Abstract
Aims: The present study is to investigate the effect of microRNA-146a (miR-146a) and ethnic factor in the occurrence of cervical cancer in Uygur women in Xinjiang Uygur Autonomous Region. Methods: A total of 620 pieces of cervical tissues were obtained between September 2010 and September 2013, including 208 cases of cervicitis, 207 cases of cervical intraepithelial neoplasia, and 205 cases of cervical cancer. The relative expression of miR-146a in tissues was measured using quantitative real-time polymerase chain reaction. Polymerase chain reaction - restriction fragment length polymorphism was used to determine the genotypes of miR-146a (rs2910164). Differences between two groups and multiple groups were compared using t-test and one-factor analysis of variance, respectively. Comparison of genotype compositions and genetic balance examinations were performed using χ2 test. Results: Uygur women had earlier age of marriage, more times of pregnancy, and more childbirths than Han women. The miR-146a (rs2910164) genotype composition was significantly different between Uygur and Han, with the ratio of GG genotype in Uygur being higher than that in Han. Logistic regression analysis showed that miR-146a (rs2910164) genotypes were significantly correlated to ethnic factor and tumor sizes. The expression of miR-146a was elevated in cervical intraepithelial neoplasia and cervical cancer, especially for Uygur women, with the GG genotype being the most highly expressed. Conclusions: The miR-146a (rs2910164) polymorphism is significantly correlated to ethnic factor and tumor diameters. miR-146a has differential expression in cervical tissues. Allele G of miR-146a (rs2910164) is related to the high expression of miR-146a, and the progression of cervical cancer.
Keywords: Cervical cancer, Uygur, microRNA-146a, rs2910164
Introduction
Cervical cancer is the second commonest malignant tumor in women, with 530 thousand new cases occurring each year in the world [1]. The morbidity and mortality of cervical cancer are very high in Xinjiang Uygur women, and become the most important health issue at present [2-4]. Epidemiological studies show that the occurrence of cervical cancer is closely related to early childbirth, multiple child birth, high-risk sex partner and suppression of immune function, and the risk for cervical cancer is enhanced as the times of childbirth are increased [5]. Human papilloma virus (HPV) infection, especially high-risk continuous infection, is recognized as the main cause of precancerous cervical lesions and cervical cancer. However, some other studies show that the pathologic mechanism of cervical cancer is also related to genetic variation [6].
MicroRNA (miRNA or miR) is a kind of endogenous non-coding single-strand RNA with 22 nucleotides. It widely exists in eukaryotic cells and participates in the regulation of gene expression by inducing degradation or inhibiting translation of target mRNA [7]. miR-146a exhibits differential expression in lung cancer [8], hepatic ischemia-reperfusion injury [9], and myasthenia gravis [10]. In addition, miR-146a (rs2910164) gene polymorphism is related to the occurrence and development of papillary thyroid carcinoma [11] and prostate cancer [12]. In the present study, we investigate the specific expression of miR-146a in the cervical tissues from Uygur and Han women residing in Xinjiang Uygur Autonomous Region, and the relationship of miR-146a (rs2910164) gene polymorphism to the occurrence and development of cervical cancer.
Materials and methods
Patients
A total of 620 pieces of cervical tissues were obtained from Uygur and Han patients admitted in the Department of Pathology, The First Affiliated Hospital of Xinjiang Medical University from September 2010 to September 2013. The 620 tissue samples included 208 cases of cervicitis (control), 207 cases of cervical intraepithelial neoplasia (CIN), and 205 cases of cervical cancer. The pathological sections were independently reviewed by two experienced pathologists. The inclusion criteria were: sexual life history; Uygur and Han patients with pathological diagnosis of cervicitis, CIN or cervical cancer; complete clinical and pathological data; no chemotherapy or radiotherapy before surgery. Exclusion criteria were: other types of malignant tumors; autoimmune diseases; pregnancy or lactation. All procedures were approved by the Ethics Committee of Xinjiang Medical University. Written informed consents were obtained from all patients or their families.
