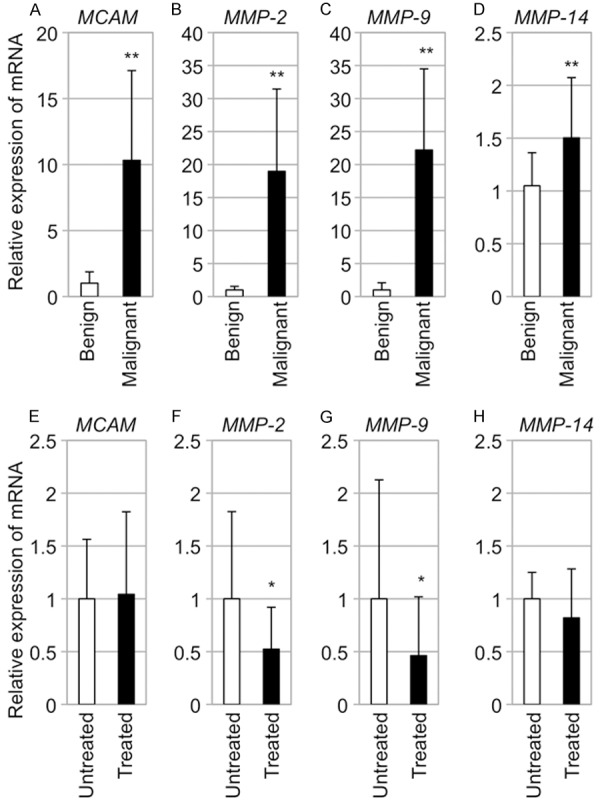
Transcript expression levels of MCAM and MMPs in melanomas from RET-mice. (A-D) Relative transcript expression levels (means ± SD) of MCAM (A), MMP-2 (B), MMP-9 (C) and MMP-14 (D) in melanocytic benign tumors (Benign; n=6) and melanomas (Malignant; n=6) from RET-mice. (E-H) Relative transcript expression levels (means ± SD) of MCAM (E), MMP-2 (F), MMP-9 (G) and MMP-14 (H) in untreated (Untreated; n=6) and NEAPP-treated (Treated; n=6) melanomas from RET-mice are presented. Statistical significance was analyzed by the Mann-Whitney U test. *P<0.05; **P<0.01.
Transcript expression levels of MMP-2 and -9 in melanomas from RET-mice were significantly decreased by single NEAPP irradiation (Figure 2F and 2G). There was no statistical difference in MCAM and MMP-14 transcript expression levels between untreated and NEAPP-treated melanomas (Figure 2E and 2H).
Discussion
We demonstrated for the first time that single NEAPP irradiation decreases expression levels of cell cycle regulators (Cyclin E1, G1 and G2) and invasion regulators (MMP-2 and -9) in melanoma from RET-mice with limited effects on temperature and histopathological appearance.
After repeated NEAPP irradiation to benign melanocytic tumors in original RET-mice for 11 weeks, the effect was evaluated 12 weeks after the first irradiation in our previous studies [12,15,18]. However, survival for 12 weeks after the first irradiation might not be easy for RET-mice with melanoma, and we therefore chose single NEAPP irradiation to melanoma in RET-mice as our first trial. To our knowledge, this is the first study showing the effect of single NEAPP irradiation on spontaneously developed melanoma in mice with an intact immune system.
We showed decreased expression levels of CyclinE1, G1 and G2 in NEAPP-treated melanomas after showing increased expression levels of CyclinD, E and G in melanomas from RET-mice. Since cell cycle promoters such as CyclinE and G have been suggested to be involved in the growth of tumors including melanoma in humans and mice [12,20], our results suggest a potentially suppressive effect of single NEAPP irradiation on melanoma growth. We further showed decreased expression levels of MMP-2 and -9 in NEAPP-treated melanomas after showing increased expression levels of MMP-2 and -9 in the melanomas from RET-mice. Since the major role of MMP-2, -9 and -14 and MCAM has been reported to be regulation of the invasion of various cancers including melanoma in humans and mice [9,21], our results suggest a potentially inhibitory effect of single NEAPP irradiation on melanoma invasion.
Expression levels of CyclinD1, D2 and E2, PCNA, MCAM and MMP-14 were comparable in untreated and NEAPP-treated melanomas. Moreover, not only temperature but also microscopic appearance of melanoma was comparable before and after NEAPP irradiation. These results suggest that NEAPP irradiation-mediated decrease in the expression levels of regulatory molecules for tumor growth and invasion may be independent of NEAPP irradiation-mediated tissue damage.
Our results suggest that NEAPP irradiation is a novel candidate for therapy of melanoma without BRAFV600E mutation because there were no melanomas with BrafV600E mutation in RET-mice. Our results are encouraging for further studies aimed at clinical application of NEAPP irradiation for melanoma therapy.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (A) (No. 15H01743 and 15H02588), (B) (No. 24390157 and 24406002) and (C) (No. 25340052 and 25461717), Grant-in-Aid for Challenging Exploratory Research (No. 26670525), Grant-in-Aid for Scientific Research on Innovative Areas (No. 24108001) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Toyoaki Scholarship Foundation, the Mitsui & Co., Ltd. Environment Fund and Foundation from Center for Advanced Medical and Clinical Research Nagoya University Hospital.
