Abstract
In human, proximal convoluted tubules and thin limbs of Henle show expression of αB crystallin. Renal cell carcinoma also showed expression of αB crystallin in previous reports. We aimed to study the association between αB crystallin expression and renal cell carcinoma and urothelial carcinoma. Furthermore, we also investigated αB crystallin expression depending on the subtype of renal cell carcinoma and examined the relationship between αB crystallin expression and survival in patients with renal cell carcinoma. In our study, αB crystallin expression was different according to the type of tumor. A greater proportion of the clear cell type (52/77, 67.5%) and papillary type (4/4, 100%) showed reactivity compared to the chromophobe type (0/10, 0%). In the present study, a significantly greater number of renal cell carcinomas showed strong expression of αB crystallin (56/91, 61.5%) compared to urothelial carcinoma (P=7.967e-07). Therefore, αB crystallin might be a significant marker of renal cell carcinoma and might help to determine the type of renal tumor in cases of poorly differentiated kidney lesions and metastatic lesions. αB crystallin expression was not related to overall survival in univariate and multivariate models. In our study, alpha B crystallin could not be considered a prognostic marker of renal cell carcinoma.
Keywords: Alpha B crystallin, renal cell carcinoma, urothelial carcinoma, subtype, overall survival
Introduction
Renal cell carcinoma is estimated to account for 2-3% of adult malignancies. Approximately 80% of renal cell carcinomas are clear cell type, 15% are papillary type, and 5% are others [1]. αB crystallin is a small heat-shock protein that functions as a cytoprotective molecular chaperone. αB crystallin expression has been reported in renal epithelial tissues [2,3]. In a previous report on immunohistochemical analysis of αB crystallin in rat nephrogenesis, αB crystallin expression was initially found to be focal in the capsular epithelium during the late embryonic stage. At birth, αB crystallin expression was observed in the thin limbs of the loop of Henle and the inner medullary collecting duct also became positive for expression of αB crystallin. At adult stages, the proximal convoluted tubules in the S3 segment, thin limbs of loop of Henle, and medullary collecting ducts showed strong positivity for αB crystallin. In the developing rat kidney, αB crystallin showed restricted distribution in tubules according to stage. The expression of αB crystallin was detected from the outer capsule to inner medulla and increased in response to osmotic challenge. These findings indicate that αB crystallin has a function in the development and maintenance of tubules in the rat [4,5]. In human, proximal convoluted tubules and thin limbs of Henle show expression of αB crystallin. [6] αB crystallin has been reported in several human epithelial tumors such as head and neck squamous cell carcinoma, breast cancer, and thyroid cancer [2,7-9]. Renal cell carcinoma also showed expression of αB crystallin in previous reports [6,10,11]. Therefore we aimed to elucidate the association between αB crystallin expression and renal cell carcinoma and urothelial carcinoma. Furthermore, we also investigated αB crystallin expression depending on the subtype of renal cell carcinoma and examined the relationship between αB crystallin expression and survival in patients with renal cell carcinoma.
Materials and methods
Patients and tissue sampling
A total of 108 formalin-fixed paraffin-embedded kidney tissues were obtained from 91 patients with stage I to IV renal cell carcinoma and 17 patients with stage I to III urothelial carcinoma of the renal pelvis. All patients underwent radical nephrectomy at Samsung Changwon hospital between 2003 and 2012. All clinical information was acquired through medical records. None of the patients received adjuvant treatment. For patients with renal cell carcinoma, 61 were male and 30 were female, and 52 (57.1%) were aged 60 or younger. Patients with suspicion of distant metastasis at the time of nephrectomy were excluded from this study. The proportions of T/N stages were as follows: T1a: 31 patients (34.1%), T1b: 35 (38.4%), T2a: 6 (6.6%), T2b: 3 (3.3%), T3a: 14 (15.4%), T4: 2 (2.2%)/N0: 89 (97.8%), N1: 2 (2.2%). The stages of the tumor were determined according to the TNM system of the American Joint Committee on Cancer (AJCC), 7th edition. Renal cell carcinoma subtyping was performed based on previous reports. The reviews were conducted by two experienced pathologists (E. H. Lee and H. W. Lee). The tumor subtypes were categorized as follows: clear cell, 77 (84.6%); papillary, 4 (4.4%); chromophobe, 10 (11%). The mean follow-up duration was 1,830.2 days. The median overall survival was 1,591 days and mean overall survival was 1,830.198 days. The clinical characteristics of 91 patients with renal cell carcinoma and 17 patients with urothelial carcinoma of pelvis are summarized in Table 1.