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP)
DNA was extracted using DNeasy FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer’s manual. DNA concentration and purity were determined by NANODROP 1000 (Thermo Fisher Scientific, Waltham, MA, USA). C1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA) was used for PCR. Polymerase chain reaction (PCR) system (25 μl) included 2× Taq PCR Master mix (12.5 μl) (Lifefeng Biotech Co., Ltd., Wuhan, China), forward primer (0.5 μl), reverse primer (0.5 μl), template DNA (2.5 μl) and water (9 μl). miR-146a primer sequences were: forward, 5’-TTC CAT GGG TTG TGT CAG TGT CAG ACG T-3’; reverse, 5’-TTT CTC ACA GGA ACT CAC ACT CCT T-3’. PCR conditions were: 95°C for 3 min; 95°C for 10 s; 34 cycles of 59.0°C for 30 s and 72°C for 30 s; 72°C for 10 min; 4°C forever. Digestion reaction system (25 μl) was composed of Sac I enzyme (0.5 μl) (NEW ENGLAND BioLabs, Ipswich, MA, USA), 1× NEBuffer (2.5 μl), PCR product (10 μl) and double-distilled water (12 μl). The system was incubated at 37°C overnight (Model DNP-9082, Jinghong Laboratory Instrument Co., Ltd., Shanghai, China). The digestion product was subject to 3% agarose gel electrophoresis (100 V, 110 mA, 60 min) for genotyping analysis. The gels were imaged by Universal Hood II (Bio-Rad, Hercules, CA, USA).
Reverse transcription PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR)
Total RNA was obtained using RNeasy FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer’s manual. RNA concentration and purity were determined by NANODROP 1000 (Thermo Fisher Scientific, Waltham, MA, USA). The integrity of RNA was examined using 1% denaturing agarose gel electrophoresis.
For RT-PCR system (Thermo Scientific, Waltham, MA, USA), total RNA (200 ng), buffer (4 μl), dNTP (2 μl), RNase inhibitor (1 μl), M-MuLV reverse transcriptase (1 μl), and reverse transcription primer (1 μl) were added before addition of water to reach a total volume of 20 μl. The reaction condition was: 42°C for 60 min and 70°C for 5 min. The cDNA was stored at -20°C. Reverse transcription primers for miR-146a were specific stem-loop primers with a sequence of 5’-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACA ACC C-3’ (Sangon Biotech, Shanghai, China). Reverse transcription reaction of U6 was carried out using Oligo (dT) (0.5 μg/μl) (Sangon Biotech, Shanghai, China).
The iQ5 Multicolor Real-Time PCR Detecter System (Bio-Rad, Hercules, CA, USA) was used for qRT-PCR. The system of qRT-PCR (25 μl) was composed of 2× SYBR Green I Master mix (12.5 μl) (Thermo Scientific, Waltham, MA, USA), forward primer (1 μl), reverse primer (1 μl), reverse transcription product (3 μl) and water (7.5 μl). miR-146a primers were: forward, 5’-AGC AGT GAG AAC TGA ATT CCA T-3’; reverse, 5’-GTG CAG GGT CCG AGG T-3’. U6 primers were: forward, 5’-CTC GCT TCG GCA GCA CA-3’; reverse, 5’-AAC GCT TCA CGA ATT TGC GT-3’ (Sangon Biotech, Shanghai, China). qRT-PCR conditions were: 95°C for 3 min; 40 cycles of 95°C for 10 s and 52.8°C for 30 s; 81 cycles of 55°C for 10 s and 95°C for 10 s. Each sample was tested in triplicate. Ct values were determined by the number of cycles for the reaction to reach set fluorescence intensity thresholds. N = 2-ΔΔCt was used to determine the expression levels of miR-146a, where ΔΔCt = (CtmiR-146a - CtU6)cervical cancer - (average CtmiR-146a - average CtU6)cervicitis.
Statistical analysis
The results were analyzed using SPSS 17.0 software (IBM, Armonk, NY, USA). The data were expressed as means ± standard deviation. Differences between two groups and multiple groups were compared using t-test and one-factor analysis of variance, respectively. Comparison of genotype compositions and genetic balance examinations were performed using χ2 test.
Results
Uygur women have earlier age of marriage, more times of pregnancy, and more childbirths than Han women
To acquire basic knowledge about the conditions of the patients, their maternity information was compared among cervicitis, CIN and cervical cancer groups. The data showed significant differences in age of marriage, times of pregnancy, and parity among the three groups (P < 0.05), but no significant difference in means of delivery among the three groups (P > 0.05). In addition, there was no significant difference in ages between Uygur and Han women (P > 0.05). Compared with Han women, Uygur women had lower age of marriage, more times of pregnancy and parity, and higher percentage of vaginal delivery (P < 0.05) (Table 1). The results indicate that Uygur women have earlier age of marriage, more times of pregnancy, and more childbirths than Han women.
Table 1.
