Abstract
Phosphaturic mesenchymal tumor (PMT) has been established as a tumor that causes tumor-induced osteomalacia (TIO) associated with mesenchymal neoplasm. Its lineage of differentiation has not been elucidated. Previously, the presence of lymphatic vessels inside PMTs has been documented using an anti-podoplanin antibody; the tumor cells of PMTs were reported to not react with it. In this study of 14 cases of PMTs, we used immunohistochemistry of D2-40, a relatively specific lymphatic endothelial marker, to see if they stained PMTs or not, with particular interest in its reaction with microcystic structures containing lymph-like fluid. We report that most of the PMTs (12 out of 14 cases; 86%) were immunostained by D2-40 in their tumor cells; D2-40-positive lymphatic vessels inside the tumor were also observed. We used a proportion score (0-4+), an intensity score (0-3+), and a total score (the sum of the proportion score and the intensity score) to quantitate our results. We report that 50% of cases (7 out of 14 cases) had a total score ≥ 4+; immunostaining of D2-40 in cases with a total score ≥ 4+ was easy to observe at a glance. Most of the tumor cells lining the microcystic structures were immunostained with D2-40. Thus, D2-40 could be a useful diagnostic marker of PMTs and it might also indicate that PMTs take a lymphatic endothelial immunophenotype.
Keywords: Phosphaturic mesenchymal tumor, immunohistochemistry, D2-40
Introduction
Tumor-induced osteomalacia (TIO) has been recognized as a minor cause of osteomalacia [1]. TIO is caused by tumors producing phosphatonins, hormones that disrupt phosphate reuptake in the kidney [2]. Among the phosphatonins, fibroblast growth factor 23 (FGF23) is the most common, acting primarily on proximal renal tubular cells and being involved in phosphate homeostasis. Under normal circumstances, osteocytes are the main source of its secretion [3].
TIO cases are primarily associated with mesenchymal neoplasms, but rare epithelial neoplasms that lead to TIO have been documented, particularly in prostate carcinoma [4]. Various mesenchymal neoplasms have been describe as a cause of TIO; however, it has recently been revealed that a histologically uniform neoplasm, phosphaturic mesenchymal tumor (PMT), is the most common mesenchymal neoplasm-associated cause of TIO, but its lineage of differentiation has not been elucidated [5,6].
PMTs can arise from various sites in the body, including soft tissues, bone, and the sinonasal region [5]. PMTs typically consist of bland, spindled to stellate cells, with osteoclast-like giant cells sometimes observable. In the background of tumor cells, PMTs exhibit a particularly well developed capillary network, with the larger vessels sometimes taking on the morphology of a staghorn pattern [5]. The matrix in PMTs typically exhibits grungy or flocculent calcification [5]. Microcystic changes are sometimes encountered [6,7].
It has been previously documented by Williams et al. that lymphatic vessels are present in PMTs [8]. They emphasized the importance of their presence because lymphatic vessels are not observed in solitary fibrous tumors, which was the most frequent misdiagnosis given to the tumors now known as PMTs before PMTs had been established as a distinct tumor entity [5,8]. To confirm the presence of lymphatic vessels, Williams et al. conducted immunohistochemical analysis using two antibodies, anti-podoplanin and LYVE-1; the anti-podoplanin antibody they used is the same one that was tested on histological specimens in 1999 [9]. They also reported that the tumor cells of PMTs are not immunostained by anti-podoplanin and LYVE-1 [8].
We experienced that D2-40, a commercially available mouse monoclonal antibody to an Mr 40,000 O-linked sialoglycoprotein and one of the lymphatic endothelial markers [10,11], was immunostained in the aforementioned microcystic changes of PMTs containing lymph-like fluid. Hence, we supposed that the tumor cells constituting the wall of the microcystic structures may show a similar immunophenotype to lymphatic endothelial cells, and that, if so, the tumor cells might be said to partly show differentiation toward lymphatic endothelium-like cells. In order to prove this, we chose to use the commercially available D2-40 antibody. In this study, D2-40 staining of PMT tumor cells is examined in detail.