Table 1.
Clinical characteristics of 91 renal cell carcinoma patients and 17 urothelial carcinoma patients
| Clinicopathologic feature | Renal cell carcinoma | Urothelial carcinoma | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | % | N | % | ||||
| Gender | Male | 61 | 67 | 11 | 64.7 | ||
| Female | 30 | 33 | 6 | 35.3 | |||
| Age | ≤60 | 52 | 57.1 | 4 | 23.5 | ||
| >60 | 39 | 42.9 | 13 | 76.5 | |||
| T stage | T1a | 31 | 34.1 | Ta | 2 | 11.8 | |
| T1b | 35 | 38.4 | Tis | 2 | 11.8 | ||
| T2a | 6 | 6.6 | T1 | 1 | 5.9 | ||
| T2b | 3 | 3.3 | 2 | 3 | 17.6 | ||
| T3a | 14 | 15.4 | 3 | 7 | 41.2 | ||
| T3b | 0 | 0 | 4 | 2 | 11.8 | ||
| T3c | 0 | 0 | |||||
| T4 | 2 | 2.2 | |||||
| N stage | N0 | 89 | 97.8 | NO | 16 | 94.1 | |
| N1 | 2 | 2.2 | N1 | 0 | |||
| N2 | 1 | 5.9 | |||||
| N3 | 0 | ||||||
| M | M0 | 91 | 100 | M0 | 16 | 94.1 | |
| M1 | 0 | 0 | M1 | 1 | 5.9 | ||
| Tumor type | Clear cell | 77 | 84.6 | Papillary | 17 | 100 | |
| Papillary | 4 | 4.4 | |||||
| Chromophobe | 10 | 11 | |||||
Immunohistochemical staining
Formalin-fixed paraffin-embedded (FFPE) tissue samples from patient tumors were collected retrospectively. To construct the tissue microarray for 91 renal cell carcinomas, evaluation of primary H&E-stained slides was conducted by two experienced pathologists (E. H. Lee and H. W. Lee). The most representative tumor areas were sampled. Each tumor core was 2 tissue cores, 6 mm in diameter. A total of 20 TMA blocks were created. For 17 urothelial carcinoma samples, a representative area was selected by H&E slide review by two experienced pathologists (E. H. Lee and H. W. Lee) without TMA construction. Representative sections from the standard tissue blocks and TMA blocks were cut with a microtome at 4-μm thickness and dried overnight at 37°C on a salinized slide. Immunochemical staining was performed using a Benchmark XT slide stainer (Ventana, Inc.) according to the manufacturer’s instructions. The antibody used for immunohistochemical staining of αB crystallin was mouse IgG1 monoclonal antibody, clone 1B6.1-3G4 (Enzo Life Sciences, Inc.; 1:200 dilution, 1 hour incubation at room temperature). To evaluate αB crystallin protein expression, the intensity was scored using a scoring system from 0-3 (0: negative; 1: weak; 2: moderate; 3: strong) and multiplied by the percentage of positive cells. The total score range was therefore 0-300. For αB crystallin expression, cytoplasmic and membranous staining was considered positive and nuclear staining was excluded from the scoring. Human pilocytic astrocytoma tissue was used as a positive control for αB crystallin. The immunohistochemical staining was evaluated by experienced pathologists (E. H. Lee and H. W. Lee) who were blinded to the patient information.
Statistical analysis
Statistical analysis was conducted using R software (version 3.1.0) with packages named “prettyR” and “survival”. To identify associations between αB crystallin and clinicopathologic characteristics, we used Fisher’s exact test and t-test. To examine difference of αB crystallin expression in renal cell carcinoma and urothelial carcinoma, we used Fisher’s exact test. And to analyze αB crystallin expression according to type of renal cell carcinoma, we used Fisher’s exact test. To determine relationships between survival and αB crystallin expression with respect to clinicopathologic characteristics, we performed analysis by univariate and multivariate Cox regression models. Kaplan-Meier analysis was performed to estimate the overall survival of the αB crystallin positive group and negative group. Log rank test was used to compare the survival distribution of the two groups (positive vs. negative αB crystallin expression). A P value < 0.05 was considered statistically significant (Figure 2).