Maternity information analysis
| Ethnic groups | Groups | Cervicitis group | CIN group | Cervical cancer group | χ2 | P | |
|---|---|---|---|---|---|---|---|
| Han | Age of marriage | < 20 | 3.1% (5/163) | 2.9% (4/138) | 4.3% (5/116) | 0.550 | 0.759 |
| ≥ 20 | 96.9% (158/163) | 97.1% (134/138) | 95.7% (111/116) | ||||
| Uygur | < 20 | 24.4% (10/41) | 32.8% (22/67) | 83.5% (71/85) | 50.757 | < 0.001 | |
| ≥ 20 | 75.6% (31/41) | 67.2% (45/67) | 16.5% (14/85) | ||||
| Han | Times of pregnancy | < 6 | 96.7% (146/151) | 100.0% (136/136) | 92.2% (106/115) | 7.223 | 0.027 |
| ≥ 6 | 3.3% (5/151) | 0.0% (0/136) | 7.8% (9/115) | ||||
| Uygur | < 6 | 90.0% (36/40) | 82.1% (55/67) | 57.8% (48/83) | 13.114 | 0.001 | |
| ≥ 6 | 10.0% (4/40) | 17.9% (12/67) | 42.2% (35/83) | ||||
| Han | Parity | 1-3 | 98.6% (140/142) | 97.8% (131/134) | 92.8% (103/111) | 7.465 | 0.024 |
| ≥ 4 | 1.4% (2/142) | 2.2% (3/134) | 7.2% (8/111) | ||||
| Uygur | 1-3 | 97.4% (37/38) | 75.0% (45/60) | 52.4% (43/82) | 24.181 | < 0.001 | |
| ≥ 4 | 2.6% (1/38) | 25.0% (15/60) | 47.6% (39/82) | ||||
| Han | Vaginal delivery | 85.2% (121/142) | 85.8% (115/134) | 93.7% (104/111) | 2.954 | 0.228 | |
| Caesarean | 14.8% (21/142) | 14.2% (19/134) | 6.3% (7/111) | ||||
| Uygur | Vaginal delivery | 94.7% (36/38) | 100% (60/60) | 95.1% (78/82) | 1.570 | 0.456 | |
| Caesarean | 5.3% (2/38) | 0% (0/60) | 4.9% (4/82) | ||||
Note: In cervicitis group, 2 and 14 Han women were unmarried and never pregnant, respectively, while 2 and 3 Uygur women were unmarried and never pregnant, respectively; in CIN group, 2 and 4 Han women were unmarried and never pregnant, respectively, while all Uygur women were married and used to be pregnant; in cervical cancer group, 3 and 4 Han women were unmarried and never pregnant, respectively, while 1 and 3 Uygur women were unmarried and never pregnant, respectively. CIN, cervical intraepithelial neoplasia.
miR-146a (rs2910164) genotype composition is significantly different between Uygur and Han, with the ratio of GG genotype in Uygur being higher than that in Han
For the analysis of miR-146a (rs2910164) genotyping, PCR-RFLP was performed. After Sac I digestion, there were three types of DNA fragments: the type of fragment with 147 bp band corresponded to GG genotype; the type of fragments with 122 bp and 25 bp bands (not shown) corresponded to CC genotype; the type of fragments with 147 bp, 122 bp and 25 bp bands (not shown) corresponded to CG genotype (Figure 1). The composition of genotypes of miR-146a (rs2910164) showed no significant difference among cervicitis group, CIN group and cervical cancer group in Han or Uygur women (For Han, χ2 = 6.557, P = 0.161; for Uygur, χ2 = 0.126, P = 0.998). Of note, the miR-146a (rs2910164) genotype composition was significantly different between Han and Uygur (χ2 = 57.927, P < 0.001), with the percentage of GG genotype in Uygur being significantly higher than that in Han, and the percentage of CC genotype in Uygur being significantly lower than that in Han (Table 2). For miR-146a (rs2910164) in Uygur women, allele G accounted for 64.55%, while allele C accounted for 35.45%. For miR-146a (rs2910164) in Han women, allele G accounted for 41.8%, while allele C accounted for 58.2%. The results suggest that miR-146a (rs2910164) genotype composition is significantly different between Uygur and Han, with the ratio of GG genotype in Uygur being higher than that in Han.
Figure 1.