Materials and methods
Patient samples
First, the samples used in this study were the same as those used in another paper which we were reporting [12]. The computerized pathological database of The University of Tokyo Hospital was searched between 2000 and 2015 pick up cases of PMT. Seventeen cases were identified; formalin-fixed, paraffin-embedded blocks were available for 14 cases. The computerized medical record system was referred to for clinical information of these 14 cases.
Enzyme-linked immunosorbent assay
The serum FGF23 concentration was measured using an enzyme-linked immunosorbent assay (ELISA) that detects only full-length FGF23 (Intact FGF23 ELISA, Kainos Laboratories, Tokyo, Japan). Based on this assay, the normal level of serum FGF23 ranges from 10 to 50 pg/ml. Preoperative and postoperative serum FGF23 levels were measured.
Histopathological evaluation
Each surgically obtained specimen was fixed in 10% buffered-formalin and then embedded in paraffin. Specimens obtained from bone had been subjected to decalcification using an ethylenediaminetetraacetic acid (EDTA) (10% EDTA 2Na; MUTO PURE CHEMICALS, Tokyo, Japan)-based reagent for the duration of approximately 1 week before paraffin-embedding. Four-micrometer-thick sections were obtained from paraffin-embedded blocks in all 14 cases and were stained with hematoxylin and eosin.
Immunohistochemistry
Four-micrometer thick sections were obtained from paraffin-embedded blocks. Immunohistochemistry was performed using D2-40 (clone D2-40, Signet Laboratories, Dedham, MA, USA) at a dilution of 1:100. Immunohistochemistry was conducted using a BenchMark XT Autoimmune Stainer (Roche Ventana Medical Systems Inc., Tokyo, Japan) and an I-View DAB detection kit (Roche Ventana Medical Systems Inc.). The proportion of immunoreactive tumor cells was scored according to the following standard-0: no staining; 1+: 1-25%; 2+: 26-50%; 3+: 51-75%; and 4+: 76-100%. The intensity of immunostaining was assigned one of four scores as follows: a score of 3+ (strong intensity) was given if the immunopositivity of the tumor cells was strong on low-power view (40x); a score of 1+ (weak intensity) was given if the immunopositivity was weak on low-power view, but obviously stained on high-power view (400×); a score of 2+ (moderate intensity) was given for moderate intensity immunostaining between 1+ and 3+; and a score of 0 was given if tumor cells were not immunostained. The total score was the sum of the proportion score and the intensity score, ranging from 0 to 7+. In cases containing microcystic structures, the immunoreactivity of tumor cells lining the walls was evaluated as follows: if at least a part of the microcystic structures was immunostained, the evaluation was positive (+); if there was at least one microcystic structure completely non-immunostained, the evaluation was negative (-).
Ethics
This study was approved by the ethics committees and governance boards of The University of Tokyo (10461-2).
Results
Clinical findings
The clinical characteristics of the 11 cases are summarized in Table 1. The mean patient age, the male to female ratio, and the mean preoperative serum FGF23 level were the same as described in the previous report [12]. For the eight cases where diameter could be measured, the mean tumor diameter was 24 mm, with a range of 9 mm to 60 mm. The mean preoperative serum FGF23 level was 1204 pg/ml, with serum levels ranging from 88.9 pg/ml to 9165 pg/ml. Postoperatively, serum FGF23 levels of all of the patients dropped to variable extent.
Table 1.