Figure 2.
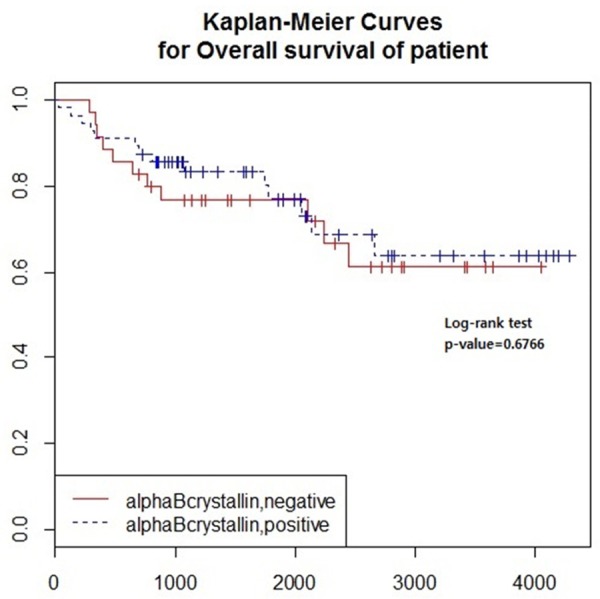
Kaplan-Meier curves and Log rank tests for overall survival according to αB crystallin expression were performed. αB crystallin showed no relationship to overall survival in renal cell carcinoma (P=0.6766).
Results
Immunohistochemical staining of αB crystallin
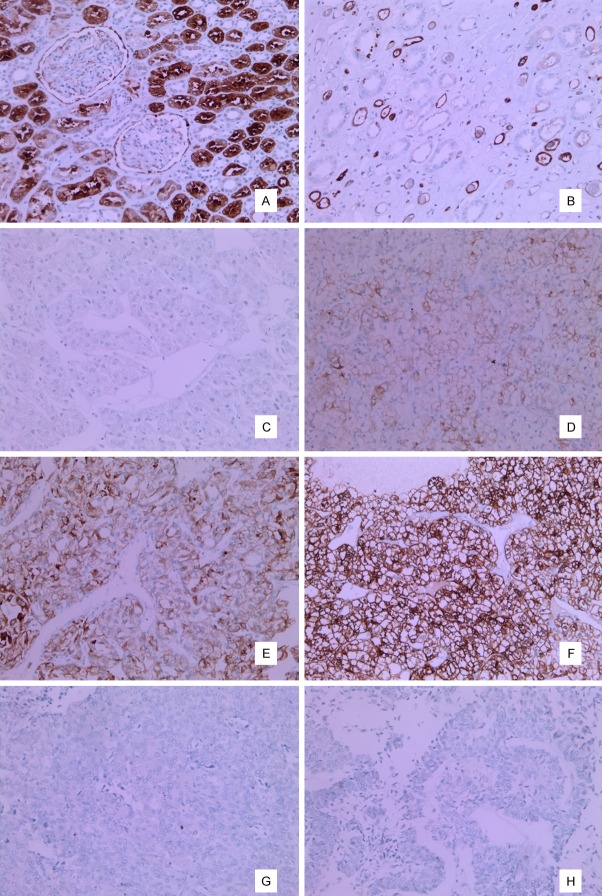
In immunohistochemical staining, αB crystallin expression was positive in the proximal convoluted tubules (Figure 1A) and thin limbs of Henle of the normal kidney (Figure 1B). The intensity of staining in renal carcinomas was found to be negative (Figure 1C), weak (Figure 1D), moderate (Figure 1E), and strong (Figure 1F). All cases of urothelial carcinoma of pelvis were clearly negative (Figure 1G and 1H). The intensity score multiplied by the percentage of positive cells in the tumor yielded a mean total score of 154.336 and median total score of 180. The cut-off value was determined by the median score (180) and the study population was divided into a positive group and negative group on the basis of median score. The percentage of stained cells in the positive group was 60-100%. The results of staining are summarized in Table 2.
Figure 1.