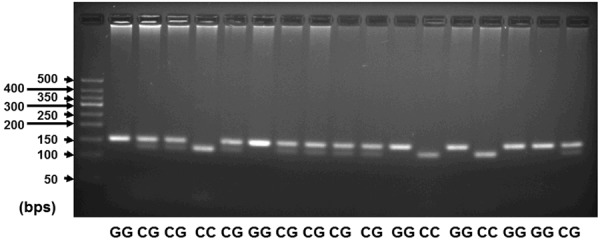
Examples of genotyping of miR-146a (rs2910164). The digestion product was subject to 3% agarose gel electrophoresis (100 V, 110 mA, 60 min) for genotyping analysis. The gels were imaged by Universal Hood II (Bio-Rad, Hercules, CA, USA).
Table 2.
Composition of genotypes of miR-146a (rs2910164)
| Genotypes | Ethnic groups | χ2 | P | |
|---|---|---|---|---|
|
| ||||
| Han (N %) | Uygur (N %) | |||
| GG | 67 (15.8%) | 76 (38.8%) | 57.927 | < 0.001 |
| CC | 137 (32.2%) | 19 (9.7%) | ||
| CG | 220 (52.0%) | 101 (51.5%) | ||
| Total | 424 (100%) | 196 (100%) | - | - |
The selected Uygur and Han populations are representative of the whole ethnic groups of Uygur and Han according to Hardy-Weinberg equilibrium
To examine whether bias exists in the selection of control population of Han or Uygur women, Hardy-Weinberg equilibrium examination was performed. After comparing the observed values of miR-146a (rs2910164) genotypes for Uygur and Han in the three groups with theoretical values, no statistically significant difference was found (P > 0.05). The data indicate that the selected Uygur and Han populations are representative of the whole ethnic groups of Uygur and Han.
miR-146a (rs2910164) genotypes are significantly correlated to ethnic factor and tumor sizes
To study the relationship of miR-146a (rs2910164) gene polymorphism with age, ethnic factor, pathological types, invasion depth, lymph node metastasis, uterine transfer, or intervascular invasion, Logistic regression analysis was performed. The data showed that miR-146a (rs2910164) gene polymorphism is correlated with ethnic factor and the diameter of tumors. GG/CG genotypes are significantly correlated to Uygur (OR = 3.332, 95% CI: 1.411-7.868) and tumor diameter that was greater than 4 cm (OR = 3.792, 95% CI: 1.162-12.376) (Table 3). The result suggests that miR-146a (rs2910164) genotypes are significantly correlated to ethnic factor and tumor sizes.
Table 3.
miR-146a (rs2910164) gene polymorphism and the pathologic characteristics of cervical cancer
| Groups | Genotypes | OR (95% CI) | P | ||
|---|---|---|---|---|---|
|
| |||||
| GG/CG | CC | ||||
| Ethnic groups | Han | 74 (62.2%) | 45 (37.8%) | 1.000 | 0.006 |
| Uygur | 78 (90.7%) | 8 (9.3%) | 3.332 (1.411-7.868) | ||
| Tumor diameter | < 4 cm | 123 (71.5%) | 49 (28.5%) | 1.000 | 0.027 |
| ≥ 4 cm | 29 (87.9%) | 4 (12.1%) | 3.792 (1.162-12.376) | ||
Note: OR, odd ratio; CI, confidence interval.
Expression of miR-146a is elevated in CIN and cervical cancer, especially for Uygur women, with the GG genotype being the most highly expressed
To investigate the relationship between miR-146a (rs2910164) gene polymorphism and relative expression of miR-146a, we determined the levels of miR-146a in cervical tissues from 163 cases. The data showed that the expression of miR-146a was elevated as the severity of cervical lesions was aggravated, and miR-146a expression in CIN and cervical cancer groups was higher than that in cervicitis group (P < 0.01) (Figure 2). In cervical cancer group, the levels of miR-146a in Uygur were significantly higher than those in Han (Z = -2.008, P = 0.045) (Table 4). The levels of GG genotype of miR-146a in CIN and cervical cancer groups were significantly higher than that of CC genotype (tCIN = 6.649, P = 0.014; tcervical cancer = 4.971, P = 0.040) (Table 5). The results suggest that the expression of miR-146a is elevated in CIN and cervical cancer, especially for Uygur women, with the GG genotype being the most highly expressed.
Figure 2.