Clinical characteristics
| Case* | Age | Sex | Diameter | FGF23 (pg/ml) | Location |
|---|---|---|---|---|---|
| 2 | 63 | M | N/A | 9165 | First lumbar vertebra |
| 7 | 58 | M | N/A | 3066 | Right femoral bone |
| 1 | 68 | F | N/A | 2236 | Right siatic bone |
| 6 | 51 | M | 22 mm | 482 | Left sole |
| 9 | 68 | M | 25 mm | 446 | Right humeral bone |
| 12 | 41 | F | N/A | 423 | Sphenoid bone |
| 14 | 77 | M | 20 mm | 242 | Left parotid gland |
| 4 | 61 | M | 18 mm | 225 | Dura |
| 5 | 40 | F | N/A | 162 | Right knee |
| 10 | 64 | F | 27 mm | 111 | Right iliac bone |
| 11 | 64 | F | 60 mm | 111 | Right iliac bone |
| 8 | 58 | M | N/A | 98.9 | Left femoral bone |
| 13 | 46 | M | 13 mm | 88.9 | Left femoral bone |
| 3 | 67 | M | 9 mm | 53 | Right femoral bone |
F: female; M: male; N/A: not available.
The case numbers are the same as those in the previous report [12].
Histological findings
A common histological finding in all cases was the monotonous proliferation of tumor cells within a background of prominent microvessels. Another characteristic finding, staghorn vessels, was observed in 11 cases (79%) (Figure 1A). The unique grungy or flocculent calcification was observed in patches in eight cases (57%) (Figure 1B). The tumor cells were spindle-shaped with scant eosinophilic cytoplasm and bland nuclei with indistinct nucleoli. Osteoclast-like giant cells were present in patches in 10 cases (71%) (Figure 1C). Microcystic structures were observed in seven cases (50%), all of which had lymph-like fluid (Figure 1D). None of the cases showed histological atypia that is indicative of malignancy.
Figure 1.
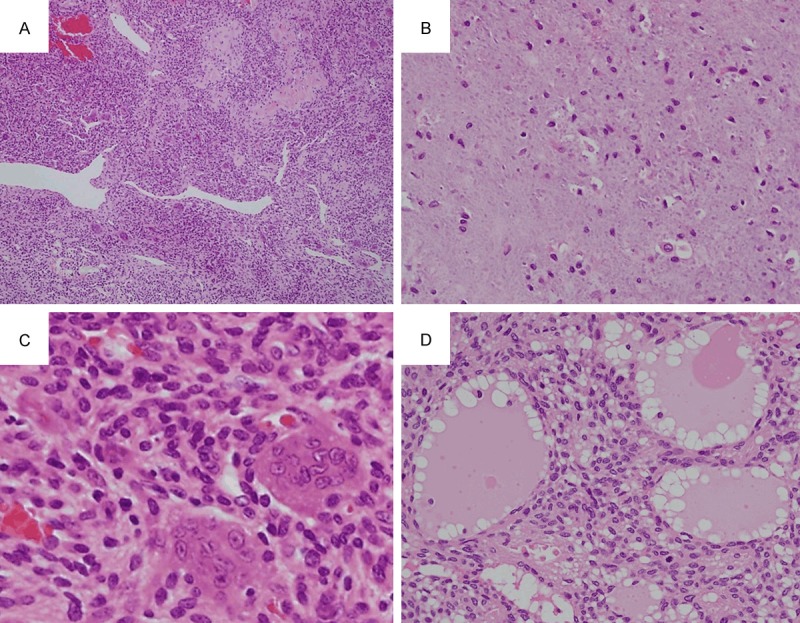
Histological findings. A. Case 8. Monotonous proliferation of tumor cells with a background of prominent microvessels are observed along with the presence of staghorn vessels (×40). B. Case 6. The unique grungy or flocculent calcification is observed (×400). C. Case 8. The tumor cells are spindle-shaped with scant eosinophilic cytoplasm, and bland nuclei with indistinct nucleoli. Osteoclast-like giant cells are also present (×600). D. Case 7. Microcystic structures are observed, in which lymph-like fluid is present (×400).