A. In immunohistochemical staining, αB crystallin expression was positive in the proximal convoluted tubules of the normal kidney. B. αB crystallin expression was positive thin limbs of Henle of the normal kidney. C. Negative expression of αB crystallin in renal cell carcinoma. D. Weak (1+) expression of αB crystallin in renal cell carcinoma. E. Moderate (2+) expression of αB crystallin in renal cell carcinoma. F. Strong (3+) expression of αB crystallin in renal cell carcinoma. G, H. Negative expression of αB crystallin in urothelial carcinoma.
Table 2.
Results of immunohistochemical staining in renal cell carcinoma
| Score (0-3) | |||
| 0 (no) | 1 (weak) | 2 (moderate) | 3 (strong) |
| 9 (9.9%) | 0 (0%) | 9 (9.9%) | 73 (80.2%) |
| Proportion (%) | |||
| 0-25 | 26-50 | 51-75 | 75-100 |
| 20 (22%) | 11 (12.1%) | 19 (20.8%) | 41 (45.1%) |
αB crystallin expression in renal cell carcinoma and urothelial carcinoma
A greater proportion of renal cell carcinoma samples showed strong reactivity to αB crystallin (56/91, 61.5%) compared to urothelial carcinoma and this difference was statistically significant (P=7.967e-07). αB crystallin expression was different according to the type of tumor. A greater proportion of the clear cell type (52/77, 67.5%) and papillary type (4/4, 100%) showed reactivity compared to the chromophobe type (0/10, 0%) and urothelial carcinoma (0/17, 0%). This difference was statistically significant (P=1.639e-11). These results are summarized in Table 3.
Table 3.
αB crystallin expression in renal cell carcinoma and urothelial carcinoma
| αB crystallin | Renal cell carcinoma (mean score) | Urothelial carcinoma (mean score) | p value | ||
|
| |||||
| Positive | 56 (259.7321429) | 0 (NA) | 7.967e-07 | ||
| Negative | 35 (61.4) | 17 (0) | |||
|
| |||||
| αB crystallin | Clear cell (mean score) | Papillary (mean score) | Chromophobe (mean score) | Urothelial carcinoma (mean score) | |
|
| |||||
| Positive | 52 (257.5) | 4 (288.75) | 0 (NA) | 0 (NA) | 1.639e-11 |
| Negative | 25 (79) | 0 (NA) | 10 (17.4) | 17 (0) | |
Univariate analysis of αB crystallin expression of renal cell carcinoma and clinicopathologic characteristics
Results of univariate analysis of αB crystallin expression and clinicopathologic characteristics are shown in Table 4. Sex, cancer type, and T stage were associated with αB crystallin expression. A total of 56 cases showed strong αB crystallin expression. αB crystallin overexpression was observed significantly more frequently in the clear cell type and papillary type compared to the chromophobe type (clear cell: 52/77 [68%]; papillary: 4/4 [100%]; chromophobe: 0/10 [25%]; P=1.426e-05). The proportion of alpha B crystallin expression was different between T stages (P=0.04431) and was higher in females than in males (male: 32/61 [52%], female: 24/30 [80%]).
Table 4.
αB crystallin expression in renal cell carcinoma according to clinicopathologic characteristics
| αB crystallin | αB crystallin | 95% confidence interval | P value | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Positive | Negative | Lower | Upper | |||
| Age | Mean | 57.80357 | 56.65714 | -6.762666 | 4.469809 | 0.686 |
| Sex | Male | 32 (52%) | 29 (48%) | 0.0816431 | 0.8321968 | 0.0125 |
| Female | 24 (80%) | 6 (20%) | ||||
| T stage | 1a | 24 (77%) | 7 (23%) | 0.04431 | ||
| 1b | 21 (60%) | 14 (40%) | ||||
| 2a | 4 (67%) | 2 (33%) | ||||
| 2b | 2 (67%) | 1 (33%) | ||||
| 3a | 4 (29%) | 10 (71%) | ||||
| 3b | 0 (NA) | 0 (NA) | ||||
| 3c | 0 (NA) | 0 (NA) | ||||
| 4 | 1 (50%) | 1 (50%) | ||||
| N stage | 0 | 55 (62%) | 34 (38%) | 0.02002464 | 129.11977469 | 1 |
| 1 | 1 (50%) | 1 (50%) | ||||
| Type | Clear cell | 52 (68%) | 25 (32%) | 1.426e-05 | ||
| Papillary | 4 (100%) | 0 (0%) | ||||
| Chromophobe | 0 (0%) | 10 (100%) | ||||
| Overall survival (days) | Mean | 1837.661 | 1818.257 | -514.4767 | 475.6696 | 0.9381 |
Analysis of overall survival according to αB crystallin expression and clinicopathologic characteristics of renal cell carcinoma by Cox-regression model
Univariate and multivariate Cox-regression models of overall survival with respect to αB crystallin expression and clinicopathologic characteristics are summarized in Table 5. αB crystallin expression was not related to overall survival in the univariate and multivariate model (univariate P=0.677; multivariate, P=0.4401). N stage showed an association with overall survival only in the univariate model (P=0.000122). However, age and T stage were associated with overall survival in both the univariate and multivariate model (T stage, P=0.000734 and 0.0294; age, P=0.0127 and 0.0409).