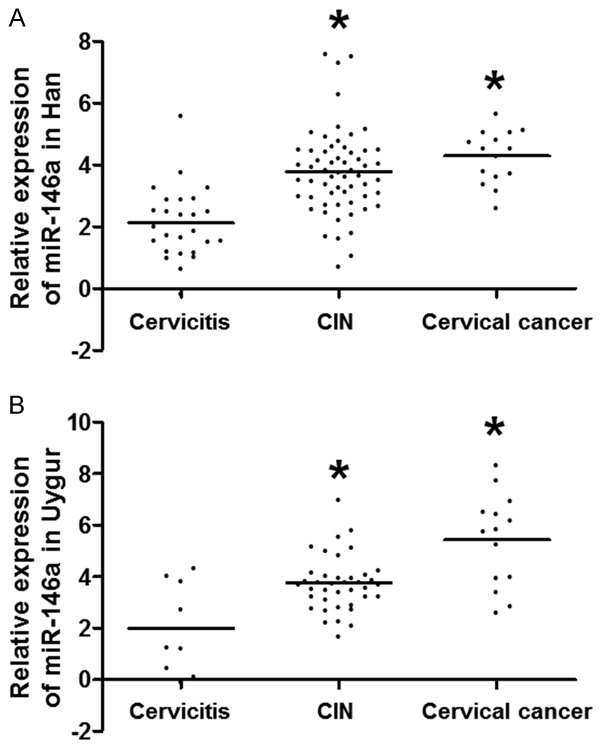
Relative expression of miR-146a in cervical tissues from (A) Han and (B) Uygur. qRT-PCR was performed to measure the levels of miR-146a. For Han women, 26, 60 and 15 cases were included in the cervicitis, CIN and cervical cancer groups, respectively. For Uygur women, 9, 39 and 14 cases were included in the cervicitis, CIN and cervical cancer groups, respectively. *P ≤ 0.001 compared with cervicitis group. CIN, cervical intraepithelial neoplasia.
Table 4.
Expression of miR-146a in cervical tissues from Uygur and Han women
| Ethnic groups | Groups | Statistics | P | ||
|---|---|---|---|---|---|
|
| |||||
| Cervicitis group | CIN group | Cervical cancer group | |||
| Han | 2.127 ± 1.165 | 3.787 ± 1.359 | 4.293 ± 0.855 | F = 20.148 | < 0.001 |
| Uygur | 2.005 ± 1.760 | 3.753 ± 1.064 | 5.433 ± 1.792 | χ2 = 14.993b | 0.001 |
| Statistics | Z = -0.226a | T = 0.134 | Z = -2.008a | - | - |
| P | 0.838 | 0.894 | 0.045 | ||
Mann-Whitney test;
Kruskal-Wallis test.
Table 5.
Expression of miR-146a with GG/CG/CC genotypes
| Genotypes | Groups | F | P | ||
|---|---|---|---|---|---|
|
| |||||
| Cervicitis group | CIN group | Cervical cancer group | |||
| GG | 2.333 ± 1.290 | 4.097 ± 1.158 | 5.628 ± 1.718 | 13.292 | < 0.001 |
| CG | 2.217 ± 1.231 | 3.825 ± 1.275 | 4.481 ± 1.048 | 15.479 | < 0.001 |
| CC | 1.654 ± 1.546 | 3.162 ± 1.099 | 3.940 ± 0.920 | 7.341 | 0.003 |
| F | 0.689 | 2.990 | 3.744 | - | - |
| P | 0.509 | 0.055 | 0.037 | ||
| GG | 2.333 ± 1.290 | 4.097 ± 1.158 | 5.628 ± 1.718 | - | - |
| CC | 1.654 ± 1.546 | 3.162 ± 1.099 | 3.940 ± 0.920 | ||
| t | 0.873 | 6.649 | 4.971 | ||
| P | 0.366 | 0.014 | 0.040 | ||
Discussion
A study shows that the infection rate of HPV in Uygur women (8.27%) was significantly lower than that in Han women (16.1%) [13]. Therefore, genetic susceptibility and features of social life may be important reasons for the high incidence of cervical cancer in Xinjiang Uygur women. The present study showed that Uygur women, especially those with precancerous cervical lesions and cervical cancer, usually have early marriage, multiple times of pregnancy, and multiple childbirths. The occurrence of cervical cancer in Uygur women might be related to precocious sex life, locally insufficient immune function in cervical tissues, and weak defense against HPV infection, cervical trauma caused during early vaginal delivery and endocrinological changes caused by pregnancy.