Immunohistochemical findings
The immunohistochemistry results for D2-40 are summarized in Table 2. EDTA-based decalcification did not affect the quality of immunostaining. The combined immunohistochemical results of the 14 cases are summarized in Table 3. Tumor cells in 12 cases (86%) were immunostained with D2-40, with a proportion score ranging from 1+ (three cases, 21%), 2+ (five cases, 36%), to 3+ (four cases, 29%) and an intensity sore ranging from 1+ (three cases, 21%), 2+ (four cases, 29%), to 3+ (five cases, 36%; Figure 2A). The total scores were as follows: 0 (two cases, 14%), 1+ (none, 0%), 2+ (one case, 7%), 3+ (four cases, 29%), 4+ (one case, 7%), 5+ (three cases, 21%), 6+ (three cases, 21%), and 7+ (none, 0%). Lymphatic vessels strongly positive for D2-40 were present preferentially in areas where D2-40-positive tumor cells were present (Figure 2B). In areas without D2-40-postive tumor cells, lymphatic vessels tended to be sparse or absent (Figure 2C). In the aforementioned seven cases in which microcystic structures were observed, most of the tumor cells lining the wall of the structures were immunostained with D2-40 (Figure 2D); occasionally, microcystic structures lacking circumferential immunopositivity for D2-40 were observed, but at least part of all of the microcystic structures were immunostained with the antibody. The relationship between the total score and the presence or absence of microcystic structures, with information about the evaluation of D2-40-positive microcystic structure-lining tumor cells, is summarized in Table 4. All of the cases contained at least some D2-40-positive lymphatic vessels. The D2-40-immunoreactivity of tumor cells lining microcystic structures was weaker than that of the endothelial cells of the lymphatic vessels.
Table 2.
Immunohistochemical results of D2-40 immunostaining for each case
| Case | Decalcification | Proportion | Intensity | Total score |
|---|---|---|---|---|
| 1 | + | 3+ | 2+ | 5+ |
| 2 | + | 3+ | 3+ | 6+ |
| 3 | + | 2+ | 1+ | 3+ |
| 4 | - | 2+ | 2+ | 4+ |
| 5 | + | 0 | 0 | 0 |
| 6 | - | 1+ | 2+ | 3+ |
| 7 | + | 3+ | 3+ | 6+ |
| 8 | + | 0 | 0 | 0 |
| 9 | + | 2+ | 1+ | 3+ |
| 10 | + | 3+ | 3+ | 6+ |
| 11 | + | 2+ | 3+ | 5+ |
| 12 | + | 1+ | 2+ | 3+ |
| 13 | + | 1+ | 1+ | 2+ |
| 14 | - | 2+ | 3+ | 5+ |
Table 3.
Combined immunohistochemical results of all cases
| Cases/total (%) | |
|---|---|
| Positive cases (%) | 12/14 (86%) |
| Proportion | |
| 4+ | 0/14 (0%) |
| 3+ | 4/14 (29%) |
| 2+ | 5/14 (36%) |
| 1+ | 3/14 (21%) |
| 0 | 2/14 (14%) |
| Intensity | |
| 3+ | 5/14 (36%) |
| 2+ | 4/14 (29%) |
| 1+ | 3/14 (21%) |
| 0 | 2/14 (14%) |
| Total score | |
| 6+ | 3/14 (21%) |
| 5+ | 3/14 (21%) |
| 4+ | 1/14 (7%) |
| 3+ | 4/14 (29%) |
| 2+ | 1/14 (7%) |
| 1+ | 0/14 (0%) |
| 0 | 2/14 (14%) |
Figure 2.