Table 5.
Overall survival according to clinicopathologic characteristics
| Univariate Cox regression | Multivariate Cox regression | ||||
|---|---|---|---|---|---|
|
|
|||||
| Independent variable | Reference | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value |
| αB crystallin = positive | Negative | 0.8451 (0.3828-1.865) | 0.677 | 1.48075 (0.5466-4.011) | 0.4401 |
| Age | 1.04533 (1.01-1.082) | 0.0127 | 1.04269 (1.0017-1.085) | 0.0409 | |
| Sex = female | Male | 0.6914 (0.2885-1.657) | 0.408 | 0.73627 (0.2826-1.918) | 0.5309 |
| Papillary | Clear cell | 0.8624 (0.1156-6.432) | 0.885 | 1.20829 (0.1515-9.636) | 0.8582 |
| Chromophobe | 1.1569 (0.3448-3.882) | 0.813 | 1.52048 (0.3790-6.101) | 0.5544 | |
| T stage | T1 | 1.4565 (1.171-1.812) | 0.000734 | 1.35175 (1.0306-1.773) | 0.0294 |
| N stage | N0 | 23.6984 (4.716-119.1) | 0.000122 | 2.68901 (0.2997-24.125) | 0.3769 |
Discussion
Heat shock proteins act as molecular chaperones to protect the kidneys against stress, such as heat, ischemia, hypertension, inflammation, and drugs [13,14]. αB crystallin is one of the small heat-shock proteins and has an antiapoptotic function. In vitro, αB crystallin induced resistance to oxidative stress-induced apoptosis in rabbit lens epithelial cells [12]. αB crystallin expression was reported during renal tubulogenesis and probably has a cytoprotective function in kidney development [5,6]. αB crystallin was also found in rat renal tubules, including the pars recta of proximal tubules, thin limbs of loops of Henle, and inner medullary collecting ducts [5,6]. In humans, αB crystallin was detected in the proximal tubules and thin limbs of Henle, but not in the distal tubules and glomerular components [6]. Renal cell carcinoma originates in the renal tubules and accounts for 80-85% of malignant renal tumors. A pathological classification of renal cell carcinomas was proposed in 1986 [13]. Approximately 80% of renal cell carcinomas are clear cell type, 15% are papillary type, and 5% are others. Clear cell carcinoma is thought to have originated in the proximal convoluted tubule. Papillary renal cell carcinomas are derived from proximal convoluted tubule and distal convoluted tubule. And chromophobe renal cell carcinomas are derived from intercalated cell of cortical collecting duct [14]. In our study, αB crystallin expression was different according to the type of tumor. A greater proportion of the clear cell type (52/77, 67.5%) and papillary type (4/4, 100%) showed reactivity compared to the chromophobe type (0/10, 0%). These findings probably due to cell origin of renal cell carcinoma. In human kidney, proximal convoluted tubule shows reactivity of αB crystallin so clear cell type and papillary type renal cell carcinoma have a tendency of αB crystallin expression. And in human kidney, collecting duct shows negative reactivity of αB crystallin. Chromophobe type is derived from intercalated cell of cortical collecting duct. So all chromophobe type shows negative reactivity for αB crystallin in our study. In the present study, a significantly greater number of renal cell carcinomas showed strong expression of αB crystallin (56/91, 61.5%) compared to urothelial carcinoma (P=7.967e-07). Therefore, αB crystallin might be a significant marker of renal cell carcinoma subtype and our findings might help to determine the type of renal tumor in cases of poorly differentiated kidney lesions and metastatic lesions. αB crystallin expression was not related to overall survival in univariate and multivariate models. In our study, alpha B crystallin was not related to overall survival and could not be considered a prognostic marker of renal cell carcinoma. Our study has limitations of the small number of cases and the use of only immunohistochemical staining as a protein detection method.