miRNA participates in many complex life processes, including cell differentiation [14], tumor growth [15], and angiogenesis [16,17]. A research shows that decreased levels of CC genotype of miR-146a (rs2910164) inhibit target genes, leading to higher risk of papillary thyroid carcinoma [11]. Mutation of allele G to C leads to down-regulated miR-146a expression, increasing the risk for prostate cancer in man [12]. In addition, allele C is related to enhanced risk for laryngo-carcinoma [18]. Other studies indicate that miR-146a (rs2910164) polymorphism is not significantly correlated to the risks for bladder cancer [19], kidney cancer [20], and congenital heart disease [21]. A study on the relationship between gastric cancer and miR-146a (rs2910164) proposed that allele G of miR-146a (rs2910164) is a risk factor for gastric cancer, with patients carrying GG having higher risks than those carrying GC or CC [22]. Dai et al. show that the risks for breast cancer are elevated in Caucasian populations with GG genotype of miR-146a (rs2910164) and dominant gene expression, being in contrast to the results obtained from Asian populations [23]. Liu et al. also demonstrate that populations with allele G have higher risks for hepatocellular carcinoma than those with allele C [24], being consistent with the report by Wang et al. [25]. However, a study in central south China shows that populations carrying CC genotype of miR-146a (rs2910164) have higher risks for nasopharyngeal carcinoma than those carrying CG or GG [26]. In addition, other studies propose that miR-146a (rs2910164) does not enhance the risks for type II diabetes [27], systemic lupus erythematosus and rheumatoid arthritis [28], or immune thrombocytopenia [29]. In recent years, several studies were carried out regarding miR-146a (rs2910164) polymorphism in Asian and Caucasian populations. A meta-analysis on prostate cancer, breast cancer, gastric cancer and liver cancer shows that GG genotype ratio in Caucasian populations from USA, Italy and India is significantly higher than that in Asian populations from Korea, Japan and China [30]. Another meta-analysis on all disease types showed that the percentages of allele G of miR-146a (rs2910164) in Caucasian patient group (5761 cases) and control group (6243 cases) were 75.07% and 76.08%, respectively, while those in Asian patient group (6140 cases) and control group (7957 cases) were 50.42% and 47.88%, respectively [31]. In the present study, miR-146a (rs2910164) composition is not significantly different among cervicitis group, CIN group and cervical cancer group. However, miR-146a (rs2910164) composition between Uygur and Han women was significantly different, with GG genotype ratio in Uygur being significantly higher than that in Han and CC genotype ratio in Uygur being significantly lower than that in Han. The percentage of allele G of miR-146a (rs2910164) in Uygur and Han women are 64.55% and 41.8%, respectively. Logistic regression analysis of miR-146a (rs2910164) genotypes in cervical cancer group showed that miR-146a (rs2910164) polymorphism is significantly correlated to ethnicity, with the ratio of GG/CG in Uygur being 3.332 times of that in Han. These data suggest that miR-146a (rs2910164) polymorphism has obvious ethnic specificity.
A study demonstrates that elevated expression of miRNA-146a in cervical cancer tissues might promote the growth of tumor cells [32]. Yue et al. propose that miR-146a expression in cervical cancer tissues with GG is significantly lower than that with CG or CC genotypes, having elevated risk for cervical cancer compared with those with CG or CC [33]. The present study shows that the levels of miR-146a in CIN and cervical cancer groups were significantly higher than that in cervicitis group. In CIN and cervical cancer groups, the expression of GG genotype was significantly higher than CC genotype. Especially, miR-146a expression in Uygur with cervical cancer was significantly higher than that in Han with cervical cancer. In addition, Logistic regression analysis of miR-146a (rs2910164) genotypes against factors like ages, pathological types, invasion depths, lymph node metastasis, parametrial metastasis, and vascular space invasion showed that tumor sizes ≥ 4 cm was significantly correlated to miR-146a (rs2910164) GG/CG genotypes (OR = 3.792, 95% CI: 1.162-12.376). The risk for tumor sizes ≥ 4 cm in cervical cancer patients with GG/CG genotypes is 3.792 times of that in cervical cancer patients with CC genotype, indicating that patients carrying allele G of miR-146a (rs2910164) are more likely to have larger tumor sizes and more severe conditions. This observation suggests that allele G of miR-146a (rs2910164) is possibly related to the progression of cervical cancer. Forloni [34] et al. shows that miR-146a significantly promotes the growth of melanoma, in which the carcinogenic ability of allele G of miR-146a (rs2910164) is stronger than allele C. Another study shows that CG or CC genotype reduces the risk for lung cancer in nonsmokers (OR = 0.66, 95% CI: 0.45-0.96, P = 0.03), and expression of miR-146a in GG genotype is significantly higher than that in CC or CG genotypes (P = 0.02) [35].