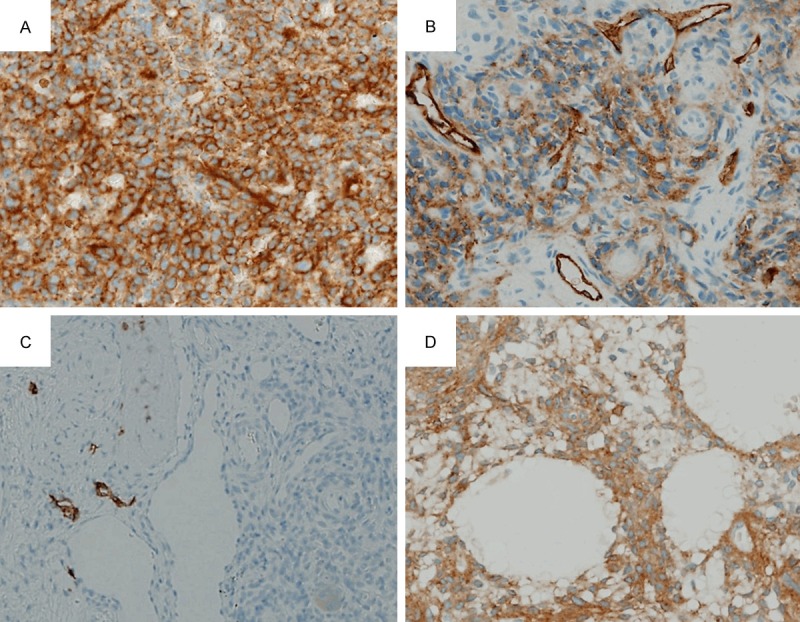
Immunohistochemical findings for D2-40. A. Case 14 showing strong intensity (×400). B. Case 4 showing moderate intensity. Note immunopositive lymphatic vessels with strong intensity (×400). C. Case 8 showing no staining of tumor cells. Note immunopositive lymphatic vessels present outside the tumor area (left half of the field) (×400). D. Case 7 showing microcystic structures, with the tumor cells lining them moderately immunostained (x400).
Table 4.
Relationship between D2-40 expression and microcystic structure (MS)
| Case | Total score | MS | D2-40 in MS-lining cells |
|---|---|---|---|
| 1 | 5+ | + | + |
| 2 | 6+ | + | + |
| 3 | 3+ | - | N.D. |
| 4 | 4+ | - | N.D. |
| 5 | 0 | - | N.D. |
| 6 | 3+ | - | N.D. |
| 7 | 6+ | + | + |
| 8 | 0 | - | N.D. |
| 9 | 3+ | + | + |
| 10 | 6+ | + | + |
| 11 | 5+ | + | + |
| 12 | 3+ | - | N.D. |
| 13 | 2+ | - | N.D. |
| 14 | 5+ | + | + |
N.D.: No data.
Discussion
Most TIO-associated tumors display relatively uniform histological features, as was demonstrated by Folpe et al. in 2004, which distinguished PMT as a distinct tumor entity [5]. Several tumor entities are included in the differential diagnosis of PMT, as shown by the various misdiagnoses previously provided for PMT in the review by Folpe et al. [5]. Solitary fibrous tumor is the most confusing tumor to be distinguished from PMT, especially in the situation where the characteristic grungy or flocculent calcification and osteoclast-like giant cells are not apparent in PMT. This is because PMT harbor proliferating small vessels sometimes accompanied by staghorn vessels, and a high microvessel density with staghorn vessels is one of the characteristic features of solitary fibrous tumors [5,14]. Williams et al. showed that proliferative vessels in solitary fibrous tumors are not immunostained with anti-podoplanin antibody, but some of those in PMTs are [8]. These findings mean that the presence or absence of lymphatic vessels inside a tumor can be used as a diagnostic differential when PMTs and solitary fibrous tumors are considered in the final diagnosis [8]. In this study, all of the cases contained lymphatic vessels immunoreactive for D2-40. This finding confirms the observation by Williams et al.
D2-40 reactivity to tumor cells of PMTs is a previously undescribed finding. Williams et al. did not observe immunostaining of the tumor cells using the anti-podoplanin antibody [8]; it was thus predicted that the cells would also be immunonegative for D2-40. Interestingly, tumor cells of 12 out of our 14 cases (86%) were immunostained with D2-40; the degree of staining was variable, ranging from a case showing a limited staining area and weak staining (case 13; proportion score 1+, intensity score 1+) to cases showing a widespread staining area and strong staining (cases 2, 7, and 10; proportion score 3+, intensity score 3+). Fifty percent of the cases had a total score ≤ 3+ and the rest had a total score ≥ 4+; in cases with a total score ≥ 4+, the D2-40 immunostaining in the tumor cells was obvious at a glance. These findings regarding tumor cell immunopositivity of D2-40 imply that it is a useful marker for the diagnosis of PMTs, as well as being useful for detecting the presence of lymphatic vessels inside PMTs.