Disclosure of conflict of interest
None.
References
- 1.Lito P, Rosen N, Solit DB. Tumor adaptation and resistance to RAF inhibitors. Nat Med. 2013;19:1401–1409. doi: 10.1038/nm.3392. [DOI] [PubMed] [Google Scholar]
- 2.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 3.Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, Vasilets V, Friedman G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chemistry and Plasma Processing. 2007;27:163–176. [Google Scholar]
- 4.Tanaka H, Mizuno M, Ishikawa K, Nakamura K, Kajiyama H, Kan H, Kikkawa F, Hori M. Plasma-activated medium selectively kills glioblastoma brain tumor cells by downregulating a survival signaling molecule, AKT kinase. Plasma Med. 2011;1:265–277. [Google Scholar]
- 5.Iseki S, Nakamura K, Hayashi M, Tanaka H, Kondo H, Kajiyama H, Kano H, Kikkawa F, Hori M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl Phys Lett. 2012;100:113702. [Google Scholar]
- 6.Utsumi F, Kajiyama H, Nakamura K, Tanaka H, Mizuno M, Ishikawa K, Kondo H, Kano H, Hori M, Kikkawa F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One. 2013;8:e81576. doi: 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marghoob AA. Congenital melanocytic nevi. Evaluation and management. Dermatol Clin. 2002;20:607–616. doi: 10.1016/s0733-8635(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 8.Kato M, Takeda K, Kawamoto Y, Tsuzuki T, Hossain K, Tamakoshi A, Kunisada T, Kambayashi Y, Ogino K, Suzuki H, Takahashi M, Nakashima I. c-Kit-targeting immunotherapy for hereditary melanoma in a mouse model. Cancer Res. 2004;64:801–806. doi: 10.1158/0008-5472.can-03-2532. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, Iwamoto T, Suzuki H, Nakashima I. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 10.Thang ND, Yajima I, Nakagawa K, Tsuzuki T, Kumasaka MY, Ohgami N, Ly TB, Iwamoto T, Watanabe D, Kato M. A novel hairless mouse model for malignant melanoma. J Dermatol Sci. 2012;65:207–212. doi: 10.1016/j.jdermsci.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Végran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, Chalmin F, Rébé C, Dérangère V, Ryffel B, Kato M, Prévost-Blondel A, Ghiringhelli F, Apetoh L. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol. 2014;15:758–766. doi: 10.1038/ni.2925. [DOI] [PubMed] [Google Scholar]
- 12.Yajima I, Iida M, Kumasaka MY, Omata Y, Ohgami N, Chang J, Ichihara S, Hori M, Kato M. Non-equilibrium atmospheric pressure plasmas modulate cell cycle-related gene expressions in melanocytic tumors of RET-transgenic mice. Exp Dermatol. 2014;23:424–425. doi: 10.1111/exd.12415. [DOI] [PubMed] [Google Scholar]
- 13.Shi H, Liu L, Liu LM, Geng J, Chen L. Inhibition of tumor growth by β-elemene through downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9 in a murine intraocular melanoma model. Melanoma Res. 2015;25:15–21. doi: 10.1097/CMR.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 15.Iida M, Yajima I, Ohgami N, Tamura H, Takeda K, Ichihara S, Hori M, Kato M. The effects of non-thermal atmospheric pressure plasma irradiation on expression levels of matrix metalloproteinases in benign melanocytic tumors in RET-transgenic mice. Eur J Dermatol. 2014;24:392–394. doi: 10.1684/ejd.2014.2330. [DOI] [PubMed] [Google Scholar]
- 16.Zigler M, Villares GJ, Dobroff AS, Wang H, Huang L, Braeuer RR, Kamiya T, Melnikova VO, Song R, Friedman R, Alani RM, Bar-Eli M. Expression of Id-1 is regulated by MCAM/MUC18: a missing link in melanoma progression. Cancer Res. 2011;71:3494–3504. doi: 10.1158/0008-5472.CAN-10-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 18.Omata Y, Iida M, Yajima I, Takeda K, Ohgami N, Hori M, Kato M. Non-thermal atmospheric pressure plasmas as a novel candidate for preventive therapy of melanoma. Environ Health Prev Med. 2014;19:367–369. doi: 10.1007/s12199-014-0399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzerling L, Kühnapfel S, Meckbach D, Baiter M, Kaempgen E, Keikavoussi P, Schuler G, Agaimy A, Bauer J, Hartmann A, Kiesewetter F, Schneider-Stock R. Rare BRAF mutations in melanoma patients: implications for molecular testing in clinical practice. Br J Cancer. 2013;108:2164–2171. doi: 10.1038/bjc.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgieva J, Sinha P, Schadendorf D. Expression of cyclins and cyclin dependent kinases in human benign and malignant melanocytic lesions. J Clin Pathol. 2001;54:229–235. doi: 10.1136/jcp.54.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dye DE, Medic S, Ziman M, Coombe DR. Melanoma biomolecules: independently identified but functionally intertwined. Front Oncol. 2013;3:252. doi: 10.3389/fonc.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]