In conclusion, the present study demonstrates that early marriage, multiple times of pregnancy and childbirths are important external factors that induce higher morbidity of cervical cancer in Uygur women. miR-146a (rs2910164) polymorphism is significantly correlated to ethnic factor and tumor diameters. miR-146a has differential expression in cervical tissues, and participates in the occurrence and development of cervical cancer. Therefore, miR-146a may be a risk factor for the high morbidity of cervical cancer in Uygur women.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81160278) and the Open Issues of State Key Lab Incubation Base of Xinjiang Major Diseases Research (No. SKLIB-XJMDR-2012-5).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y, Lalai S, Zhou K, Wang Z, Fang X, Wang L. Clinical analysis of 4505 cases of cervical cancer. Chin J Obstet Gynecol. 2003;38:764–765. [Google Scholar]
- 3.Jiang S, Wang T, Tu S, Zhou J, Mairemu S, Xu X, Hairini S, Ruxian G, Shalai M, Aimu R, Deng X. Epidemiological investigation of cervical cancer in Xinjiang Cele County. Chin J Pract Gynecol Obstet. 2006;20:379–381. [Google Scholar]
- 4.Lalai S, Maimaiti A. HPV DNA examination in the biopsy of cervical cancer tissues from Xinjiang Uygur women. Chin J Obstet Gynecol. 1997;32:405–408. [Google Scholar]
- 5.Feng Y, Sheng K, Ma D, Kong B, Li L. Obstetrics and Gynecology. Beijing: People’s Medical Publishing House; 2010. [Google Scholar]
- 6.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Yang H, Li Y, Liu Y, Chen S, Qi C, Zhang Q, Lan T, He X, Guan XY, Wang L. microRNA-146 up-regulation predicts the prognosis of non-small cell lung cancer by miRNA in situ hybridization. Exp Mol Pathol. 2014;96:195–199. doi: 10.1016/j.yexmp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Kong L, Xu X, Geng Q, Tang W, Jiang W. Down-regulation of microRNA-146a in the early stage of liver ischemia-reperfusion injury. Transplant Proc. 2013;45:492–496. doi: 10.1016/j.transproceed.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Yan M, Wang Y, Zhang J, Yang H, Tian FF, Zhou W, Zhang N, Li J. Altered expression of miR-146a in myasthenia gravis. Neurosci Lett. 2013;555:85–90. doi: 10.1016/j.neulet.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, de la Chapelle A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2008;105:7269–7274. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu B, Feng NH, Li PC, Tao J, Wu D, Zhang ZD, Tong N, Wang JF, Song NH, Zhang W, Hua LX, Wu HF. A functional polymorphism in Pre-miR-146a gene is associated with prostate cancer risk and mature miR-146a expression in vivo. Prostate. 2010;70:467–472. doi: 10.1002/pros.21080. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Mayineur N, Chen F, Zhu K, Liu X, Reziya M, Sun Z, Zhao L, Zhang X, Pan Q, Liu X, Wu Y, Qiao Y. A cross-sectional study: The prevalence and distribution characteristic of HPV infection in Uygur women in Xinjiang. Oncology Progress. 2010;8:114–119. [Google Scholar]
- 14.Kawasaki H, Taira K. Retraction: Hes1 is a target of microRNA-23 during retinoic-acid-induced neuronal differentiation of NT2 cells. Nature. 2003;426:100. doi: 10.1038/nature02141. [DOI] [PubMed] [Google Scholar]
- 15.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW, Yee AJ, Ang LC, He C, Shan SW, Yang BB. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene. 2011;30:806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- 17.Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D, Dong W, Lu M, Xing G, Dong J, Zhang W. Relationship between microRNA-146a precursor region gene polymorphism and genetic susceptibility of laryngo-carcinoma in Jiangsu Han populations. Journal of Otolaryngology and Ophthalmology of Shangdong University. 2014;28:46–50. [Google Scholar]
- 19.Yang H, Dinney CP, Ye Y, Zhu Y, Grossman HB, Wu X. Evaluation of genetic variants in microRNA-related genes and risk of bladder cancer. Cancer Res. 2008;68:2530–2537. doi: 10.1158/0008-5472.CAN-07-5991. [DOI] [PubMed] [Google Scholar]