Acknowledgements
This work was supported by a grant from Samsung Biomedical Research Institute.
Disclosure of conflict of interest
None.
References
- 1.Reuter VE, Presti JC Jr. Contemporary approach to the classification of renal epithelial tumors. Semin Oncol. 2000;27:124–137. [PubMed] [Google Scholar]
- 2.Pinder S, Balsitis M, Ellis I, Landon M, Mayer R, Lowe J. The expression of alpha B crystallin in epithelial tumours: A useful tumour marker? J Pathol. 1994;174:209–215. doi: 10.1002/path.1711740310. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwaki T, Iwaki A, Liem R, Goldman JE. Expression of alpha B-crystallin in the developing rat kidney. Kidney Int. 1991;40:52–56. doi: 10.1038/ki.1991.178. [DOI] [PubMed] [Google Scholar]
- 5.Michl M, Ouyang N, Fraek M, Beck F, Neuhofer W. Expression and regulation of αB-crystallin in the kidney in vivo and in vitro. Pflügers Archiv. 2006;452:387–395. doi: 10.1007/s00424-005-0033-6. [DOI] [PubMed] [Google Scholar]
- 6.Takashi M, Katsuno S, Sakata T, Kato K, Ohshima S. Different concentrations of two small stress proteins, αB crystallin and HSP27 in human urological tumor tissues. Urol Res. 1998;26:395–399. doi: 10.1007/s002400050075. [DOI] [PubMed] [Google Scholar]
- 7.Chin D, Boyle GM, Williams RM, Ferguson K, Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG, Coman WB. Alpha B Crystallin, a New Independent Marker for Poor Prognosis in Head and Neck Cancer. Laryngoscope. 2005;115:1239–1242. doi: 10.1097/01.MLG.0000164715.86240.55. [DOI] [PubMed] [Google Scholar]
- 8.Chan S, Lui PC, Tan P, Yamaguchi R, Moriya T, Yu A, Shao M, Hliang T, Wong S, Tse GM. Increased alphaB crystallin expression in mammary metaplastic carcinomas. Histopathology. 2011;59:247–255. doi: 10.1111/j.1365-2559.2011.03882.x. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov O, Chen F, Wiley EL, Keswani A, Diaz LK, Memmel HC, Rademaker A, Gradishar WJ, Morrow M, Khan SA. αB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2008;111:411–417. doi: 10.1007/s10549-007-9796-0. [DOI] [PubMed] [Google Scholar]
- 10.Ho PY, Chueh SC, Chiou SH, Wang SM, Lin WC, Lee IL, Yang HY, Peng HC, Lai MK. αB-Crystallin in clear cell renal cell carcinoma: Tumor progression and prognostic significance. Urol Oncol. 2013;31:1367–1377. doi: 10.1016/j.urolonc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Holcakova J, Hernychova L, Bouchal P, Brozkova K, Zaloudik J, Valik D, Nenutil R, Vojtesek B. Identification of alphaB-crystallin, a biomarker of renal cell carcinoma by SELDI-TOF MS. Int J Biol Markers. 2008;23:48–53. doi: 10.1177/172460080802300108. [DOI] [PubMed] [Google Scholar]
- 12.Mao YW, Xiang H, Wang J, Korsmeyer S, Reddan J, Li DW. Human bcl-2 gene attenuates the ability of rabbit lens epithelial cells against H2O2-induced apoptosis through down-regulation of the alpha B-crystallin gene. J Biol Chem. 2001;276:43435–43445. doi: 10.1074/jbc.M102195200. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakopoulos CE, Chittoria N, Choueiri TK, Kroeger N, Lee JL, Srinivas S, Knox JJ, Bjarnason GA, Ernst SD, Wood LA, Vaishampayan UN, Agarwal N, Pal SK, Kanesvaran R, Rha SY, Yuasa T, Donskov F, North SA, Heng DY, Rini BI. Outcome of patients with metastatic sarcomatoid renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clin Genitourin Cancer. 2015;13:e79–85. doi: 10.1016/j.clgc.2014.08.011. [DOI] [PubMed] [Google Scholar]