The presence of microcytic structures was positively correlated with the total score; all six cases with a total score ≥ 5+ contained them; among the eight cases with a total score ≤ 4+, only one case (total score 3+, case 9) contained them. Most of the tumor cells lining the microcystic structures were immunostained with D2-40. Microcystic structures contain a lymph-like fluid; it is thus presumed that the tumor cells show immunophenotype of lymphatic endothelium and that microcystic structures may have some relationship with this phenotypic change. However, microcystic structures are not equivalent to lymphatic vessels, because D2-40-immunostaining in microcystic structures was weaker than in that of lymphatic vessels.
In conclusion, most cases of PMTs have tumor cells immunoreactive for D2-40 and contain D2-40-positive lymphatic vessels. Microcystic structures appeared in half of the PMTs and their presence positively correlated with the degree of D2-40-staining of tumor cells of PMTs. As microcystic structures contained lymph-like fluid, PMTs taking immunophenotype of lymphatic endothelium may show some extent of differentiation toward lymphatic lineage. D2-40 is a useful diagnostic marker of PMTs.
Disclosure of conflict of interest
None.
References
- 1.Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011;18:R53–77. doi: 10.1530/ERC-11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism--unique biological characteristics of FGF23. Bone. 2007;40:1190–1195. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab. 2012;23:610–618. doi: 10.1016/j.tem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakahama H, Nakanishi T, Uno H, Takaoka T, Taji N, Uyama O, Kitada O, Sugita M, Miyauchi A, Sugishita T, et al. Prostate cancer-induced oncogenic hypophosphatemic osteomalacia. Urol Int. 1995;55:38–40. doi: 10.1159/000282746. [DOI] [PubMed] [Google Scholar]
- 5.Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, Econs MJ, Inwards CY, Jan de Beur SM, Mentzel T, Montgomery E, Michal M, Miettinen M, Mills SE, Reith JD, O’Connell JX, Rosenberg AE, Rubin BP, Sweet DE, Vinh TN, Wold LE, Wehrli BM, White KE, Zaino RJ, Weiss SW. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Folpe AL. Phosphaturic mesenchymal tumour. Lyon: IARC; 2013. [Google Scholar]
- 7.Carter JM, Caron BL, Dogan A, Folpe AL. A novel chromogenic in situ hybridization assay for FGF23 mRNA in phosphaturic mesenchymal tumors. Am J Surg Pathol. 2015;39:75–83. doi: 10.1097/PAS.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 8.Williams K, Flanagan A, Folpe A, Thakker R, Athanasou NA. Lymphatic vessels are present in phosphaturic mesenchymal tumours. Virchows Arch. 2007;451:871–875. doi: 10.1007/s00428-007-0471-y. [DOI] [PubMed] [Google Scholar]
- 9.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, Yeger H, Banerjee D, Baumal R. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569–578. doi: 10.1038/sj.bjc.6690393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 12.Tajima S, Takashi Y, Ito N, Fukumoto S, Fukuyama M. ERG and FLI1 are useful immunohistochemical markers in phosphaturic mesenchymal tumors. Med Mol Morphol. 2015 doi: 10.1007/s00795-015-0115-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Evangelou E, Kyzas PA, Trikalinos TA. Comparison of the diagnostic accuracy of lymphatic endothelium markers: Bayesian approach. Mod Pathol. 2005;18:1490–1497. doi: 10.1038/modpathol.3800457. [DOI] [PubMed] [Google Scholar]
- 14.van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol. 1994;18:814–820. doi: 10.1097/00000478-199408000-00008. [DOI] [PubMed] [Google Scholar]