- 20.Horikawa Y, Wood CG, Yang H, Zhao H, Ye Y, Gu J, Lin J, Habuchi T, Wu X. Single nucleotide polymorphisms of microRNA machinery genes modify the risk of renal cell carcinoma. Clin Cancer Res. 2008;14:7956–7962. doi: 10.1158/1078-0432.CCR-08-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Liu R, Gu H, Nie Y, Zhang H, Liu R, Zhang H, Hu S. Association study of miR-146a rs2910164 C > G polymorphism and risk of congenital heart disease. Journal of Clinical and Experimental Medicine. 2013;12:729–733. [Google Scholar]
- 22.Xie WQ, Tan SY, Wang XF. MiR-146a rs2910164 polymorphism increases risk of gastric cancer: a meta-analysis. World J Gastroenterol. 2014;20:15440–15447. doi: 10.3748/wjg.v20.i41.15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai ZJ, Shao YP, Wang XJ, Xu D, Kang HF, Ren HT, Min WL, Lin S, Wang M, Song ZJ. Five common functional polymorphisms in microRNAs (rs2910164, rs2292832, rs11614913, rs3746444, rs895819) and the susceptibility to breast cancer: evidence from 8361 cancer cases and 8504 controls. Curr Pharm Des. 2015;21:1455–1463. doi: 10.2174/1381612821666141208143533. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Xie B, Chen S, Jiang F, Meng W. Association study of two inflammation-related polymorphisms with susceptibility to hepatocellular carcinoma: a meta-analysis. BMC Med Genet. 2014;15:92. doi: 10.1186/s12881-014-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Zhang L, Shi X, Xu H, Wang T, Bian J. Association between two common polymorphisms and risk of hepatocellular carcinoma: evidence from an updated meta-analysis. Biomed Res Int. 2014;2014:468605. doi: 10.1155/2014/468605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang GL, Chen ML, Li YZ, Lu Y, Pu XX, He YX, Tang SY, Che H, Zou Y, Ding C, He Z. Association of miR-146a gene polymorphism with risk of nasopharyngeal carcinoma in the central-southern Chinese population. J Hum Genet. 2014;59:141–144. doi: 10.1038/jhg.2013.135. [DOI] [PubMed] [Google Scholar]
- 27.Wang TT, Chen YJ, Sun LL, Zhang SJ, Zhou ZY, Qiao H. Affection of single-nucleotide polymorphisms in miR-27a, miR-124a, and miR-146a on susceptibility to type 2 diabetes mellitus in Chinese Han people. Chin Med J (Engl) 2015;128:533–539. doi: 10.4103/0366-6999.151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YH, Bae SC. The miR-146a polymorphism and susceptibility to systemic lupus erythematosus and rheumatoid arthritis: A meta-analysis. Z Rheumatol. 2015;74:153–156. doi: 10.1007/s00393-014-1509-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Zhang Y, Xue F, Xu J, Fang Z. Has-mir-146a rs2910164 polymorphism and risk of immune thrombocytopenia. Autoimmunity. 2014;47:173–176. doi: 10.3109/08916934.2014.883503. [DOI] [PubMed] [Google Scholar]
- 30.Yin Z, Yan L, Cui Z, Li X, Ren Y, Zhou B. Effects of common polymorphisms rs2910164 in miR-146a and rs3746444 in miR-499 on cancer susceptibility: a meta-analysis. Mol Biol Rep. 2013;40:3003–3013. doi: 10.1007/s11033-012-2372-7. [DOI] [PubMed] [Google Scholar]
- 31.Wang AX, Xu B, Tong N, Chen SQ, Yang Y, Zhang XW, Jiang H, Liu N, Liu J, Hu XN, Sha GZ, Chen M. Meta-analysis confirms that a common G/C variant in the pre-miR-146a gene contributes to cancer susceptibility and that ethnicity, gender and smoking status are risk factors. Genet Mol Res. 2012;11:3051–3062. doi: 10.4238/2012.August.31.2. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yue C, Wang M, Ding B, Wang W, Fu S, Zhou D, Zhang Z, Han S. Polymorphism of the pre-miR-146a is associated with risk of cervical cancer in a Chinese population. Gynecol Oncol. 2011;122:33–37. doi: 10.1016/j.ygyno.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Forloni M, Dogra SK, Dong Y, Conte D Jr, Ou J, Zhu LJ, Deng A, Mahalingam M, Green MR, Wajapeyee N. miR-146a promotes the initiation and progression of melanoma by activating Notch signaling. Elife. 2014;3:e01460. doi: 10.7554/eLife.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeon HS, Lee YH, Lee SY, Jang JA, Choi YY, Yoo SS, Lee WK, Choi JE, Son JW, Kang YM, Park JY. A common polymorphism in pre-microRNA-146a is associated with lung cancer risk in a Korean population. Gene. 2014;534:66–71. doi: 10.1016/j.gene.2013.10.014. [DOI] [PubMed] [Google Scholar]


